Many young children living in urban poverty today are vulnerable to malnutrition( 1 ). In Brazil, the Northeast (NE) region is the poorest in the country and where young children living in low-income urban slums are at increased risk of anaemia, micronutrient deficiencies and morbidity( Reference Prado, Strina and Barreto 2 – Reference Martins, Santos and Assis 4 ). Together, these deleterious effects can have a long-lasting adverse impact on the cognitive and developmental potential of young children( Reference Grantham-McGregor, Cheung and Cueto 5 ).
Numerous diet and host-related factors may contribute to micronutrient malnutrition among urban slum pre-schoolers in NE Brazil. Their staple diets are based on cereals and legumes, and are devoid of expensive micronutrient-dense animal-source foods. Thus, the adequacy of key essential micronutrients such as bioavailable Fe and Zn, preformed vitamin A and vitamin B12 may be compromised by low intakes and/or poor bioavailability from these plant-based diets( Reference Antunes, Sichieri and Salles-Costa 6 ). In urban slum settings, the water supply and sanitation are poor( Reference Prado, Strina and Barreto 2 ), so intestinal parasitic infections are often widespread( Reference Barreto, Genser and Strina 7 ). Such infections can exacerbate poor micronutrient bioavailability by increasing permeability and reducing transit time in the intestine( Reference Müller and von Allmen 8 ). Furthermore, in Salvador, NE Brazil, 80 % of the population is of West African descent( 9 ). Hence, some disadvantaged pre-schoolers in Salvador are likely to be at risk for genetic Hb disorders, particularly thalassaemias and sickle cell disease( Reference Bain 10 ), which can be associated with anaemia and Zn deficiency, respectively( Reference Zemel, Kawchak and Fung 11 , Reference Fung 12 ).
Over recent decades, Brazil has implemented several key pro-poor policies to improve the micronutrient status, health, physical and cognitive development of urban pre-schoolers living in low-income environments. Policies include supplementation of young children with vitamin A and Fe( 13 ), mandatory fortification of wheat and corn flour with Fe and folic acid( 13 ), implementation of sewage and sanitation programmes( Reference Barreto, Genser and Strina 7 ), and promotion of day-care facilities for the care and education of pre-schoolers( 14 ).
Nevertheless, there have been few comprehensive studies that have examined the micronutrient status of disadvantaged urban pre-schoolers attending day care in NE Brazil since the implementation of these policies. Consequently, little is known about the prevalence of micronutrient deficiencies among children in these settings today. In earlier reports, we have examined the growth, intestinal parasitic infections and adequacy of the nutrient supply from day-care meals in a group of pre-schoolers living in urban slums in Salvador, NE Brazil( Reference Lander, Lander and Houghton 15 , Reference Lander 16 ). Here, we extend the research by investigating the prevalence of anaemia and micronutrient deficiencies in these same children and exploring the major predictors of Hb and micronutrient biomarkers.
Methods
Study sites and participants
The present cross-sectional study was conducted between August and November 2010 in Salvador, in the state of Bahia, NE Brazil. Seven philanthropic pre-school day-care centres within the city centre and peri-urban areas participated, as described earlier( Reference Lander, Lander and Houghton 15 ). These day-care centres were selected based on the criteria that all the children in attendance were from poor communities and participated full-time (i.e. 07.30–17.00 hours). The service provided was without charge and provided five meals per day, with an on-site dietitian.
The children (n 376) enrolled in the day-care centres (maximum class size of twenty-five children) were from low-income families and attended day care five days per week, except holidays, until school age. The day-care centres supplied filtered drinking water and the majority of the weekday food intake for the children, and provided flush toilets, hand basins and shower facilities. Inclusion criteria for the study were apparently healthy children enrolled in the day-care classes for 3- and 4-year-olds in the 2010 school year (February to December). The study protocol was approved by the Human Ethics Committees of the Federal University of Bahia, Salvador and the University of Otago, New Zealand. Informed written permission to participate in the study was given by the parents or primary guardians of the children.
Questionnaire and assessment of children's growth, parasite status and day-care meals
Data on sociodemographic status and health of the participants were collected via a culturally appropriate structured questionnaire, administered by trained research assistants. Methodological details are provided elsewhere( Reference Lander, Lander and Houghton 15 ). Sociodemographic data were used to calculate an overall socio-economic status (SES) score for each participant based on a model designed to assess the poverty level of Brazilian families in low socio-economic urban populations( Reference Issler, Guigliani and Kreutz 17 ). Briefly, points were assigned for family and housing size and structure, parental education and occupation, marital status, ownership of house and household assets, toilet and sewage facilities, type of drinking water, availability of electricity and susceptibility of the house to flooding during heavy rain, and used to calculate two SES categories: extremely low and low SES, based on scores ≤34 and ≥35, respectively.
Health status variables of the child supplied by maternal report included coffee intake within the past 24 h, smoking in the home and history of asthma in the mother or siblings. The mother also reported on the use of Fe supplements and deworming treatment within the past 6 months, whereas details of vitamin A supplementation was obtained from the child's health card. Ethnicity of the child was determined by skin colour, hair and facial characteristics, which were recorded by the research assistants, as performed in the national census( 9 ).
Weight and height measurements were taken with children wearing light clothes and no shoes using standardized techniques and calibrated equipment( 18 ). Standardized Z-scores for height-for-age (HAZ), weight-for-age (WAZ), weight-for-height (WHZ) and BMI (BMIZ) were calculated using the WHO 2006/2007 growth reference data( 18 , Reference de Onis, Onyango and Borghi 19 ). Overweight and obesity in the children was defined as BMIZ >1 to ≤2 and BMIZ >2, respectively.
Of the pre-schoolers, 86 % (325/376) provided a stool sample which was examined by microscopy for helminths and protozoan intestinal parasites. Positive samples for Giardia intestinalis were identified by an enzyme immunoassay. Major food sources and adequacy of the micronutrient supply from twenty daily day-care menus was evaluated by procedures described earlier( Reference Lander 16 ).
Biochemical assessment
Morning fasting peripheral venepuncture blood samples were obtained with participants in a sitting position, after applying a topical local vasodilator anaesthetic (amethocaine; Ametop™) to minimize any discomfort. Blood was drawn into a trace-element-free evacuated tube (Becton Dickinson, Franklin Lakes, NJ, USA) for the micronutrient and infection biomarkers and into a paediatric evacuated tube containing EDTA (Becton Dickinson) for a complete blood count and for testing for genetic Hb disorders. All blood samples were refrigerated immediately following collection( Reference Tamura, Johnston and Freeberg 20 ), protected from UV light, and the serum separated within 2 h using trace-element-free techniques. Aliquots of serum and washed red blood cells were frozen within 10 min, initially at −30°C and later at –70°C. An aliquot of EDTA-anticoagulated whole blood was haemolysed by a 1:10 dilution in 1 % ascorbic acid solution (w/w) and frozen for erythrocyte folate analysis( Reference Thurlow, Winichagoon and Green 21 ). Frozen samples were shipped on dry ice to the University of Otago, New Zealand for analysis.
Serum ferritin was determined on an Elecsys 2010 auto-analyser (Roche, New Zealand; CV = 3 %) using a Ferritin Elecsys reagent kit (Roche Diagnostics GmbH, Mannheim Germany) and soluble transferrin receptor (sTfR) via an enzyme immunoassay (Ramco Laboratories Inc., Houston, TX, USA; CV = 7 %). Serum Zn was analysed by flame atomic absorption spectrophotometry (ContrAA 700; Analytik Jena AG, Jena, Germany; CV = 5 %)( Reference Smith, Butrimovitz and Purdy 22 ) and serum Se by electrothermal atomic absorption spectrophotometry (AA-800, Perkin Elmer 2690; Ebos Group Ltd, Auckland, New Zealand; CV = 7 %)( Reference Jacobson and Lockitch 23 ). Serum retinol was analysed by HPLC( Reference Thurnham, Smith and Flora 24 ) (CV = 2 %) and serum vitamin B12 by an electrochemiluminescence immunoassay (CV = 4 %) using a Vitamin B12 Elecsys reagent kit (Roche Diagnostics GmbH) on an Elecsys 2010 auto-analyser (Roche, New Zealand). Serum and whole-blood folate concentrations were measured by microbiological assay( Reference Molloy and Scott 25 ) in ninety-six-well microtitre plates using chloramphenicol-resistant cryopreserved Lactobacillus rhamnosus (ATCC 27773; American Type Culture Collection, Manassa, VA, USA) and 5-methyltetrahydrofolate as the calibrator (Merck & Cie, Schaffhausen, Switzerland; CV = 14 %). Erythrocyte folate concentrations were calculated from whole blood values by using individual packed cell volumes and correction for serum folate concentration. Serum C-reactive protein (CRP) and α1-glycoprotein (AGP) concentrations were assayed by immunoturbidimetry (Roche Diagnostics GmbH) on a Cobas Mira II auto-analyser (CV = 10 % and 3 %, respectively). The precision of the biochemical assays was checked using a pooled serum sample and their accuracy established using certified reference materials or appropriate manufacturers’ controls; values fell within the certified ranges.
The complete blood count was determined using an automatic electronic analyser (Coulter LH 750 Hematology Analyzer; Beckman Coulter Inc., São Paulo, Brazil) in the Professor Edgar Santos Hospital haematology laboratory, Salvador. Haemoglobinopathies were detected by alkaline and acid Hb electrophoresis analysis (Sebia Hydrasys Electrophoresis Analyzer; Sebia Inc., Norcross, GA, USA), which separated normal haemoglobins (A, A2 and F) and detected major Hb variants, including the heterozygous variant of Hb S (Hb AS) and Hb C (Hb AC). The presence of α3·7 thalassaemia was determined by using a multiplex PCR reaction on extracted DNA( Reference Tan, Quah and Low 26 ). Positive Hb and DNA controls were used for the electrophoresis analysis and PCR reactions, respectively. A negative control confirmed the absence of contamination for the PCR results, as described elsewhere( Reference Alsaleh 27 ).
Anaemia and other haematological disturbances were defined using the following interpretive criteria: Hb <110 g/l and <115 g/l for children aged <5 years and ≥5 years, respectively( 28 ); mean cell volume (MCV) <73 fl and <74 fl for children aged <5 years and ≥5 years, respectively( 28 ); and red cell distribution width (RDW) >14 %( Reference Gibson 29 ). For storage Fe depletion, serum ferritin was defined as <12 μg/l and <15 μg/l for children aged <5 years and ≥5 years, respectively( 28 ). Tissue Fe deficiency was defined as sTfR >8·5 mg/l( Reference Cook, Skikne and Baynes 30 ). Hb AS and Hb AC, and α3·7 thalassaemia, homozygous or heterozygous, were identified as present or absent.
Interpretive criteria to define micronutrient deficiencies were: serum Zn <9·9 μmol/l( Reference Hotz, Peerson and Brown 31 ), Se ≤0·82 μmol/l( Reference Thomson 32 ), retinol <0·70 μmol/l( Reference de Pee and Dary 33 ) and vitamin B12 <150 pmol/l( Reference de Benoist 34 ). Marginal vitamin A status was defined as serum retinol ≥0·7 but <1·05 μmol/l( Reference Ballew, Bowman and Sowell 35 ). Low serum folate concentration was defined as <6·8 nmol/l and low erythrocyte folate as <317 nmol/l( Reference Pfeiffer, Johnson and Jain 36 ). Acute and chronic inflammation were assessed by serum CRP >5 mg/l( Reference Thurnham, Mburu and Mwaniki 37 ) and AGP >1·0 g/l( 38 ), respectively.
Statistical analysis
Selected characteristics of the children and households, and prevalence of intestinal parasites and genetic Hb disorders are presented as percentages. Means and standard deviations were calculated for haematology and micronutrient biomarkers, and adjusted where necessary for inflammation (i.e. ferritin and retinol)( Reference Thurnham, McCabe and Northrop-Clewes 39 , Reference Thurnham, McCabe and Haldar 40 ). Associations between elevated AGP concentrations (>1 g/l) and BMIZ >1 and between the presence of genetic Hb disorders and ethnicity were investigated using Fisher's exact test. AGP was used as a dichotomized variable because the precision of the assay precluded using the data as a continuous variable. Data were log-transformed if the variable had a strongly skewed distribution (e.g. ferritin, sTfR, retinol and serum folate). Multiple regression models included variables if associations using univariate regression analysis were P < 0·2. The sandwich estimator was used to obtain robust standard errors, to account for the sampling procedure. Statistical analyses were carried out using the statistical software package STATA version 11.
Results
Sociodemographic, health, growth and parasite status
Of the 438 eligible children, 378 (86 %) were recruited. Reasons for non-participation included children on the roll who had moved or were moving during the study, children who were chronically ill and not in regular day-care attendance, and parental refusal. Mean age of the children was 4·2 (sd 0·61) years and 52 % were boys (Table 1). Of the households, 48·4 % (182/376) were classified as extremely low SES and 51·6 % as low SES. Most of the pre-schoolers were black (42·2 %) or mixed race (i.e. brown; 51·8 %); only 6·0 % were white. More than 50 % had received vitamin A supplements and deworming treatment, but less than 20 % had reportedly been supplemented with Fe.
Table 1 Sociodemographic, growth, health, parasite status, genetic Hb disorders and elevated biomarkers of inflammation among pre-schoolers aged 3–6 years from disadvantaged households, Salvador, Northeast Brazil, August–November 2010
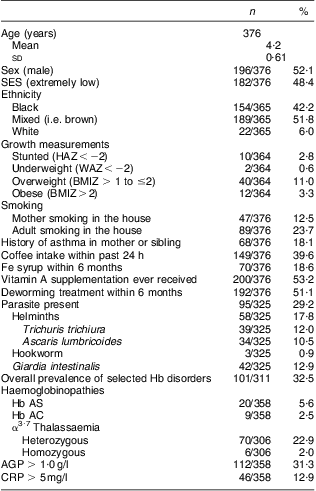
SES, socio-economic status; HAZ, height-for age Z-score; WAZ, weight-for-age Z-score; BMIZ, BMI Z-score; AGP, α1-glycoprotein; CRP, C-reactive protein.
Prevalence of stunting and underweight was low (≤5 %), whereas that of overweight and obesity was 11·0 % and 3·3 %, respectively (Table 1). Stool samples were provided by 86 % (325/376) of the pre-schoolers, of whom nearly 30 % were infected with at least one intestinal parasite (Table 1). No significant differences in age, sex, sociodemographic status and deworming treatment were found between participants who provided a stool sample and those participants who did not (n 51), except for the use of vitamin A supplements. Daily supply of almost all micronutrients from the day-care meals appeared adequate except for vitamin A, where risk of adequacy was ≤50 %, also reported earlier( Reference Lander 16 ). Intake of animal-source foods from the day-care meals was 80 g/d.
Genetic Hb disorders, biomarkers of infection and haematology
Almost a third of the pre-schoolers had at least one genetic Hb disorder, of which the most prevalent was heterozygous α3·7 thalassaemia (23 %). Prevalence of Hb AS and Hb AC was low (Table 1). Four children had both a haemoglobinopathy and α3·7 thalassaemia. Prevalence of genetic Hb disorders was independent of ethnicity. Hb AS tended to be higher in the black children (60 %, 12/20) compared with those of mixed race (i.e. 30 %, 6/20) or white (10 %, 2/20), although the difference was not significant (P = 0·084). Only 13 % had elevated CRP, although nearly a third had elevated AGP (Table 1). Overweight (i.e. BMIZ > 1) was more prevalent among those with AGP > 1 g/l compared to those with AGP ≤ 1 g/l (i.e. 49 %, 24/49 v. 29 %, 87/303; P = 0·007).
Significant differences existed across the Hb variants for Hb, MCV and RDW (P < 0·001), with Hb AA children having greater mean Hb and MCV, and lower RDW, compared with children with Hb AS and Hb AC and/or α3·7 thalassaemia. There were no significant differences across the groups for serum ferritin and sTfR (Table 2).
Table 2 Mean values (and standard deviation) for haematological variables and iron biomarkers for normal Hb type (Hb AA) and the four major abnormal Hb variants among pre-schoolers aged 3–6 years from disadvantaged households, Salvador, Northeast Brazil, August–November 2010
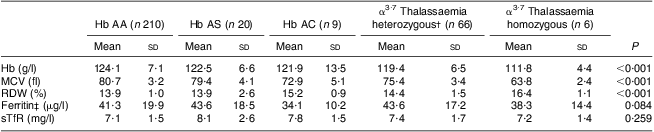
MCV, mean cell volume; RDW, red cell distribution width; sTfR, soluble transferrin receptor.
†Participants with concomitant Hb AS or Hb AC were excluded (n 4).
‡Adjusted for infection( Reference Thurnham, McCabe and Northrop-Clewes 39 ).
Anaemia and micronutrient deficiencies
Mean concentrations of Hb and the micronutrient biomarkers and the prevalence of anaemia and micronutrient deficiencies are shown in Table 3. Prevalence of anaemia and micronutrient deficiencies was <10 %, and ranged from 0 % for folate to 9·5 % for Se (Table 3); 13 % (46/358) had at least one micronutrient deficiency. The prevalence of anaemia was higher among children with at least one Hb disorder than among those without Hb disorders (i.e. 69 %, 9/13 v. 31 %, 4/13; P = 0·006). Of the few children with depleted Fe stores (2 %, 7/358), only one child had an Hb disorder. However, 15 % (54/358) had elevated sTfR indicative of tissue Fe deficiency, of whom 30 % (16/54) had an abnormal Hb variant.
Table 3 Prevalence (% and 95 % confidence interval) of anaemia and micronutrient deficiencies, and mean values (and standard deviation) of specific micronutrient biomarkers, including adjusted mean values for those impacted by infection, among pre-schoolers aged 3–6 years from disadvantaged households, Salvador, Northeast Brazil, August–November 2010
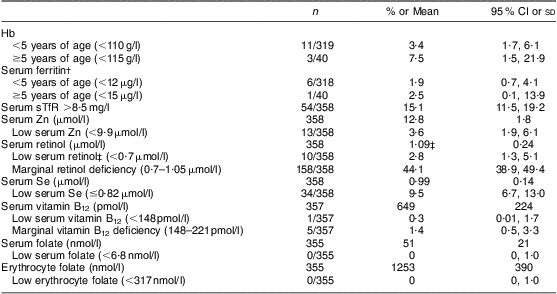
sTfR, soluble transferrin receptor.
†Adjusted for infection( Reference Thurnham, McCabe and Northrop-Clewes 39 ).
‡Adjusted for infection( 38 ).
Predictors of Hb and micronutrient biomarkers
Table 4 shows the significant predictors of Hb and biomarkers of Fe status. Both homozygous and heterozygous α3·7 thalassaemia variants were negative determinants of Hb (P ≤ 0·001), whereas Se and retinol were positive determinants of Hb; serum ferritin was not a significant determinant of Hb. BMIZ > 1 and Hb AS were positive determinants of sTfR but had no effect on ferritin. Elevated AGP was a positive determinant of ferritin.
Table 4 Predictors of Hb and two biomarkers of iron status based on both univariate and multiple regression models, as shown by β coefficient (and 95 % confidence interval), among pre-schoolers aged 3–6 years from disadvantaged households, Salvador, Northeast Brazil, August–November 2010
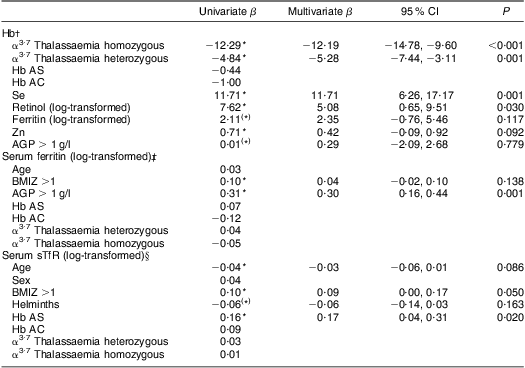
AGP, α1-glycoprotein; BMIZ, BMI Z-score.
All variables with P < 0·2 in the univariate analysis were included in the multivariate regression.
For all binary categorical variables, the reference was the absence of the condition specified.
(*) P < 0·2 based on univariate regression; *P < 0·05 based on univariate regression.
†R 2 for multivariate regression model = 0·242.
‡R 2 for multivariate regression model = 0·098.
§R 2 for multivariate regression model = 0·083.
In contrast, AGP was a negative determinant of retinol, whereas Zn was a positive predictor of retinol (Table 5). Being male was positively associated with Se, whereas helminths were a negative predictor of Se and vitamin B12. G. intestinalis was a positive predictor of serum but not erythrocyte folate. The associations between either Hb or micronutrient biomarkers and age, sex (with the exception of Se), ethnicity, supplementation with Fe or vitamin A, deworming treatment, smoking in the home, family history of asthma and recent coffee intake were not statistically significant.
Table 5 Predictors of micronutrients based on both univariate and multiple regression models, as shown by β coefficient (and 95 % confidence interval), among pre-schoolers aged 3–6 years from disadvantaged households, Salvador, Northeast Brazil, August–November 2010

AGP, α1-glycoprotein.
All variables with P < 0·2 in the univariate regression were included in the multivariate regression.
For all binary categorical variables, the reference was the absence of the condition specified.
(*) P < 0·2 based on univariate regression; *P < 0·05 based on univariate regression.
†R 2 for multivariate regression model = 0·111.
‡R 2 for multivariate regression model = 0·041.
§R 2 for multivariate regression model = 0·073.
∥R 2 for multivariate regression model = 0·063.
¶R 2 for multivariate regression model = 0·036.
Discussion
Our findings highlight the low prevalence of anaemia and micronutrient deficiencies in these disadvantaged pre-schoolers attending philanthropic day-care centres. To our knowledge, these data are the first in Brazil to examine six micronutrient biomarkers in this age group concurrently and to investigate the complex interrelationships between Hb, micronutrient status, parasitic infections and genetic Hb disorders.
The low prevalence of anaemia reported here (i.e. <4 %) was unexpected. Earlier studies of urban pre-schoolers in NE Brazil( Reference Vieira, Diniz and Cabral 41 ), including Salvador( Reference Assis, Barreto and Gomes 3 ), have reported much greater anaemia rates, sometimes as high as 40 %( Reference Jordão, Bernardi and Filho 42 ). Several factors may account for our unexpected finding. The most frequent genetic Hb disorder among these pre-schoolers was heterozygous α3·7 thalassaemia (23 %), which, unlike the homozygous variant, is relatively benign and is not always associated with anaemia.
Moreover, review of the day-care meals indicated that the supply of all the micronutrients with a major role in the maintenance of normal haematopoietic function (i.e. Fe, folate and vitamin B12), with the exception of vitamin A, met the requirements of the pre-schoolers. For example, animal-source foods provided in the day-care meals (80 g/d), provision of legumes and use of fortified cereal flours in meal preparation( Reference Lander 16 ) and some supplementation sources of Fe and vitamin A (albeit minimal coverage)( Reference Lander, Lander and Houghton 15 ) contributed to the daily nutrient requirements in these children. Despite the seeming lack of impact of mandatory Fe fortification alone on anaemia in Brazilian pre-schoolers( Reference Assunção, Santos and Barros 43 ), these multiple strategies were most likely responsible, at least in part, for the lower prevalences of anaemia and vitamin A deficiency reported here compared with earlier studies of pre-schoolers attending day-care centres in NE Brazil( Reference Vieira, Diniz and Cabral 41 , Reference Paiva, Rondo and Gonçalves-Carvalho 44 ).
Notwithstanding the apparently low prevalence of micronutrient deficiencies, there was some evidence of marginal vitamin A (45 %) and Se (9·5 %) status. The existence of suboptimal vitamin A status was attributed to an inadequate supply of vitamin A from day-care meals together with rather poor coverage of vitamin A supplementation (i.e. 50 %). Zn status may have also played a role in vitamin A status in view of the positive association between Zn and retinol observed here. This relationship is not unexpected; Zn has a role in the hepatic synthesis of retinol-binding protein, and thus in the transport and utilization of retinol, although in other studies where a similar relationship has been observed, the prevalence of Zn deficiency has been higher( Reference Muñoz, Rosado and Lopez 45 ).
Reasons for the suboptimal Se status are uncertain. It could be associated with low soil Se levels and thus low levels of Se in the major locally grown plant-based staples. However, we were unable to locate values for the concentrations of Se in locally grown foods, so the supply of Se from the day-care meals is unknown.
It is of interest that both serum retinol and Se each had independent and significant positive associations with Hb (Table 4). The positive relationship between retinol and Hb is attributed to the role of vitamin A in the mobilization of Fe from the spleen or liver into the circulation( Reference Fishman, Christian and West 46 ), a mechanism that may account for the low prevalence of storage Fe depletion (i.e. <3 %) reported here. There are several plausible mechanisms whereby Se status might impact on Hb, including its affect on the activity of thioredoxin reductase, a selenoenzyme postulated to be implicated in the up-regulation of hepatic haem oxygenase-1 involved in haem catabolism( Reference Mostert, Hill and Burk 47 ). In addition, reduced activity of glutathione peroxidase, a selenoenzyme that may protect Hb against oxidation in red blood cells( Reference Nagababu, Chrest and Rifkind 48 ), can result in increased inflammation and oxidative stress. This inflammatory response may also be induced by intestinal parasites and may account for the independent and negative impact of helminths on Se concentrations.
During inflammation, pro-inflammatory cytokines such as IL-6 and leptin stimulate an increase in circulating hepcidin produced by both the liver and adipose tissue, which in turn down-regulates Fe absorption independent of Fe status, leading to functional Fe deficiency( Reference Yanoff, Menzie and Denkinger 49 ). Chronic inflammation also accompanies overweight and obesity and thus may account for the observed positive relationship between overweight and sTfR (Table 4).
Parasitic infections are also known to play a role in the aetiology of anaemia. In the present study parasitic infections were not predictors of Hb, but instead were associated with vitamin B12 and folate status of the pre-schoolers. Their effect, however, although negative for vitamin B12, was positive for folate status. The negative relationship between vitamin B12 status and infection with helminths, and to a lesser extent G. intestinalis, is well recognized( Reference Solomons 50 , Reference Allen, Rosado and Casterline 51 ). Such infections are not only associated with changes in small intestine morphology that lead to reductions in the absorptive surface( Reference Müller and von Allmen 8 ), but also with increased bacterial overgrowth that causes malabsorption, specifically of vitamin B12 ( Reference Allen, Rosado and Casterline 51 ). Risk of bacterial overgrowth is especially high for Brazilian pre-school children living in urban slum environments( Reference dos Reis, de Morais and Oliva 52 ), emphasizing the importance of the adequate supply of animal-source foods in the day-care meals.
The positive association between serum folate status and Giardia infection observed here was unexpected but is not implausible. Mandatory folate fortification of cereal flours may have created a folate-rich environment which provided optimal conditions for the adherence of Giardia trophozoites to the intestinal epithelium( Reference Sousa, Gonçalves and Bairos 53 , Reference Khademi, Ghaffarifar and Asl 54 ). Alternatively, high levels of circulating unmetabolized folic acid from folate fortification of flour( Reference Smith 55 ) have been associated with decreased innate immune function( Reference Troen, Mitchell and Sorensen 56 ). This finding was attributed to reduced natural killer cell cytotoxicity, a mechanism responsible for targeting invading pathogens. However, to date these findings have been observed only in postmenopausal women and need to be replicated in other studies. Clearly, further research is warranted to confirm the positive association noted here between serum folate and Giardia infection, and establish the underlying biological mechanism.
Our findings are based on a cross-sectional study and hence preclude causal inferences from being made. Sampling was restricted to seven philanthropically funded day-care centres that provide a free service, so results may not apply to other day-care settings. Unfortunately, very limited data exist from the Salvador city registration on the number of philanthropic, public and private day-care centres for children of this age group. To our knowledge, the philanthropic day-care centres included here are unique to Salvador. The public government day care is also usually free of charge, but like the private day care, provides fewer meals (i.e. four and three, respectively) than the philanthropically funded day-care centres studied here. Furthermore, although we investigated several genetic Hb disorders, we were unable to measure all genetic factors, including glucose-6-phosphate dehydrogenase deficiency, which may have affected Hb and micronutrient levels, especially among the pre-schoolers of West African descent. Nevertheless, we did investigate Hb, six micronutrients, micronutrient supply from day-care meals, acute and chronic inflammation, parasite and health status variables, which together provide a greater understanding of factors influencing Hb and micronutrient status in these disadvantaged pre-schoolers.
Conclusions
In conclusion, even though the pre-schoolers were living in urban slum settings, impaired growth and anaemia and micronutrient deficiencies were uncommon. These results are most likely a reflection of the provision of micronutrient-rich day-care meals fortified with Fe and folic acid, parasite control and vitamin A supplementation, and coverage of these programmes should be expanded. Nevertheless, the existence of functional Fe deficiency associated with overweight in these pre-schoolers is of concern, and highlights the importance of strengthening efforts to prevent the emerging problem of overweight in disadvantaged pre-schoolers in NE Brazil.
Acknowledgements
Sources of funding: The work was funded in part by the University of Otago Research Fund, but the Board administering the Fund had no role in the design of the study, the data analysis or the writing of this article. Conflicts of interest: None. Authors’ contributions: R.L.L. contributed to the research project design, conduct of the study, statistical analysis and manuscript draft; K.B.B., A.G.L. and A.A.A. contributed to the analysis of the specimens; H.C.C.-R. and A.P.M. contributed to the research study oversight and recruitment of the day-care centres; D.B.L. assisted with the conduct of the study; L.A.H. and I.M.M. provided oversight of analytical procedures and contributed to the manuscript draft; S.M.W. contributed to the statistical analysis and manuscript draft; R.S.G. contributed to the research project design, study oversight and manuscript draft. All authors have read and approved the final draft. Acknowledgements: The authors thank the day-care organizations Santa Casa de Misericordia and Mansão do Caminho for their support during planning and implementation of this study, the parents of the participating children, and the coordinators of the seven day-care centres. They also thank all nutritionists from the Fima Lifshitz Research Unit of the Hospital Universitário Professor Edgard Santos who assisted with the data collection.







