Children of mothers with psychiatric disorders during pregnancy are at high risk for developing physical and psychiatric disorders later in life.Reference Goodman, Rouse, Connell, Broth, Hall and Heyward1,Reference Sanger, Iles, Andrew and Ramchandani2 Maternal psychopathology and stress during pregnancy are among the most common intrauterine exposures associated with negative outcomes in offspring, with prevalence rates of 10–15% for depression and anxiety disorders.Reference Bennett, Einarson, Taddio, Koren and Einarson3–Reference Dennis, Falah-Hassani and Shiri5 Psychiatric disorders are associated with alterations in basal cortisol levels and disturbed variability of the stress response owing to dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis.Reference Booij, Bouma, de Jonge, Ormel and Oldehinkel6,Reference Zorn, Schur, Boks, Kahn, Joels and Vinkers7 Accumulating research suggests placental transfer of maternal cortisol might play a mediating role in the effects of maternal psychopathology on the neurocognitive and physical development of the foetus,Reference Monk, Feng, Lee, Krupska, Champagne and Tycko8 and increased prenatal maternal cortisol levels have been repeatedly linked to adverse child outcomes in the short and long term, such as lower birth weight, small for gestational age, intellectual disability and behavioural problems.Reference Buitelaar, Huizink, Mulder, de Medina and Visser9–Reference Talge, Neal and Glover11 As most previous research has focused on less-affected individuals, the specific aim of this study is to explore the effect of severe and long-lasting psychiatric disorders during pregnancy on early hair cortisol concentrations (HCCs) in mother–infant dyads.
Hair cortisol is a reliable biomarker reflecting chronic systemic cortisol levels,Reference Russell, Kirschbaum, Laudenslager, Stalder, de Rijke and van Rossum12,Reference Manenschijn, Koper, Lamberts and van Rossum13 as well as stress exposure.Reference Raul, Cirimele, Ludes and Kintz14,Reference Mustonen, Karlsson, Scheinin, Kortesluoma, Coimbra and Rodrigues15 Maternal HCC at 6 weeks’ postpartum probably reliably reflects cortisol exposure in the preceding 3 months, which allows us to quantify cortisol exposure from the last 6 weeks of pregnancy to the first 6 weeks’ postpartum.Reference Kirschbaum, Tietze, Skoluda and Dettenborn16,Reference Hollanders, van der Voorn, Kieviet, Dolman, de Rijke and van den Akker17 In very young infants, less is known about the exact timeframe of exposure, but supposedly HCC reflects cortisol exposure in the intrauterine milieu during late pregnancy and early postpartum cortisol exposure.Reference de Kruijff, Noppe, Kieviet, Choenni, Lambregtse-van den Berg and Begijn18
HPA axis and psychiatric disorders
The relationship between psychopathology and HPA axis deviations in general has not been fully elucidated. Specific psychiatric diagnoses, such as major depressive disorder, bipolar disorder and schizophrenia, have been linked to higher basal cortisol levels, whereas anxiety disorders have been associated with a combined profile of higher levels of cortisol during acute stress and lower baseline cortisol levels.Reference Staufenbiel, Penninx, Spijker, Elzinga and van Rossum19,Reference Olff, Güzelcan, de Vries, Assies and Gersons20 The latter finding of lower baseline cortisol levels has also been found in studies on borderline personality disorder.Reference Thomas, Gurvich, Hudaib, Gavrilidis and Kulkarni21 Post-traumatic stress disorder has been generally linked to lower cortisol levels; however, cortisol appears to be elevated when the traumatic event has happened more recently, or when traumatic circumstances are still present.Reference Steudte-Schmiedgen, Kirschbaum, Alexander and Stalder22,Reference van den Heuvel, Stalder, du Plessis, Suliman, Kirschbaum and Seedat23 It has been proposed there is a non-linear, two-stage timeline with regard to cortisol dysfunction in relation to trauma: the severity of traumatisation and more temporally distant traumatisation were related to lower HCC, whereas higher HCC was found in more recently traumatised individuals.Reference Gao, Zhong, Xie, Wang, Jin and Deng24 Similarly, in depression, it has been found that recurring episodes are associated with lower HCC. This evidence leads to the hypothesis that chronic overactivation of the stress response leads to blunted HPA axis activity over time, indicating that the severity and duration of stress activity might be a more important determinant of basal cortisol levels in patients with severe and long-lasting psychiatric disorders than the nature of the psychiatric diagnosis.Reference Danese, Moffitt, Harrington, Milne, Polanczyk and Pariante25,Reference Nystrom-Hansen, Andersen, Khoury, Davidsen, Gumley and Lyons-Ruth26
Maternal HPA axis functioning and its influence on the foetus
Altered HPA axis activity in mothers who suffer from severe psychiatric disorders can influence the foetus through intrauterine programming of the HPA axis.Reference Staufenbiel, Penninx, Spijker, Elzinga and van Rossum19,Reference Van den Bergh, van den Heuvel, Lahti, Braeken, de Rooij and Entringer27 There is some evidence that these early alterations in HPA axis functioning contribute to vulnerability to psychiatric disease in offspring later in life,Reference Molenaar, Tiemeier, van Rossum, Hillegers, Bockting and Hoogendijk28 by early fine-tuning of the HPA axis set point.
Under normal conditions, in the absence of psychopathology or severe stress, maternal and infant cortisol and cortisol responses appear to be positively correlated shortly after birth.Reference Sethre-Hofstad, Stansbury and Rice29,Reference Stenius, Theorell, Lilja, Scheynius, Alm and Lindblad30 This finding is mainly based on research with saliva cortisol, but has been confirmed in animal studies on hair cortisolReference Meise, von Engelhardt, Forcada and Hoffman31 and in studies on healthy mother–infant dyads.Reference Hollanders, van der Voorn, Kieviet, Dolman, de Rijke and van den Akker17
Studies evaluating the effects of stress and psychopathology on maternal and foetal HCC during pregnancy and beyond are rapidly emerging, but results are inconclusive.Reference Orta, Tworoger, Terry, Coull, Gelaye and Kirschbaum32–Reference Kim, Kim and Son38 This might be partly explained by differences in study sample (i.e. healthy versus depressed mothers) and different definitions of ‘stress’ (i.e. perceived stress versus psychiatric symptom scales). Inconsistent results have emerged on the association between maternal prenatal cortisol levels and self-reports of prenatal psychological distress, elevated symptoms of prenatal depression, anxiety and antidepressant use. Evidence in different studies does show that excess maternal cortisol during pregnancy is associated with decreased infant cortisol levels, as measured in infant hair, shortly after birthReference Van der Voorn, Hollanders, Kieviet, Dolman, de Rijke and van Rossum33,Reference Romero-Gonzalez, Caparros-Gonzalez, Gonzalez-Perez, Delgado-Puertas and Peralta-Ramirez39 and at 12 months’ postpartum.Reference Galbally, van Rossum, Watson, de Kloet and Lewis34 In 2-year-old children, Bryson et alReference Bryson, Mensah, Goldfeld, Price and Giallo40 found a significant association between maternal and infant HCC that was not mediated by measures of early childhood adversity. Furthermore, two studies found that elevated maternal HCC during pregnancy mediated disrupted mother–child interaction in early infancy.Reference Nystrom-Hansen, Andersen, Khoury, Davidsen, Gumley and Lyons-Ruth26,Reference Khoury, Bosquet Enlow, Patwa and Lyons-Ruth41 These results suggest that maternal stress (i.e. psychiatric symptoms) is inconsistently related to maternal and/or infant HCC, but independently, maternal HCC seems to influence infant cortisol and mother–child interaction both in the early postpartum period and beyond.
The underlying mechanism of transmission of maternal psychopathology during pregnancy through cortisol remains unclear, as cortisol attunement between mother and foetus is composed of complex intrauterine interactions between the maternal, placental and foetal endocrine systems. The placental barrier is not completely impenetrable for transfer of cortisol, as a small proportion (10–20%) of maternal cortisol does reach the foetus.Reference Zhu, Wang, Zuo and Sun42 However, in stressful situations, more cortisol can cross the placental barrier.Reference Reynolds43,Reference Cottrell and Seckl44 Thus, it has been proposed that in stressed mothers, the excess of maternal cortisol levels leads to downregulation of cortisol production in the foetal adrenal,Reference Van der Voorn, Hollanders, Kieviet, Dolman, de Rijke and van Rossum33 altering the set point of HPA axis functioning in the foetus. Because psychiatric disorders are associated with altered HPA axis activity, and this is associated with suboptimal HPA axis functioning of the infant, more insight into this process is needed in clinical and healthy dyads, to understand contributing factors and ultimately prevent adverse outcomes for offspring.
Hypotheses
In the current study, we assessed the association between severe and long-lasting psychiatric disorders and HCCs of mothers and infants. We compared HCCs of patient dyads to healthy control dyads at 6 weeks’ postpartum, to further elucidate mechanisms associated with the transgenerational transmission of psychopathology. In accordance with previous studies demonstrating that psychiatric disorders are associated with differential HPA axis disturbances, we expected larger variance in cortisol concentrations in our patient group than in controls. Subsequently, we assessed the effect of the severity of current maternal symptoms on infant HCC.
Also, we expected infant HCC to be associated with maternal perinatal HCC in healthy dyads. Previous research shows that in healthy mother–infant dyads, maternal and infant HCC appear to be positively correlated. Therefore, we expected to find an attuned association of HCC in control dyads. Because maternal psychopathology is associated with both increased and decreased maternal cortisol levels, influenced by the nature, chronicity and genetic heritability of the psychiatric disorder, we expected to find a divergence of this pattern in mothers and infants who were subject to maternal severe psychiatric disorders.
Method
Study procedure and design
The current study was embedded in an observational study on parenting capacity of mothers with severe psychiatric disorders and their infant's cognitive and socio-emotional development (the Infant Caregiving Assessment Scales (INCAS) study). All mothers fulfilled criteria for a current severe psychiatric disorder. A common definition of severe psychiatric disorders, or ‘severe mental illness’, consists of having any psychiatric diagnosis with a treatment duration of 2 years or more, together with dysfunction, as indicated by lower scores on the Global Assessment of Functioning scale.Reference Jones, Thorncroft, Coffey and Dung45 Common disorders that are referred to are schizophrenia, mood disorders (chronic depression, bipolar disorder), chronic anxiety and personality disorders.Reference Ruggeri, Leese, Thornicroft, Bisoffi and Tansella46
During pregnancy, mothers with severe psychiatric disorders were recruited from specialised psychiatry-obstetrics-paediatric secondary and tertiary out-patient clinics and other specialised mental healthcare institutions where pregnant women who suffer from psychiatric disorders are treated. Healthy control mothers, without current or a history of psychiatric symptoms, were recruited during pregnancy at midwifery practices in the central western part of the Netherlands, consisting primarily of the four largest Dutch cities and their surrounding areas.
The INCAS study was approved by the Erasmus University Medical Center Medical Research Ethics Committee (approval number NL42662.078.12); written informed consent was obtained from all mothers for their own and their infant's participation, and from fathers with legal guardianship.
Exclusion criteria
In the current study, exclusion criteria for both groups were insufficient amount of hair necessary for cortisol analysis; use of locally administered and systemic corticosteroids during or after pregnancy; use of illicit drugs or alcohol in the last trimester of pregnancy; and perinatal complications, including prematurity. Additionally, control dyads were excluded from analysis when maternal global score on the Brief Symptom Inventory (BSI) was in the clinical range or when mothers used psychotropic medication.Reference De Beurs and Zitman47
Inclusion of clinical sample
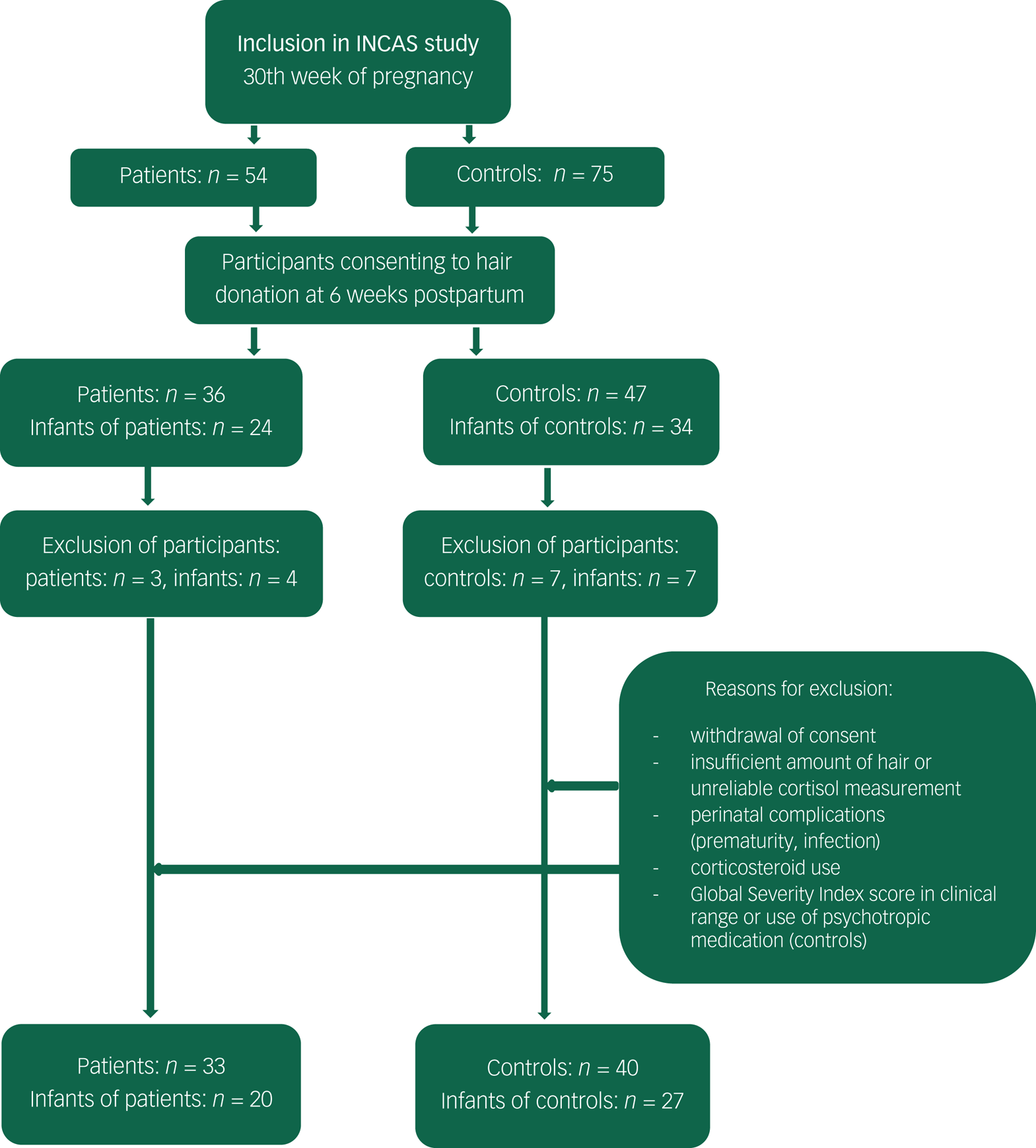
From June 2013 to January 2016, patients and control mothers were included in the INCAS study during their third trimester of pregnancy (N = 129). A total of 64% of participating mothers (n = 83) agreed on hair donation at 46 ± 8.5 days’ postpartum (range 34–84 days) for themselves, and 45% for their infant (n = 58). After exclusion based on the aforementioned exclusion criteria, HCCs were available for a total of 73 mothers (patient n = 33, control n = 40) and 47 infants (infant of patient n = 20, control infant n = 27) (see flowchart in Appendix).
Non-response analyses, comparing mothers and infants who did and did not donate hair for cortisol measurement, showed no differences with regards to maternal age, ethnicity, educational level, psychiatric symptoms, and infant birth weight or gestational age.
Measures
HCCs
Mother and child HCCs were determined from hair strands collected 6 weeks’ postpartum (46 days ± 8.5, range 34–84 days). All samples were collected according to researcher protocol. In adults, scalp hair has a predictable growth rate of approximately 1 cm per month, making it possible to have an estimate of long-term exposure to cortisol.Reference Kirschbaum, Dettenborn, Stalder, Foley, Steudte and Tietze48,Reference Lee, Kim and Choi49 When collected at 6 weeks’ postpartum, HCC in the proximal 3 cm of maternal hair reflects the maternal HPA axis activity over the first 6 weeks after childbirth and the last 6 weeks of pregnancy.Reference Kirschbaum, Tietze, Skoluda and Dettenborn16
A small strand of hair was cut from the posterior vertex of the scalp, as close as possible to the scalp. Hair strands were taped to a piece of paper with the scalp end marked, and stored in an envelope at room temperature until further analysis. The proximal 3 cm of maternal hair samples were weighed and minced. For infants, the full length of the hair was analysed with a minimum of 1.25 mg, for reliable measurement. For extraction of cortisol, LC-grade methanol was used at 25°C, for 18 h, in the presence of labelled glucocorticoids as internal standard. The extraction was centrifuged and cleaned. Cortisol concentrations were quantified by liquid chromatography with tandem mass spectrometry (Waters XEVO-TQ-S system; Waters Corporation, Milford, MA, USA). Measurements were reported in picograms per milligram of hair, and log-transformed (10log) to approach normality.Reference Noppe, Rijke, Dorst, Akker and Rossum50
Psychiatric diagnosis and current symptoms
Presence and history of psychiatric diagnoses were examined with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II), by a trained interviewer.Reference First, Spitzer and Groenestijn51,Reference First, Spitzer, Gibbon and Williams52 SCID-I and SCID-II are considered to be the gold standard of semi-structured assessment instruments for clinical psychiatric disorders, with adequate to excellent validity and interrater reliability.Reference Lobbestael, Leurgans and Arntz53
Level of current symptoms in both the patient and control group were measured with the BSI, at 6 weeks’ postpartum.Reference Derogatis and Melisaratos54 Severity of stress was indicated by the Global Severity Index (GSI).Reference Derogatis55 The BSI comprises 53 items on nine symptom dimensions (somatisation, obsession–compulsion, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation and psychoticism). The GSI presents the mean BSI score. Normative data are available for clinical and non-clinical samples. The BSI has a high internal consistency, moderate test–retest reliability and strong convergent validity with measures of emotional functioning.Reference De Beurs and Zitman47 In our sample, Cronbach's alpha was 0.93 in patients and 0.82 in controls.
Covariates and potential confounders
Demographic data; information on smoking, alcohol and illicit drug use; and exposure to psychotropic medication during pregnancy were collected during the third trimester of pregnancy (patients) and at 6 weeks’ postpartum (controls), using self-reports. Confounders were selected a priori, based on previous research.Reference Hoffman, D'Anna-Hernandez, Benitez, Ross and Laudenslager56,Reference Rippe, Noppe, Windhorst, Tiemeier, van Rossum and Jaddoe57 The following confounders were controlled for the following: child gender, gestational age and birth weight; and maternal age, ethnicity, socioeconomic status, parity (primiparity versus multiparity), tobacco use and use of psychotropic medication.
Data analyses
Demographic and clinical characteristics of the control and patient sample are reported, and differences between the samples were tested with χ 2-tests (for categorical variables) and t-tests or Mann–Whitney U-tests (for continuous variables). Differences in HCCs between patient and control mothers, for diagnostic subgroups in the patient group, and between infants, were tested using Mann–Whitney U-tests. For this purpose, HCCs were log-transformed.
We estimated the association between maternal and infant HCC in both the patient and control sample by regression analysis. Preliminary analyses did not show significant correlations between hair characteristics (e.g. hair treatment in the past 3 months, heavy transpiration, hair product use before hair collection) in mothers (P > 0.201) or infants (P > 0.577), or between timing of the hair sample (range 34–84 days’ postpartum) and infant HCC (P = 0.770); accordingly, we did not control for these variables in the regression analyses. To adjust for the effects of other potential confounders, we calculated a propensity score including all available confounders as summarised in subheading 'Covariates and potential confounders', and included the propensity score as a single covariate in all analyses.Reference Freemantle, Marston, Walters, Wood, Reynolds and Petersen58 Differences in maternal–infant HCC associations between the patient and control samples were tested with Fisher z-scores.
We also explored whether maternal symptom severity was related to HCC in mothers and infants. Therefore, we estimated the association between perinatal symptom severity levels (based on BSI scores) and maternal and infant HCCs, using regression analysis. We conducted a sensitivity analysis, repeating the regression analysis but leaving out two outliers.
Results from the regression analyses are reported as correlation coefficient (r) and s.e.Reference Altman59 Q-Q plots were used to check all data for normality of the distribution. HCC data were checked for extreme outliers (defined as below quartile 1 (Q1) − 1.5 interquartile range (IQR) or above quartile 3 (Q3) + 1.5 interquartile range (IQR)), which were removed from all analyses (n = 4). Statistical analyses were performed with SPSS version 24 for Windows (IBM, New York, USA).
Results
Background and clinical characteristics
A sample description is displayed in Tables 1 and 2. Mothers in the patient and control group did not differ with regard to age and ethnicity. Lower educational level and smoking were more common among patients. Expectedly, infants of patients had a significantly lower gestational age and birth weight compared with control infants.Reference Ruggeri, Leese, Thornicroft, Bisoffi and Tansella46,Reference Grote, Bridge, Gavin, Melville, Iyengar and Katon60 In the patient group, depressive and anxiety disorders were most common (33.3 and 51.1%, respectively), followed by bipolar disorders (18.2%). A considerable percentage of mothers (39.4%) had two or more Axis I disorders (e.g. depressive disorder and panic disorder). Furthermore, half of the patients had a comorbid personality disorder (48.4%), mostly in Cluster C (avoidant, dependent or obsessive–compulsive personality disorder). Approximately two-thirds (67.7%) of the patient group used psychotropic medication during pregnancy, which were mostly antidepressants, followed by antipsychotics and hypnotics. A smaller group of mothers used two or more psychotropic medications (19.4%).
Table 1 Demographic characteristics of patients (n = 33), control mothers (n = 40), infants of patients (n = 20) and control infants (n = 27)

GSI, Global Severity Index.
a. Mean reference range was 0.93–1.32 for Dutch clinical females and 0.29–0.45 for healthy females.
Table 2 Clinical characteristics of patients (n = 33)

SSRI, Selective Serotonin Reuptake Inhibitor; SNRI, Selective Serotonin and Noradrenalin Reuptake Inhibitor; TCA, Tricyclic Antidepressants.
a. Any exposure during pregnancy.
Differences in HCCs between patient and control dyads
HCC of patient and control dyads are displayed in Fig. 1. Median (interquartile range) and distribution of HCC were significantly different in patients compared with control mothers (U = 468.5, P = 0.03). Results did not differ in infants of patients (U = 250.0, P = 0.67).

Fig. 1 Distribution of hair cortisol concentrations (HCCs) in patient versus control mothers, and infants of patients versus infants of controls. Median (interquartile range) and distribution of HCCs were significantly different in patients compared with control mothers (U = 468.5, P = 0.03). Results did not differ in infants of patient (U = 250.0, P = 0.67).
Correlation of HCCs within clinical and control mother–infant dyads
We found a positive correlation between maternal perinatal HCC and infant HCC in the control group (n = 27, r = 0.55 (0.14), P = 0.003). The correlation between maternal perinatal HCC and infant HCC in the patient group was non-significant (n = 18, r = 0.082 (0.13), P = 0.746; see Fig. 2). The correlations between maternal perinatal HCC and infant HCC were significantly different across the patient and control group (z = −1.64, P = 0.05). The correlation analyses were repeated with the propensity score, to adjust for confounders. After adjustment, the strength of the correlation between HCCs in control mother–infant dyads increased somewhat (r = 0.65 (0.13), P = 0.001). In patient mother–infant dyads, the correlation increased greatly, but remained non-significant (r = 0.37 (0.13), P = 0.16).

Fig. 2 Correlation between mother and infant log-transformed hair cortisol concentrations (HCCs). Figure based on unadjusted results.
Correlation of current maternal symptom severity with HCCs in patient dyads
We explored if maternal symptom severity in the perinatal period is correlated with maternal and infant HCC. Results are displayed in Fig. 3.

Fig. 3 Association between maternal symptom severity by means of the Global Severity Index and log-transformed hair cortisol concentrations (HCCs) of patients (left) and infants of patients (right). Figure based on unadjusted results.
In mothers, symptom severity was not correlated with HCC (n = 23, r = −0.09 (0.12), P = 0.67). In infants, a positive correlation between maternal perinatal symptom severity and HCC was found (n = 16, r = 0.63 (0.06), P = 0.008). The correlation analyses were repeated with the propensity score, to adjust for confounders. After adjustment, the strength of the correlation between symptom severity and maternal perinatal HCC (r = 0.08 (0.13), P = 0.70) and infant HCC (r = 0.59 (0.18), P = 0.02) remained in a similar range. In the sensitivity analysis, leaving out the two outlier infants on the right, the strength of the correlation between maternal symptom severity and infant HCC remained in a similar range as our original result.
Discussion
In this study, we explored the influence of severe psychiatric disorders on HCCs of mothers and newborn infants. We found a significantly wider range of HCCs in mothers with severe psychiatric disorders compared with controls, but we did not find differences in infants. We also found that HCC of patients and infants of patients were not associated, whereas in control dyads, we found a significant positive association between mother and child HCCs. In infants of patients, HCCs were positively associated with maternal symptom severity.
HCCs in patient and control groups
In our study, a significant variation in HCC was found in mothers with psychopathology, showing both (mainly) higher and lower values than control mothers. One explanation for this finding is the nature of our clinical sample, in which women with severe and long-lasting psychiatric disorders were selected. Previous studies have shown cortisol levels in patients with mood disorders change over time. First episodes are more often associated with higher cortisol levels, whereas long-term duration and recurrence of episodes might diminish the sensitivity of the HPA axis over time.Reference Booij, Bouma, de Jonge, Ormel and Oldehinkel6,Reference Wei, Sun, Zhao, Yang, Liu and Lin61 Heterogeneity of psychiatric diagnoses and high prevalence of medication use might also be critical factors in differences of HPA axis functioning and long-term release of cortisol in affected mothers.Reference Staufenbiel, Penninx, Spijker, Elzinga and van Rossum19,Reference Dettenborn, Muhtz, Skoluda, Stalder, Steudte and Hinkelmann62
Infants of patients showed no significant differences with regard to variation in HCC compared with control infants. Previous studies have shown that higher maternal perinatal HCC predicted lower HCC in newborn infants early postpartum.Reference Van der Voorn, Hollanders, Kieviet, Dolman, de Rijke and van Rossum33,Reference Romero-Gonzalez, Caparros-Gonzalez, Gonzalez-Perez, Delgado-Puertas and Peralta-Ramirez39 We could not replicate this finding. Three factors may contribute to this inconsistence. First, the sample size of our study might not have been sufficient to uncover differences in our patient dyads. Second, the mothers in the aforementioned studies were healthy or subject to mood and anxiety disorders and only used antidepressants, whereas patients in this study had high rates of comorbidity and other medication use (including antipsychotics). Third, the absence of marked differences of HCC in infants of the patient group might reflect that foetal exposure to increased or decreased maternal cortisol concentrations during pregnancy is effectively regulated by the dynamic nature of placental 11β-hydroxysteroid dehydrogenase type II (11β-HSD-2).Reference Benediktsson, Calder, Edwards and Seckl63
Prenatal synchrony of HCCs between mother and child
In line with previous studies,Reference Hollanders, van der Voorn, Kieviet, Dolman, de Rijke and van den Akker17,Reference Romero-Gonzalez, Caparros-Gonzalez, Gonzalez-Perez, Delgado-Puertas and Peralta-Ramirez39 we found a positive association between maternal and infant HCC in healthy control dyads. This finding might reflect early physiological synchrony, which is defined as the matching of biological states between mother and child that develops via interactions among genetic predispositions, prenatal programming and postnatal behaviour.Reference Davis, West, Bilms, Morelen and Suveg64,Reference Feldman65 In mother–infant dyads subject to severe psychiatric disorders, we found a divergence of this pattern. This might indicate that synchrony of the HPA axis between mother and child might be prenatally affected by the presence of a maternal psychiatric disorder. This finding should be interpreted with caution because the two groups in this study differed with regards to relevant demographic and obstetric variables (e.g. lower education level in patients, lower birth weight and gestational age in infants of patients), but it should also be noted that the findings remained the same when these factors were controlled for.
HCCs and (self-reported) symptom severity
We did not find a correlation between maternal perinatal HCC and self-reported symptom severity at 6 weeks’ postpartum. Because of the previously mentioned blunted cortisol responses in long-lasting psychopathology, the absence of an association between HCC and maternal-reported stress levels might indicate reduced responsiveness of the HPA axis to stressful experiences.Reference Zorn, Schur, Boks, Kahn, Joels and Vinkers7 However, the relationship between the human concept of stress and HPA axis functioning is an ongoing subject of debate. A recent meta-analysis of Kalliokoski et alReference Kalliokoski, Jellestad and Murison66 on hair glucocorticoids as a measure of stress suggests that self-reported assessments of stress poorly correlate with HPA axis functioning. Furthermore, symptom assessments in our study were obtained postpartum, which is an especially chaotic transition in a woman's life. It might not be accurate to relate this to cortisol levels that presumably reflect third trimester exposure of cortisol. It also cannot be ruled out that other physical factors that are important determinants of cortisol levels, such as obesity, metabolic syndrome and cardiovascular disease, were overrepresented in the patient group compared with the control group, and therefore may have influenced the outcomes.Reference Wester, Staufenbiel, Veldhorst, Visser, Manenschijn and Koper67,Reference Stalder, Kirschbaum, Alexander, Bornstein, Gao and Miller68
Interestingly, in infants of patients, we found that higher maternal symptom severity was associated with higher infant HCC, after statistically controlling for known covariates of HCC. There are several possible explanations for this finding. It has been proposed that the placental barrier function, inactivating cortisol by 11β- HSD-2, may be impaired by stress,Reference Cottrell and Seckl44,Reference Zijlmans, Riksen-Walraven and de Weerth69 allowing an increased passage of maternal cortisol to the foetus.Reference Benediktsson, Calder, Edwards and Seckl63 Also the production of placental corticotropin-releasing hormone (CRH) might be reacting to blunting of the maternal HPA axis, leading to stimulation of the foetal adrenal.Reference Glynn, Davis and Sandman70 Stressful circumstances during delivery and in the postpartum period might also contribute to higher infant HCC. However, this finding is subject to the same limitations (i.e. maternal self-reported stress) as in mothers, and has to be interpreted with caution.
Strengths and limitations
Our study has several strengths and limitations. The foremost strength of this study is our patient sample of mothers with various severe psychiatric disorders, representing the heterogeneity of clinical populations. Further strengths are the non-invasive measurement of chronic stress in hair performed with the state-of-the-art liquid chromatography with tandem mass spectrometry method, and the availability of detailed and reliable diagnostic information, as well as the possibility to adjust for various covariates. Limitations include the limited sample size of subgroups, which only allowed for an initial exploration. Additionally, we only measured psychiatric symptom severity at 6 weeks’ postpartum, and could therefore not take into account the possible variation of symptoms over time.
Conclusions and future research
In the current study, we observed differences in the association between HCCs of patients and their infants compared with healthy controls and their infants. Where in healthy control dyads there seems to be perinatal synchrony of HPA axis functioning in mother and infant, our findings suggest there is a divergence of this pattern in mother–infant dyads subjected to long-lasting and severe psychiatric disorders. In infants, these early differences might influence lifetime HPA axis functioning, as has been suggested in previous research.Reference Molenaar, Tiemeier, van Rossum, Hillegers, Bockting and Hoogendijk28 In turn, altered HPA axis functioning may increase susceptibility to disease, both physically and mentally. Future longitudinal studies in larger clinical samples should examine how maternal and infant hair cortisol levels are intertwined perinatally, in the early postpartum period and beyond.
Data availability
Data are stored at the institutional database of the Erasmus Medical Center in Rotterdam, The Netherlands. The data-sets on which the analyses are based are available on request to the Local Ethics Committee of the Erasmus Medical Center in Rotterdam. To request the data, please contact the corresponding author, M.P.L.-V.d.B., or Dr Joke Tulen ([email protected]). The data are not publicly available due to ethical restrictions and patient confidentiality requirements.
Author contributions
C.W.B. contributed to conceptualization, data curation, formal analysis, methodology, writing - original draft, writing - review and editing. V.C. contributed to conceptualization, data curation, funding acquisition, methodology, writing - review and editing. R.K. contributed to conceptualization, data curation, funding acquisition, methodology, writing - review and editing. B.v.d.V. contributed to writing - review and editing. I.d.K. contributed to writing - review and editing. E.L.T.v.d.A. contributed to writing - review and editing. E.F.C.v.R. contributed to writing - review and editing. W.J.G.H. contributed to writing - review and editing. M.H.J.H. contributed to writing - review and editing. A.M.K. contributed to conceptualization, formal analysis, methodology, writing - review and editing. M.P.L.-V.d.B. contributed to conceptualization, data curation, formal analysis, funding acquisition, methodology, writing - review and editing.
Funding
This research has been funded by the Sophia Foundation for Scientific Research (grant no. 670).
Declaration of interest
None.
ICMJE forms are in the supplementary material, available online at https://doi.org/10.1192/bjo.2020.159.
Appendix: flowchart









eLetters
No eLetters have been published for this article.