Introduction
Palliative sedation (PS) is the therapeutic induction of sedation, resulting in loss of consciousness in patients suffering from otherwise uncontrollable symptoms in their last and very limited phase of life (Cherny and Radbruch Reference Cherny and Radbruch2009). This concept has been initially published by Enck as “terminal sedation” (Enck Reference Enck1991). Possible indications for a PS are, for example, pain, dyspnea, seizures, delirium, anxiety, and other symptoms that cannot be controlled by a specific therapeutic measure. Such refractory symptoms occur in 5% up to 35% of palliative care patients (Benítez-Rosario and Belén Reference Benítez-Rosario and Ascanio-León2020; LiPuma and DeMarco Reference LiPuma and DeMarco2016). The initiation of PS requires a mutual relationship between the palliative care physician and the patient or the patient’s caregiver because the PS will not be discontinued until death in the majority of cases.
Comprehensive information about the diagnosis, prognosis, and informed consent is mandatory. Further preconditions are an experienced team and close monitoring of the patient (Belar et al. Reference Belar, Arantzamendi and Payne2020). Several ethical questions have been discussed in the context of PS (Committee and Administration 2006; Rady and Verheijde Reference Rady and Verheijde2010; Takla et al. Reference Takla, Savulescu and Wilkinson2020). Current consensus guidelines highlight a clear border between PS and assisted death or euthanasia (Materstvedt Reference Materstvedt2020; Rady and Verheijde Reference Rady and Verheijde2010).
Most frequent indication for PS is delirium followed by pain dyspnea and psychological/existential distress (Arantzamendi et al. Reference Arantzamendi, Belar and Payne2020). Common drugs used for PS are benzodiazepines, neuroleptics, barbiturates, and propofol (Arantzamendi et al. Reference Arantzamendi, Belar and Payne2020).
Despite the existence of national and international guidelines for PS, institutional guidelines are often lacking (Gurschick et al. Reference Gurschick, Mayer and Hanson2015; Schur et al. Reference Schur, Weixler and Gabl2016). Furthermore, a close monitoring and an assessment of patients undergoing PS is necessary (Brinkkemper et al. Reference Brinkkemper, van Norel and Szadek2013).
To evaluate the current practice of PS in Pomerania and to compare the practice between inpatients at a palliative care unit (PCU) and outpatients at home or in a hospice, this prospective observational study was conducted.
In most cases, PS will be pursued until the death of the patient. Since the patients and their relatives often have preferences, which is the best place to spend the last episode of life – on a PCU, in a hospice, or at home – a PS procedure should be practicable at any of these sites. This prospective observational trial should contribute to the clarification of the following questions:
1. Can PS only be conducted in the setting of a hospital or can it also be conducted outside the hospital in a hospice or in another outpatient care concept?
2. Are there significant differences in the indications for PS and in the operational implementation between the PCU and the outpatient setting?
3. Can a PS procedure outside a PCU deliver a comparable quality and satisfaction compared to that on a PCU with a 24/7 presence of specialized medical staff members?
Patients and methods
General aspects
The present study was conducted as a prospective noninterventional observational investigation. The trial was approved by the ethics committee of Greifswald University on June 27, 2017 (http://www2.medizin.uni-greifswald.de/ethik). The investigation was noninterventional and followed the declaration of Helsinki. The data were collected between July 2017 and June 2018. The involved institutions were the PCU of Greifswald University Hospital, the hospice of Greifswald, and the specialized outpatient palliative care (SAPV) service of Greifswald-Pomerania. Participants were the staff members, patients of at least one of the institutions, and the patients’ relatives. The investigators were not involved in the patient’s treatment or in any medical decision.
For outpatient PS at home, it was mandatory that at least one relative or caregiver lived in the same household as the patient. The major differences between a PCU, a hospice, and an SAPV are shown in Table 1. The PS in the hospice and at home was always carried out by an SAPV team.
Table 1. Characteristics of PCU, hospice, and SAPV

Note: PC, palliative care; GP, general practitioner; PCU, palliative care unit; SAPV, specialized outpatient palliative care service; and 24/7, around the clock 7 days per week.
Definition of palliative sedation
Any sedation reducing the consciousness with the intention to reduce otherwise refractory symptoms was interpreted as a PS. This definition included terminal sedations at the end of life and intermittent sedations that were discontinued after successful control of intolerable symptoms by other measures.
Data collection
The total number of patients and the number of patients receiving a PS treated by each institution in the observational interval were documented. Basic data were patient’s age, gender, underlying diagnosis with time of diagnosis, performance score (Eastern Cooperative Oncology Group [ECOG]), prior palliative care treatment (when applicable), family status, children, support by caregivers at home, and prior profession of the patient. Additionally, data from the informed consent form were included in the analysis (Young et al. Reference Young, Badgery-Parker and Dobbins2015). The informed consent had to be completed and signed by the patient’s physician, a member of the nursing staff, the patient if possible, and his/her caregiver. On the informed consent, the method and the goal of the planned PS were noted, as well as the planned co-medication, the nutrition, and the liquid substitution.
After the start of the PS, a detailed protocol was conducted. Documentation included used medications, the depth of unconsciousness, the satisfaction of the patient’s caregivers and of the staff, and additional medical data at defined time points. The depth of sedation was scored using the established Richmond Agitation Sedation Scale (RASS) (Sessler et al. Reference Sessler, Gosnell and Grap2002).
It was the goal to assess the depth of sedation and the degree of satisfaction of the staff members and the patient’s relatives with the sedation procedures 3 times daily, according to one value per shift. Depth of sedation was assessed following the RASS score, and the degree of satisfaction was scored from 1 (very good) to 6 (poor) based on the grading system for German school marks (Sessler et al. Reference Sessler, Gosnell and Grap2002). The latter graduation was chosen to facilitate the scoring for the patient’s relatives, usually not familiar with medical scores. The analyses were performed with values obtained during the last 5 days prior to the death of the patient.
Data documentation and statistics
Primary documentation was paper based and carried out by staff members of the PCU, by the members of the SAPV team, and by the patient’s caregivers, when appropriate. Data were transferred by members of the investigation group to Microsoft-Excel spreadsheets, and statistical analyses were performed using the software programs SPSS and SAS. Statistical tests used for the analysis are indicated where appropriate.
The demographical data were analyzed using the chi-square test and Fisher’s exact test (where indicated). The data sets “depth of sedation” and “satisfaction” were analyzed using McNemar’s test.
Results
Patients characteristics
A total of 1,124 patients were treated during the observational period in the PCU (n = 340), in the hospice (n = 108), and by the outpatient palliative care service (n = 676). A total of 756 patients gave their consent to the investigation. Data of these patients were available for detailed analysis.
The percentage of patients that received a PS was 7.7% (PCU, n = 26), 1.9% (hospice, n = 2), and 4.6% (SAPV, n = 31), respectively. The difference between the PCU and the hospice was significant (0.037, Fisher’s exact test; Figure 1). Of all patients, 57.1% were male and 42.9% were female with PS rates of 7.9% and 7.7%, respectively (n.s.).

Fig. 1. Study population. PCU, palliative care unit; SAPV, specialized outpatient palliative care; and PS, palliative sedation.
The mean age of all patients without PS was 71.8 years (SD = ±12.3 years, range 22–97) compared to 68.1 years (SD = ±13 years, range 37–91) of patients treated with PS (p = 0.033). In detail, this difference was observed in each setting alone (PCU, hospice, and SAPV), but the difference was only significant for the group treated at the PCU (p = 0.023; Table 2).
Table 2. Demographics and diagnoses
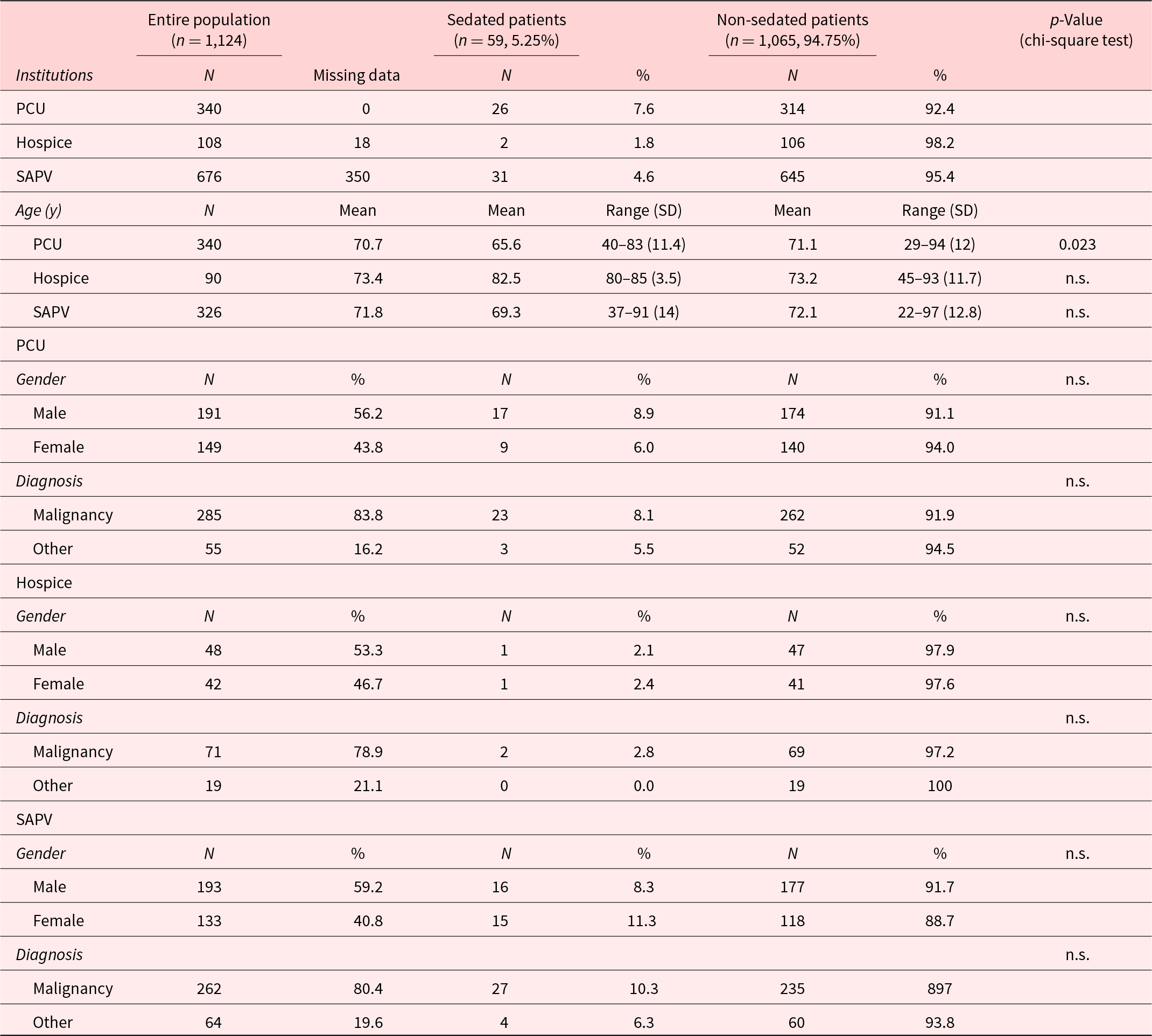
Note: PCU, palliative care unit; SAPV, specialized outpatient palliative care service; and n.s., not significant.
The underlying disease was a malignancy in 618 of 756 cases (81.7%). The differences between PCU (285/340, 83.3%), hospice (71/90, 78.9%), and SAPV (262/326, 80.4%) were only slight and nonsignificant (Table 2). The frequency of PS was neither influenced by the diagnosis of a malignancy nor by the presence of metastatic disease (data not shown). The median interval from the primary diagnosis of the underlying disease to the initiation of PS was 13.5 months (range: 0–18 years).
Four hundred thirty-two of 756 (57.1%) patients were male and 324 of 756 (42.9%) were female without significant differences between PCU, hospice, and SAPV. Gender-dependent differences on the frequency of PS were not observed. About 59.4% of all patients were married or had a committed relationship. In n = 41 (9.6%) of these patients, a PS was initiated at the end of life compared to n = 15 (5.1%) patients not living in a steady relationship (p = 0.029). The parameters children versus childlessness and the place of residence of children had no influence on PS. Most of the patients receiving the PS by SAPV at home were supported by their relatives during their last phase of life. Differences in the level of education between patients receiving PS or not could not be detected.
Indications for palliative sedation
The indications for the initiation of a PS are shown in Table 3. More than one answer was allowed. The leading diagnoses in the entire study collective were agitation (n = 42, 77.8%), anxiety (n = 31, 57.4%), delirium (n = 29, 53.7%), a poor quality of life (n = 24, 44.4%), and dyspnea and pain (each n = 22, 40.7%). A statistical comparison of subgroups was not conducted due to the low numbers of patients. However, it is noteworthy that agitation was an indication for PS in 92.3% of the patients treated in an outpatient setting (SAPV) compared to 65.4% of patients treated in the PCU (Table 3).
Table 3. Indications for palliative sedation

Notes: Empty box = 0. QOL, quality of life; and SAPV, specialized outpatient palliative care service.
Sedation procedure
The sedation protocols were available for all patients treated at the PCU and at the hospice and for 26 of 31 patients treated by SAPV in an outpatient setting (Table 4).
Table 4. Durance of PS and drugs used for PS

Notes: Empty box = 0. Data from 5 SAPV patients are lacking. PS, palliative sedation; and SAPV, specialized outpatient palliative care service.
The median duration of PS in all patients was 2.5 days with a range from 4 hours to 18 days. Sedation was conducted for less than 24 hours in 12 of 54 patients (22.2%). Eight of these patients were treated at the PCU and 4 patients in an outpatient setting. Nearly two-thirds of patients (34/54, 63.0%) received sedation over 1–7 days. In 8 cases, the sedation lasted longer than 1 week. In 3 patients sedated in the setting of SAPV, the sedation was terminated prior to death. Significant differences in the length of sedation between PCU, hospice, and SAPV were not seen.
Most patients (51/54, 94.4%) received midazolam for sedation protocol, either as a monotherapy (n = 44, 81.5%) or in combination with haloperidol, clonidine, and lorazepam (one each). Three patients received a combination of 3 drugs consisting of midazolam and clonidine plus either propofol (n = 2) or levomepromazine (n = 1). Three patients received a midazolam-free protocol consisting of propofol monotherapy (n = 1) or levomepromazine monotherapy (n = 2).
Depth of sedation and satisfaction of the staff and of the relatives
The data from the 2 patients sedated in the hospice and from the SAPV patients sedated at home were pooled for these analyses as “outpatients” since the conditions of both settings are very similar (Table 1).
A total of 338 values from 48 patients were available for analysis regarding the depth of sedation. The satisfaction of the staff and of the relatives could be analyzed with 293 and 132 scores, respectively, from 44 patients each. The lack of some measurements is, among others, owned to the facts that not all patients were sedated over 5 days and that the relatives were usually not present the whole day. The analyzed data are shown in detail in Table 5.
Table 5. Depth of sedation according to the RASS and satisfaction of the staff and patient’s relatives with sedation procedure

Notes: Satisfaction was scored from 1 (best) to 6 (worst). Data points available for analysis/patients: RASS, 338/48; satisfaction staff, 293/44; satisfaction relatives, 132/44. RASS, Richmond Agitation Sedation Scale.
The span of sedation depth varied between 4 and −5, and the sedation seemed to be lighter during the first days with a trend to an intensification from day −2 (Table 5 and Figure 2). A variate analysis with the median values from each day revealed that this observation was only a nonsignificant trend (data not shown). The sedation depth obtained from patients at the PCU was compared to those from patients treated in the hospice or in the setting of SAPV. Patients from the hospice and from the SAPV-setting were pooled for the analysis. For each patient, the median depth of sedation was calculated for every day and the comparison was conducted using the Mann–Whitney U test. Significant differences between both groups were not detected for any day (data not shown).

Fig. 2. Depth of sedation.
Since a major approach of this investigation was the comparison of PS between significantly different institutions, we had to choose a simple and robust scoring system that could be used by health-care professionals as well as by medical amateurs such as the patient’s relatives. To consider these preconditions, 3 scoring systems were chosen and each parameter should be scored 3 times per day, accordingly once per shift and once in the morning, in the afternoon, and at night. The depth of sedation was scored by staff members or – in outpatient care – by the palliative care physician according the RASS score (Sessler et al. Reference Sessler, Gosnell and Grap2002). The satisfaction with the PS procedure was scored by staff members and by patient’s relatives or caregivers from 1 (very good) to 6 (poor). The scoring by health-care professionals and by patient’s relatives considers the professional view as well as the emotional view by patient’s relatives. Furthermore, it was the goal to compare the degree of satisfaction of the staff and of the patient’s relatives with the sedation procedure. For this analysis, the median degree of satisfaction over the entire sedation period was calculated for each patient and compared between patients treated at the PCU and at the hospice or in SAPV. Comparison was conducted using the independent t-test. The degree of satisfaction of the staff was 2.3 (SD: 0.7) at the PCU and 2.3 (SD: 0.8) at the hospice or in the SAPV-setting (p = 0.86, Mann–Whitney U test). The degree of satisfaction of the patient’s relatives was similar with 2.2 (SD: 0.9) at the PCU compared to that from both other settings (hospice/SAPV) in combination with 2.2 (SD: 1.1) (p = 0.86, Mann–Whitney U test).
Discussion
The rate of PS procedures was significantly higher at the PCU compared to the hospice. Significant differences between PCU and SAPV and hospice and SAPV were not recognized. The reason for this difference is not clear. One explanation could be that the medical care at the PCU and in the setting of SAPV is realized by specialized palliative care teams, and the patients in the hospice are often treated by their general practitioner and nursed by volunteers. Another explanation could be that patients can be easily transferred from the hospice to the PCU since both are part of the University Hospital Greifswald.
Since the rate of PS was higher in patients with a spouse, it can be assumed that either the interaction of the patient with her/his spouse or of the spouse with the palliative care team may be important. This was not valid for children since no differences to childless patients were seen. Here, it should be pointed out that the spouse lives commonly in the same household with the patient and adult children living usually in their own household, not having such a close contact to the patient as the spouse has in the same household. This important point should be addressed in future investigations.
The indications for PS in the present investigation are on the whole in accordance with the literature (Arantzamendi et al. Reference Arantzamendi, Belar and Payne2020; Chater et al. Reference Chater, Viola and Paterson1998). However, some indications may appear very broad or overlapping with other. This may be owned to the fact that the indications had to be primarily documented within multiple choice options, but one additional free-text field was available. The rationale of this design was to avoid any bias of the documentation on the initiation of PS.
The predominance of midazolam in PS as well as the supplementation with drugs from other classes is common and in accordance with the international literature (Beller et al. Reference Beller, van Driel and McGregor2015; Gamblin et al. Reference Gamblin, Berry and Tresch-Bruneel2020; Maltoni et al. Reference Maltoni, Scarpi and Nanni2013, Reference Maltoni, Scarpi and Rosati2012). Major differences in the sedation protocol between PCU and outpatient care were not detected. These results support the hypothesis that a midazolam-based PS can be performed independently from the setting inpatient care or outpatient care.
A quality assessment of PS has been requested by several authors (Alessia et al. Reference Alessia, Matilde and Chrystel2022; Belar et al. Reference Belar, Arantzamendi and Payne2020; Brinkkemper et al. Reference Brinkkemper, van Norel and Szadek2013). Different scales have been employed for the evaluation of PS; however, standards have not been defined so far. Furthermore, the quality assessment is aggravated by the fact that the main person – the sedated patient – can hardly participate in the evaluation (Brinkkemper et al. Reference Brinkkemper, van Norel and Szadek2013; Maltoni et al. Reference Maltoni, Scarpi and Nanni2013).
Depth of sedation increased slightly during the last 4 days of the patient; the data did not allow a comparison between PCU and outpatient care. The median degree of satisfaction with the PS was 2 (good) over the entire sedation period, scored by health-care professionals and by patient’s relatives. The variation was broad from 1 (very good) to 6 (poor) in both groups. Significant differences in the degree of satisfaction between inpatient and outpatient care were not detected. Despite the fact that scoring by health-care professional may differ from scoring by patient’s relatives, these results support the evidence that PS can be conducted inpatient and outpatient with comparable satisfaction.
The presented investigation has some limitations and allows a future perspective: The documentation was made by the staff of the PCU for inpatients and by caregivers and relatives for outpatients. A bias due to the professional background and due to psychological factors cannot be excluded here. The RASS has been used for monitoring the depth of sedation in this investigation. This scale has achieved a good rating in a review by Krooupa et al. (Reference Krooupa, Vivat and McKeever2020); however, the authors stated the need for further research to refine the scales. The assessment of consciousness and pain during PS by nonprofessionals may not be objective, and both parameters cannot be always scored correctly with clinical methods. Here, the supplementation with neurophysiological measuring methods can improve the assessment substantially (Six et al. Reference Six, Laureys and Poelaert2021). In addition, this approach would enable a central telemetric monitoring of the patient’s parameters with a quick feedback to the outpatient treatment team, a possible reduction of bias by the caregivers, and in consequence, a possibly higher comfort for the patients.
The data from the presented investigation support the following theses: a midazolam-based PS is possible in the hospital at the PCU as well as in the outpatient setting when the patient is visited regularly by palliative care physicians. Although the score by both groups shows a wide variation, the median degree of satisfaction was good over the entire period independently from the setting of care. In consequence, an equivalent PS quality can be reached inpatient and outpatient. With the prerequisite of a professional palliative care team, the location for the terminal sedation can be chosen on the base of other medical problems of the patients, on the base of patient’s wishes, and on the base of the wishes of the patient’s relatives. An important issue to be considered is the fact that on the PCU, the relatives are relieved from any medical and nursing problems and can concentrate directly on their personal interactions with the patient. Otherwise, some patients and their relatives may prefer dying at home in their familiar environment (Kinoshita et al. Reference Kinoshita, Maeda and Morita2015; Rainsford et al. Reference Rainsford, MacLeod and Glasgow2016). In both settings, a comparable quality of PS seems possible, this is particularly important for rural areas. Telemetric monitoring of neurophysiological parameters is a possible approach for future improvement of patient’s comfort and could be helpful for a more objective evaluation of inpatient and outpatient PS.
Author contributions
BB and AJ contributed equally to this work.
Conflicts of interest
There are no conflicts of interest.










