Introduction
SARS-CoV-2 infection has been associated with a variety of psychological symptoms including low mood, anxiety and stress (Witteveen et al., Reference Witteveen, Young, Cuijpers, Ayuso-Mateos, Barbui, Bertolini, Cabello, Cadorin, Downes, Franzoi, Gasior, Gray, Melchior, Van Ommeren, Palantza, Purgato, Van Der Waerden, Wang and Sijbrandij2023), to which the COVID-19 pandemic itself has also greatly contributed through prolonged and forced isolation and strict rules determining fear, and social isolation and deprivation (Hornstein and Eisenberger, Reference Hornstein and Eisenberger2022). Hundreds of millions worldwide have been infected, and billions have been affected by the pandemic. Moreover, because these effects may have endured (Thompson et al., Reference Thompson, Stafford, Moltrecht, Huggins, Kwong, Shaw, Zaninotto, Patel, Silverwood, McElroy, Pierce, Green, Bowyer, Maddock, Tilling, Katikireddi, Ploubidis, Porteous, Timpson and Patalay2022) the impact of the pandemic on the population’s mental health (MH) remains a major public health reason of concern.
An ample and wide-ranging corpus of evidence showed that depressive symptoms, anxiety and psychological distress were common during the early stages and through the COVID-19 pandemic in the general population (Thompson et al., Reference Thompson, Stafford, Moltrecht, Huggins, Kwong, Shaw, Zaninotto, Patel, Silverwood, McElroy, Pierce, Green, Bowyer, Maddock, Tilling, Katikireddi, Ploubidis, Porteous, Timpson and Patalay2022; Witteveen et al., Reference Witteveen, Young, Cuijpers, Ayuso-Mateos, Barbui, Bertolini, Cabello, Cadorin, Downes, Franzoi, Gasior, Gray, Melchior, Van Ommeren, Palantza, Purgato, Van Der Waerden, Wang and Sijbrandij2023). Still, evidence on the long-term psychological consequences of the COVID-19 pandemic is somewhat patchy and scanty (Blankenburg et al., Reference Blankenburg, Wekenborg, Reichert, Kirsten, Kahre, Haag, Schumm, Czyborra, Berner and Armann2022; Jafri et al., Reference Jafri, Zaheer, Fatima, Saleem and Sohail2022; Larsen et al., Reference Larsen, Stiles, Shaik, Schneider, Muppidi, Tsui, Geng, Bonilla and Miglis2022; Natarajan et al., Reference Natarajan, Shetty, Delanerolle, Zeng, Zhang, Raymont, Rathod, Halabi, Elliot, Shi and Phiri2023; Osaghae et al., Reference Osaghae, Nguyen, Chung, Moffitt, Le, Suh, Prasad, Thomas, Gordon and Hwang2021). Postolache et al., (Reference Postolache, Benros and Brenner2021) suggested mechanistic underpinnings include the neurotropism of the SARS-CoV-2 virus, which would cause direct detrimental effects on the Central Nervous System (CNS). Neurological symptoms of COVID-19 and SARS-CoV-2 infection were common in infected individuals, including dysgeusia and ageusia (i.e., gustatory, and olfactory dysfunctions), myalgia, headache, confusion, delirium and dizziness (Harapan and Yoo, Reference Harapan and Yoo2021), and may relate to psychological distress (including symptoms of depression, anxiety and stress) to the body’s neuroendocrine and immune systems (Peters et al., Reference Peters, Schedlowski, Watzl and Gimsa2021; Steenblock et al., Reference Steenblock, Todorov, Kanczkowski, Eisenhofer, Schedl, Wong, Licinio, Bauer, Young, Gainetdinov and Bornstein2020) and consequent wane of the immune response (Segerstrom and Miller, Reference Segerstrom and Miller2004). Somewhat surprisingly, epidemiological evidence on the prospective association between infection (i.e., non-vaccine-induced seropositivity to SARS-CoV-2 antibodies) and psychological distress remains extremely sparse.
Leveraging epidemiological data of a population-based cohort, the aim of this study was to explore the prospective association between SARS-CoV-2 infection and facets of psychological distress (i.e., anxiety, depressive and stress symptomatology) among non-institutionalized adults aged 20 years and older, across the pandemic waves. Our hypothesis is that SARS-CoV-2 infection leads to enduring mental distress, which is lower in non-infected compared to infected individuals.
Methods
Study design and participants
This study stems out of Corona Immunitas Ticino (CIT), a prospective population-based seroprevalence study conducted in southern Switzerland during the COVID-19 pandemic. The CTT study was previously described (Amati et al., Reference Amati, Frei, Kaufmann, Sabatini, Pellaton, Fehr, Albanese and Puhan2022) and is part of Corona Immunitas (Speierer et al., Reference Speierer, Chocano-Bedoya, Anker, Schmid, Keidel, Vermes, Imboden, Levati, Franscella, Corna, Amati, Harju, Luedi, Michel, Veys-Takeuchi, Zuppinger, Nusslé, D’Acremont, Tall and Von Wyl2022; West et al., Reference West, Anker, Amati, Richard, Wisniak, Butty, Albanese, Bochud, Chiolero, Crivelli, Cullati, d’Acremont, Epure, Fehr, Flahault, Fornerod, Frank, Frei and Puhan2020), a national research program conducted to assess the population-level spread and impact of the COVID-19 pandemic in Switzerland. For this study, we focused on a representative sample, randomly drawn by the Swiss Federal Statistical Office, of adults (aged 20–64 years) and older individuals (65+ years) living in Ticino (southern Switzerland), with socio-demographic baseline data collected in July 2020, serological data on immune status collected in June 2021 and repeated psychological distress assessments (21-item Depression, Anxiety and Stress Scale [DASS-21], below) collected from December 2020 up until August 2021, that is from the first through the second and third pandemic waves in the region. All participants gave written informed consent to participate in the study.
Measurements and procedures
At study entry, we collected information on the socio-demographic and health characteristics of participants, including age, categorized into three age groups: (0) 20–49, (1) 50–64, (2) 65+ years; gender: (0) women, (1) men; education: (0) up to higher secondary/apprenticeship, (1) higher tertiary; body mass index (BMI): (0) BMI < 30 kg/m2, (1) BMI > 30 kg/m2; smoking status: (0) nonsmoker/former smoker, (1) current smoker (daily or occasional); and existing chronic conditions (‘Do you suffer from any of the following chronic conditions?’): (0) none, (1) any among hypertension, diabetes, cardiovascular disease, cancer, immunological deficiency syndromes or respiratory syndromes.
Serological testing of the CTT studies is described in detail elsewhere (Amati et al., Reference Amati, Piumatti, Franscella, Buttaroni, Camerini, Corna, Levati, Fadda, Fiordelli, Annoni, Bezani, Amendola, Fragoso Corti, Sabatini, Kaufmann, Frei, Puhan, Crivelli and Albanese2023), and all assays were previously validated in population-based samples (Fenwick et al., Reference Fenwick, Turelli, Pellaton, Farina, Campos, Raclot, Pojer, Cagno, Nusslé, D’Acremont, Fehr, Puhan, Pantaleo and Trono2021b, Reference Fenwick, Turelli, Pellaton, Farina, Campos, Raclot, Pojer, Cagno, Nusslé, D’Acremont, Fehr, Puhan, Pantaleo and Trono2021b). Briefly, we obtained sera from peripheral venous blood samples and conducted longitudinal serosurveys at five time points between July 2020 and June 2022. In this study, we used data from the third serosurvey conducted in June 2021, when the vaccination campaign in the region was already ongoing. We assessed SARS-CoV-2 specific antibodies against the spike and nucleocapsid proteins of the virus using Sensitive Anti-SARS-CoV-2 Spike Trimer Immunoglobulin Serological (SenASTrIS), a Luminex binding assay for anti-SARS-CoV-2 total immunoglobulins, purposely developed for population-based serosurveys (Amati et al., Reference Amati, Piumatti, Franscella, Buttaroni, Camerini, Corna, Levati, Fadda, Fiordelli, Annoni, Bezani, Amendola, Fragoso Corti, Sabatini, Kaufmann, Frei, Puhan, Crivelli and Albanese2023; Fenwick, Croxatto, et al., Reference Fenwick, Croxatto, Coste, Pojer, André, Pellaton, Farina, Campos, Hacker, Lau, Bosch, Gonseth Nussle, Bochud, D’Acremont, Trono, Greub and Pantaleo2021; Fenwick, Turelli, et al., Reference Fenwick, Turelli, Pellaton, Farina, Campos, Raclot, Pojer, Cagno, Nusslé, D’Acremont, Fehr, Puhan, Pantaleo and Trono2021). This assay allowed the distinction between infection and/or vaccine-induced immunity. The assay measures binding of IgG antibodies to the trimeric SARS-CoV-2 spike and the nucleocapsid proteins. The test has a high specificity (98%) and sensitivity (99%) and has been validated in samples of the general population and in specific subgroups (Fenwick et al., Reference Fenwick, Turelli, Pellaton, Farina, Campos, Raclot, Pojer, Cagno, Nusslé, D’Acremont, Fehr, Puhan, Pantaleo and Trono2021b, Reference Fenwick, Turelli, Pellaton, Farina, Campos, Raclot, Pojer, Cagno, Nusslé, D’Acremont, Fehr, Puhan, Pantaleo and Trono2021b).
Based on the serological results we classified immunological statuses as follows: (i) Infection-induced immunity (self-reported vaccination status = NO, Anti_Spike = POS and/or Anti_N = POS); (ii); Vaccine-induced immunity (self-reported vaccination status = YES, and/or Anti_Spike = POS and Anti_N = NEG); (iii) Hybrid immunity (self-reported vaccination status = YES, Anti_Spike = POS and Anti_N = POS); (iv) Seronegative (Anti_Spike = NEG e Anti_N = NEG, irrespective of vaccination status). For this study, we were interested in infections and further dichotomized the samples in never infected individuals (seronegative individuals and individuals with vaccine-induced immunity only) and infected individuals (infection-induced individuals and individuals with hybrid immunity).
For the outcome, and dependent variable in our models, we considered three complete assessments of psychological distress using the DASS-21 for depressive symptoms, anxiety and stress levels (Henry and Crawford, Reference Henry and Crawford2005) over the different seasons of the COVID-19 pandemic: winter (December 2020–February 2021); spring (March–May 2021) and summer (June–August 2021). Each DASS-21 item is self-rated on a 4-level Likert scale, from 0 (never) to 3 (almost always). The DASS-21 was used in previous research on psychological distress associated with COVID-19 (Piumatti et al., Reference Piumatti, Levati, Amati, Crivelli and Albanese2022; Wang et al., Reference Wang, Tee, Roy, Fardin, Srichokchatchawan, Habib, Tran, Hussain, Hoang, Le, Ma, Pham, Shirazi, Taneepanichskul, Tan, Tee, Xu, Xu, Vu and Kuruchittham2021). We computed the DASS-21 overall score, which ranges between 0 and 21, and we used standard cutoffs of the three subscales’ scores for mild levels of depressive symptoms (>9), anxiety (>7) and stress (>14) (Lovibond and Lovibond, Reference Lovibond and Lovibond1995; Tran et al., Reference Tran, Tran and Fisher2013) and obtained dichotomized measures of each score accordingly. We modelled the assessments of DASS-21 as a dichotomized score (Piumatti et al., Reference Piumatti, Levati, Amati, Crivelli and Albanese2022) (normal and mild levels of anxiety, depression and stress vs moderate, severe to extremely severe levels of anxiety, depression and stress), we analyzed the outcome of each subscale separate (Lovibond and Lovibond, Reference Lovibond and Lovibond1995; Tran et al., Reference Tran, Tran and Fisher2013), and we used mild conditions as a standard reference cut-off score for assessing the occurrence of anxiety, depression and stress (i.e., mild [0] vs not mild levels [1]). The DASS-21 has good convergent, discriminant and nomological validity in normative samples (Henry and Crawford, Reference Henry and Crawford2005; J. Lee et al., Reference Lee, Lee and Moon2019); Cronbach’s alpha ranged from 0.89 to 0.93 for depression, from 0.76 to 0.86 for anxiety and from 0.89 to 0.93 for stress across assessments.
We collected and recorded all data using secured online questionnaires and forms implemented in the Research Electronic Data Capture (REDCap) software, hosted at the Università della Svizzera Italiana (USI) (Reference Harris, Taylor, Minor, Elliott, Fernandez, O’Neal, McLeod, Delacqua, Delacqua, Kirby and Duda2019; Harris et al., Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde2009).
Statistical analysis
We checked data quality (i.e., straight line scoring) and analysed missing data patterns (Piumatti et al., Reference Piumatti, Levati, Amati, Crivelli and Albanese2022). We excluded responses due to straight line scoring on the DASS-21 items (<0.4% across assessments), and participants with incomplete assessment of DASS-21 i.e., less than three assessments over different seasons as mentioned above (n = 773, 37.5.5% of the study sample), and derived an analytic sample of 305 individuals in which we conducted all analysis. Next, we imputed missing values of repeated measures of depression, anxiety and stress using linear combination of available observations (Piumatti et al., Reference Piumatti, Levati, Amati, Crivelli and Albanese2022). Additionally, we compared the infected and never infected characteristics with chi2 and mean Students’t-tests, as appropriate.
We modelled moderate to severe depression, anxiety and stress as binary dependent variables in separate generalized estimating equation (GEE) models to assess variance structure and clustering error within subjects (Liang and Zeger, Reference Liang and Zeger1986; Ziegler and Vens, Reference Ziegler and Vens2010). GEE models allow the determination of how the average of a subject’s response changes with covariates while specifying variance structure for the correlation between repeated measurements in the same subject over time (Piumatti et al., Reference Piumatti, Levati, Amati, Crivelli and Albanese2022). To select the best working covariance structure for the current data, we followed a model selection method described elsewhere (Cui, Reference Cui2007; Pan, Reference Pan2001): smaller quasi-likelihood under the quasi-information criterion values was indicative of better fit. We assessed three types of covariance structure (Grady and Helms, Reference Grady and Helms1995): exchangeable, assuming responses from the same cluster are equally correlated; autoregressive, where correlations between responses decrease across time; and unstructured, considering the correlations between responses to be comparatively complex. We tested GEE univariate models with robust standard errors adjusted for age and gender. We then adjusted also for education, BMI, smoking and chronic diseases in multivariate models (Piumatti et al., Reference Piumatti, Levati, Amati, Crivelli and Albanese2022). We further tested significant between-subject effects in interaction with time and plotted results to ease interpretation. Statistical significance was considered for P < 0.05 for direct effects and P < 0.10 for interaction effects. We used Stata version 15, for all statistical analyses (StataCorp., 2015).
Results
We included only participants with at least three DASS-21 assessments (n = 733 adults; n = 450 older adults) and we excluded those with incomplete socio-demographic information (n = 20 adults; n = 13 older adults) and with no serological data in June 2021 (n = 458 adults; n = 387 older adults). We obtained a final sample of 305 participants (see Fig. 1 – flow chart).
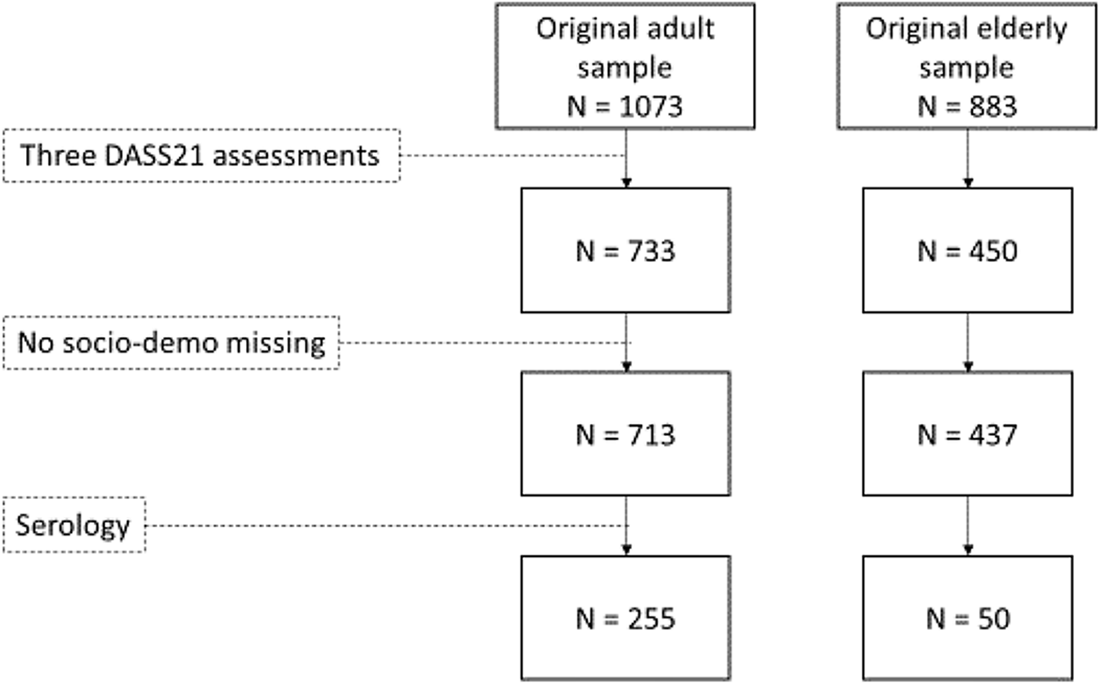
Figure 1. Participants’ flow chart.
Description of the sample
Table 1 reports the characteristics of the analytical sample by immunological status. From the total sample (n = 305) of infected and never infected (as dichotomized in the ‘Measurements and procedures’ section), 84.3% were never infected and had a mean age of 51.3 years (SD = 0.93). The infected participants were slightly younger on average (M = 46.9; SD = 2.0). 50.2% of the never infected and 45.8% of the infected were female, and in both groups the minority of the participants had a tertiary level of education or higher (28.40% and 31.25%, respectively). And 15.2% of the never infected and 18.8% of the infected participants were obese (BMI > 30 kg/m2), 24.5% and 22.9% reported a previous clinical diagnosis of at least one chronic disease and 17.5% of the never infected and 14.6% infected individuals were current smokers. The two groups did not significantly differ in any baseline socio-demographic characteristics other than age distribution (χ 2 = 6.229; P = 0.04).
Table 1. Characteristics of the analytical sample at baseline (July 2020) by infection status, Corona Immunitas Ticino (CIT) study
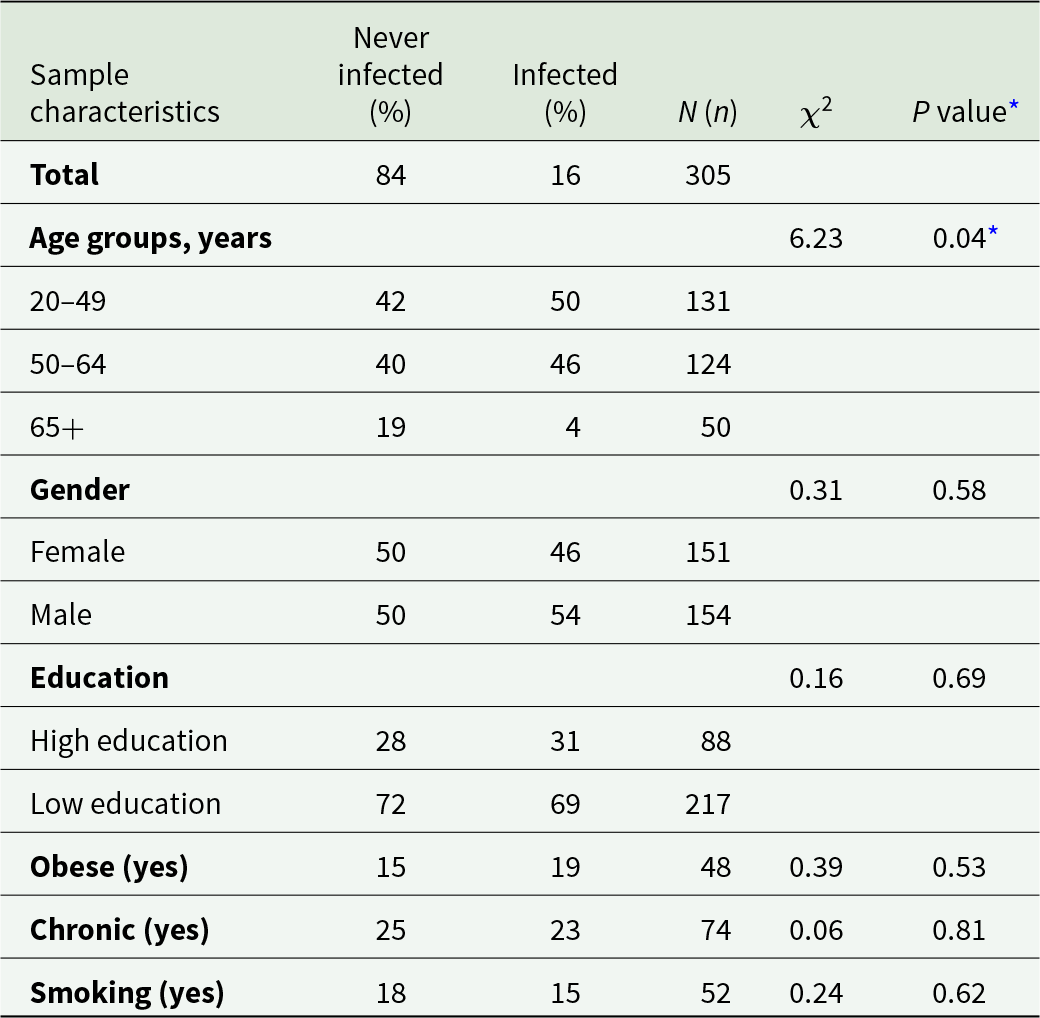
* P < 0.05 (Pearson χ 2) as significant.
SARS-CoV-2 infection and psychological distress
Table 2 reports the GEE regression results with an exchangeable variance–covariance structure, which fitted the data better than an autoregressive or unstructured solution to model the effect of infection on change in psychological distress (Piumatti et al., Reference Piumatti, Levati, Amati, Crivelli and Albanese2022). Compared with those who were never infected, infected individuals had a decreasing probability of being mildly depressed (DASS-21 sub-score >9) ([OR] = 0.64; 95% CI = 0.45, 0.91; P = 0.014) and anxious (OR = 0.50; 95% CI = 0.27, 0.94; P = 0.030) (DASS-21 sub-score > 7) through the COVID-19 pandemic waves. On the contrary, infected individuals did not show a declining probability of reporting mild stress symptoms (OR = 0.71; 95% CI = 0.47, 1.08; P = 0.113) (DASS-21 sub-score > 14). Trends over time (Fig. 2) of depressive symptoms, anxiety and stress declined faster in infected compared to never infected individuals.
Table 2. Associations (odds ratios) between seropositive immunological status and mental health between December 2020 and August 2021 in Ticino, southern Switzerland (N = 305)
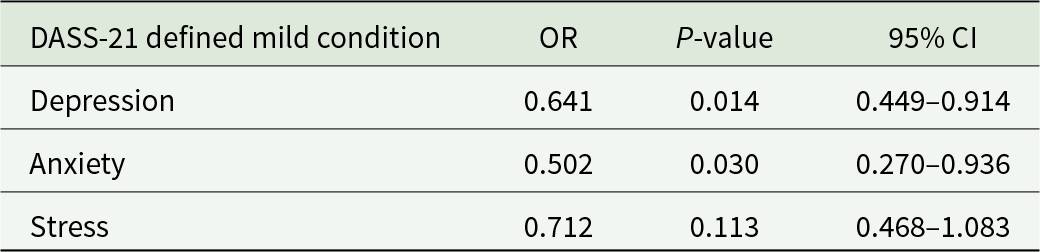
Figure 2 provides a graphical illustration of the putative effect of infection on trajectories of psychological distress, based on the GEE models results, which are presented as prevalence (95% CI) of mild depression, anxiety and stress based on the DASS-21 sub-scales with standard cut-offs for mild-conditions (depression > 9; anxiety > 75; stress > 14). None of the associations of infection status with depressive symptoms, anxiety and stress levels reached statistical significance when modelled separately at the three follow-ups (all P-values of independent, unadjusted regression models >0.051). Trends across time were in favor of infected individuals indeed, when examining the figure, it appeared that psychological distress decreased more rapidly in infected compared to never infected individuals. Similarly, in summer 2021 (i.e., 6 months after the first MH assessment) scores of depressive symptoms, anxiety and stress levels declined faster in infected compared to never infected individuals.
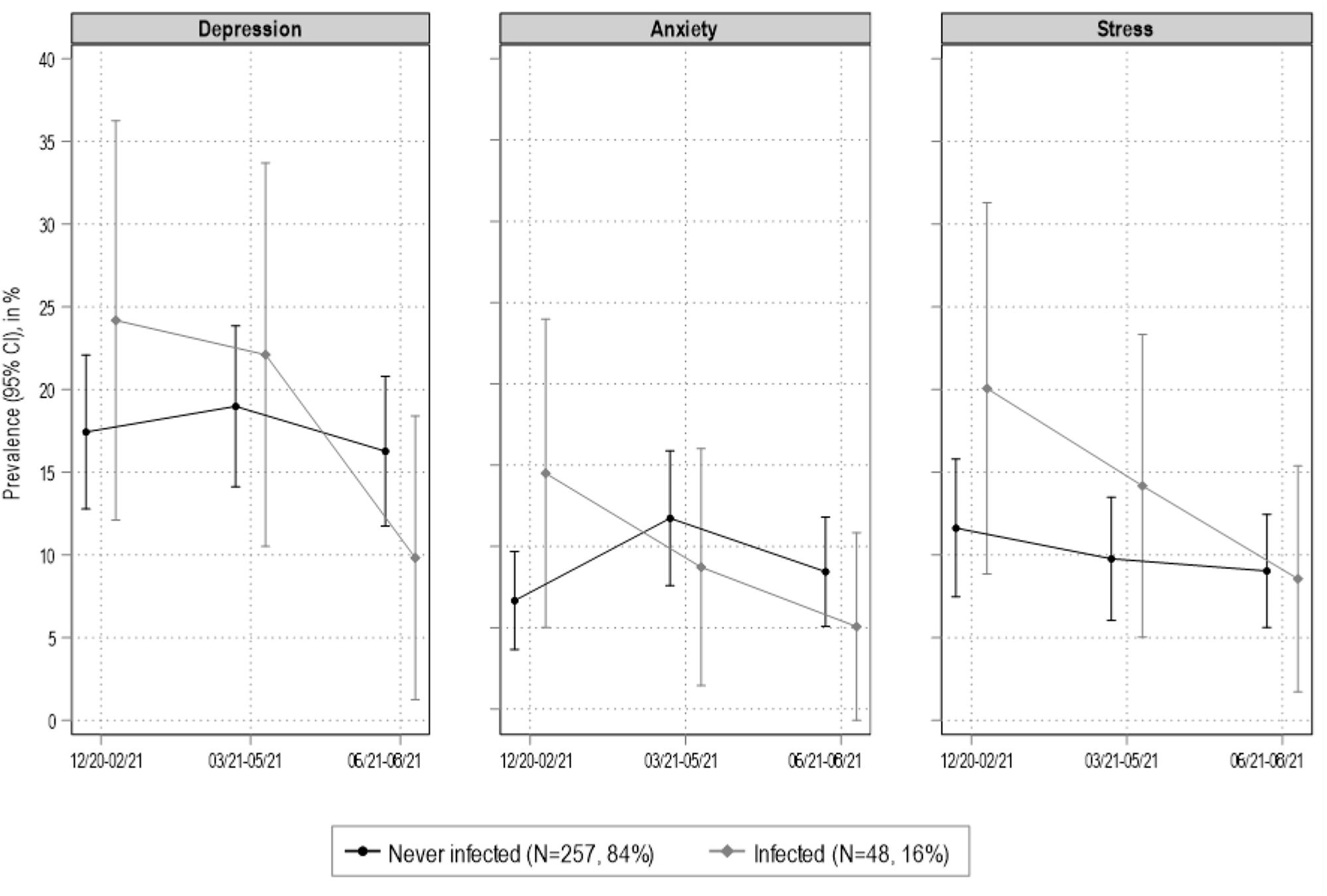
Figure 2. Generalized estimating equation (GEE) models results (DASS-21 cut-offs for mild conditions). Covariates are age, gender, education, BMI, smoking and chronic diseases. Ticino, southern Switzerland (N = 305).
In mutually adjusted GEE models, younger age (OR = 0.97; 95% CI = 0.95, 0.99; P = 0.013), self-reported chronic diseases (OR = 3.47; 95% CI = 1.71, 7.04; P = 0.001) and smoking (OR = 2.52; 95% CI = 1.23, 5.16; P = 0.011) were all significantly associated with higher levels of anxiety. Similarly, younger age (OR = 0.96; 95% CI = 0.94, 0.99; P = 0.003) and self-reported chronic conditions (OR = 2.56; 95% CI = 1.25, 5.22; P = 0.010) were significantly associated with increased stress scores. None of the associations between socio-demographic characteristics and depressive symptoms reached statistical significances (all P-values >0.140).
Discussion
The aim of this study was to explore the prospective association between SARS-CoV-2 infection and depression, anxiety and stress symptomatology. Our results may suggest a psychological pathway linking infection to distress. However, we found that, compared to never infected individuals those who were infected had a progressive improvement in psychological distress symptoms (i.e., depressive symptoms, anxiety and stress) from December 2020 to late August 2021. Moreover, younger age (age ranges: 20–49; 50–64; 65+), presence of chronic diseases and smoking habits were all independently associated with anxiety and stress symptoms over time.
Previous evidence about the impact of the COVID-19 infection on long-term MH symptoms is heterogenous but limited on the prospective association of serologically confirmed infection with psychological distress. Most studies focused on MH features in SARS-CoV-2 cases compared to non-cases (Jafri et al., Reference Jafri, Zaheer, Fatima, Saleem and Sohail2022; Magnúsdóttir et al., Reference Magnúsdóttir, Lovik, Unnarsdóttir, McCartney, Ask, Kõiv, Christoffersen, Johnson, Hauksdóttir, Fawns-Ritchie, Helenius, González-Hijón, Lu, Ebrahimi, Hoffart, Porteous, Fang, Jakobsdóttir, Lehto and Valdimarsdóttir2022; Ray et al., Reference Ray, Abdel-Mannan, Sa, Fuller, Wood, Pysden, Yoong, McCullagh, Scott, McMahon, Thomas, Taylor, Illingworth, McCrea, Davies, Whitehouse, Zuberi, Guthrie, Wassmer and Benjamin2021) not on infectious status. Moreover, although previous studies focused on diverse populations including both clinical (Ray et al., Reference Ray, Abdel-Mannan, Sa, Fuller, Wood, Pysden, Yoong, McCullagh, Scott, McMahon, Thomas, Taylor, Illingworth, McCrea, Davies, Whitehouse, Zuberi, Guthrie, Wassmer and Benjamin2021) and community-dwelling samples (Blankenburg et al., Reference Blankenburg, Wekenborg, Reichert, Kirsten, Kahre, Haag, Schumm, Czyborra, Berner and Armann2022; Fogh et al., Reference Fogh, Larsen, Hansen, Hasselbalch, Eriksen, Bundgaard, Frikke-Schmidt, Hilsted, Østergaard, Johansen, Hageman, Garred and Iversen2022; Magnúsdóttir et al., Reference Magnúsdóttir, Lovik, Unnarsdóttir, McCartney, Ask, Kõiv, Christoffersen, Johnson, Hauksdóttir, Fawns-Ritchie, Helenius, González-Hijón, Lu, Ebrahimi, Hoffart, Porteous, Fang, Jakobsdóttir, Lehto and Valdimarsdóttir2022), many were conducted in healthcare workers or settings (Grazzini et al., Reference Grazzini, Lulli, Mucci, Paolini, Baldassarre, Gallinoro, Chiarelli, Niccolini and Arcangeli2022; Osaghae et al., Reference Osaghae, Nguyen, Chung, Moffitt, Le, Suh, Prasad, Thomas, Gordon and Hwang2021). Study designs also varied, ranging from cross-sectional (Jafri et al., Reference Jafri, Zaheer, Fatima, Saleem and Sohail2022; Larsen et al., Reference Larsen, Stiles, Shaik, Schneider, Muppidi, Tsui, Geng, Bonilla and Miglis2022), case-control (Burrai et al., Reference Burrai, Barchielli, Cricenti, Borrelli, D’Amato, Santoro, Vitale, Ferracuti, Giannini and Quaglieri2021) to cohort (Blankenburg et al., Reference Blankenburg, Wekenborg, Reichert, Kirsten, Kahre, Haag, Schumm, Czyborra, Berner and Armann2022; Magnúsdóttir et al., Reference Magnúsdóttir, Lovik, Unnarsdóttir, McCartney, Ask, Kõiv, Christoffersen, Johnson, Hauksdóttir, Fawns-Ritchie, Helenius, González-Hijón, Lu, Ebrahimi, Hoffart, Porteous, Fang, Jakobsdóttir, Lehto and Valdimarsdóttir2022; Osaghae et al., Reference Osaghae, Nguyen, Chung, Moffitt, Le, Suh, Prasad, Thomas, Gordon and Hwang2021). Varying study designs, populations and time periods likely explain the marked heterogeneity of findings across studies, that reported positive (Jafri et al., Reference Jafri, Zaheer, Fatima, Saleem and Sohail2022; Thompson et al., Reference Thompson, Stafford, Moltrecht, Huggins, Kwong, Shaw, Zaninotto, Patel, Silverwood, McElroy, Pierce, Green, Bowyer, Maddock, Tilling, Katikireddi, Ploubidis, Porteous, Timpson and Patalay2022) and null (Blankenburg et al., Reference Blankenburg, Wekenborg, Reichert, Kirsten, Kahre, Haag, Schumm, Czyborra, Berner and Armann2022; Larsen et al., Reference Larsen, Stiles, Shaik, Schneider, Muppidi, Tsui, Geng, Bonilla and Miglis2022; Osaghae et al., Reference Osaghae, Nguyen, Chung, Moffitt, Le, Suh, Prasad, Thomas, Gordon and Hwang2021) associations between SARS-CoV-2 infection and psychological distress. Our findings are based on longer observational periods compared to those of a cohort study (Osaghae et al., Reference Osaghae, Nguyen, Chung, Moffitt, Le, Suh, Prasad, Thomas, Gordon and Hwang2021) in which serology was tested during spring 2020 and MH outcomes (i.e., depression and anxiety) in summer 2020 (i.e., after 6 and 16 weeks from baseline), and which similarly did not identify any significant difference in MH outcomes between seronegative and seropositive adults. Similar results were obtained in studies (Blankenburg et al., Reference Blankenburg, Wekenborg, Reichert, Kirsten, Kahre, Haag, Schumm, Czyborra, Berner and Armann2022; Larsen et al., Reference Larsen, Stiles, Shaik, Schneider, Muppidi, Tsui, Geng, Bonilla and Miglis2022) that employed other MH measures including the Patient Health Questionnaire-9 and General Anxiety Disorder-7 questionnaire (Osaghae et al., Reference Osaghae, Nguyen, Chung, Moffitt, Le, Suh, Prasad, Thomas, Gordon and Hwang2021), or investigated different population groups, such as the adolescents (Blankenburg et al., Reference Blankenburg, Wekenborg, Reichert, Kirsten, Kahre, Haag, Schumm, Czyborra, Berner and Armann2022).
Our findings that psychological distress was higher in infected compared to never infected individuals only in winter 2020–2021 might suggest a short-term, direct effect of the virus on MH and may be due to its neurotropism, but also that COVID-19 infection may cause psychological distress because of the uncertainty in the ensuing disease course and/or fear of infecting others. Nevertheless, psychological distress decreased more rapidly in infected compared to never infected individuals. This contradicts, in part, our hypothesis. It is possible that the latter feared future infections, and that this fear of infection contributed to sustained psychological distress. This is consistent with consolidated evidence on the putative causative role of fear in depression, anxiety and stress (Folayan et al., Reference Folayan, Ibigbami, Brown, El Tantawi, Aly, Ezechi, Abeldaño, Ara, Ayanore, Ellakany, Gaffar, Al-Khanati, Idigbe, Jafer, Khan, Khalid, Lawal, Lusher, Nzimande and Nguyen2022). Fear of COVID-19 infection, specifically, may underpin and cause anxiety, depressive and stress symptoms (Alimoradi et al., Reference Alimoradi, Ohayon, Griffiths, Lin and Pakpour2022; Bakioğlu et al., Reference Bakioğlu, Korkmaz and Ercan2021; Luo et al., Reference Luo, Ghanei Gheshlagh, Dalvand, Saedmoucheshi and Li2021). Because the impact of COVID-19 was greater on older than younger adults (Thompson et al., Reference Thompson, Stafford, Moltrecht, Huggins, Kwong, Shaw, Zaninotto, Patel, Silverwood, McElroy, Pierce, Green, Bowyer, Maddock, Tilling, Katikireddi, Ploubidis, Porteous, Timpson and Patalay2022), fear of infection would plausibly increase with age too. Therefore, our observations on the inverse association between age and psychological distress were somewhat unexpected and may be explained, at least to some extent, by better coping attitudes in older compared to younger adults (Derrer-Merk et al., Reference Derrer-Merk, Reyes-Rodriguez, Soulsby, Roper and Bennett2023).
Fear of infection and uncertainty on the disease course may explain why infected individuals were initially stressed and progressively, as uncertainty about the disease course diminished, less distressed compared to never infected individuals. To this end, our findings would not support current hypothesis on the short- and long-term effects of the virus. Further investigations are warranted because the neurological symptoms and consequences of the SARS-CoV-2 are well known (Brola and Wilski, Reference Brola and Wilski2022; Harapan and Yoo, Reference Harapan and Yoo2021; Hosseini et al., Reference Hosseini, Nadjafi and Ashtary2021) and they include among others, anosmia, ischaemic and haemorrhagic stroke, headache, hypoxia and meningitis. That psychological distress was not associated with infection but was pervasive in our study sample, endured several months through the pandemic waves and was explained to some extent by socio-demographic and health characteristics is worth noting and may have considerable public health implications because it suggests a prominent role of the pandemic itself, conceived as collective traumatic events of unprecedented proportions. Worldwide, billions of lives were overturned by the pandemic, significantly more than the hundreds of millions who were ultimately infected.
Our study has limitations. First, our sample was not homogenous in terms of serostatus, the never infected outnumbered the infected. However, this reflects the actual infection spreading in 2021. Second, serology and MH symptomatology were assessed concomitantly only for the third DASS-21 measurement; hence, we cannot exclude that the serology status of some participants may have changed during the study. Similarly, we cannot uncover any individual who potentially got infected but whose antibodies were not detected (false negative; asymptomatic). Further, we were unable to identify how long ago the ‘infected participants’ exactly had the infection aside from when we retrieved the serology status. Consequently, the generalizability of our results is limited by the specificity of the study sample and the constraints associated with the assessments conducted (i.e., serology status and DASS-21). Though robust, and valid, the DASS-21 scale is not a diagnostic instrument; other facets of psychological distress might have been better captured with a structured diagnostic instrument such as the Minnesota Multiphasic Personality Inventory (Butcher, Reference Butcher, Weiner and Craighead2010). Still, we intentionally focused on symptoms of common mental disorders in the general population, not on psychopathology and/or diagnosis. Third, selection bias cannot be excluded. Our results may underestimate or overestimate the true prevalence of moderate to severe depression, anxiety and stress in the target population because severe psychological distress and psychopathology could reduce individuals’ participation and increase attrition in studies involving frequent, though self-reported assessments. Compared to individuals without mood-related symptoms, those with more severe psychological distress may have been less likely to participate and more likely to drop out. This risk is outlined in the Cochrane collaboration Risk of Bias II assessment (Higgins et al., Reference Higgins, Savović, Page, Elbers, Sterne, Higgins, Thomas, Chandler, Cumpston, Li, Page and Welch2019). Fourth, our results may be generalized only to populations with MH profiles and infection status like those of our sample. Even tough, group differences in our sample were not statistically significant for any socio-demographic characteristics other than age, further research will be needed to assess the relationship between BMI and gender differences as potential confounding variables. Fifth, we did not use a formal assessment of fear or specific scales like, for example, the Corona Virus Anxiety Scale (Lee, Reference Lee2020). Our interpretation of the role of fear is speculative. Yet, the DASS-21 provides good proxies of fear-related distress. Further, the study did not consider any distinction among SARS-CoV-2 variants. However, we assessed seropositivity in mid-2021, when infections were mainly due to gamma and delta variants (Ufficio federale della anità pubblica, 2023). Finally, we did not differentiate between acute or overcome infections as it was beyond the scope of this study.
Strengths of our study include the use of a validated and comprehensive tool for measuring MH outcomes. The DASS-21 offers a comprehensive evaluation of the three main dimensions associated with psychological distress (i.e., depression, anxiety, stress), which represent the most common psychological symptoms reported during the pandemic (Alqahtani et al., Reference Alqahtani, Al-Garni, Abumelha, Alsagti, Alshehri, Alqahtani and Alkhidhran2023). Further, we performed repeated assessments in a population-based (non-clinical) sample, which was representative of the general population. Compared to studies that focused on clinical samples (Ray et al., Reference Ray, Abdel-Mannan, Sa, Fuller, Wood, Pysden, Yoong, McCullagh, Scott, McMahon, Thomas, Taylor, Illingworth, McCrea, Davies, Whitehouse, Zuberi, Guthrie, Wassmer and Benjamin2021) or specific populations, i.e., adolescents (Blankenburg et al., Reference Blankenburg, Wekenborg, Reichert, Kirsten, Kahre, Haag, Schumm, Czyborra, Berner and Armann2022) and healthcare workers and/or settings (Grazzini et al., Reference Grazzini, Lulli, Mucci, Paolini, Baldassarre, Gallinoro, Chiarelli, Niccolini and Arcangeli2022; Osaghae et al., Reference Osaghae, Nguyen, Chung, Moffitt, Le, Suh, Prasad, Thomas, Gordon and Hwang2021), our results may be less biased and have greater external validity. We applied robust statistical techniques to capture temporal variations and longitudinal patterns of psychological distress over one year of observation during the COVID-19 pandemic, and formally confirmed the goodness of fit of our statistical models. Therefore, that our initial hypothesis was not confirmed seems unlikely due to type 2 error (i.e., missing true association when present), also because we did find significant inverse associations between infection and psychological distress symptoms.
Conclusions
The public health implications of our study relate to the importance of lessening the overall impact of potential future pandemic (or similar) events conceived as a collective traumatic experience. Considering the limitations inherent in our study sample, our observations suggest that long-term MH consequences of the pandemic may not be due to the SARS-CoV-2 infection, but plausibly to the uncertainties and fears associated with the risk of infection. Our findings are novel, and replications are warranted, but our study highlights the importance of MH preventive components within preparedness strategies for potential future pandemics or other public health emergencies.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796024000507.
Acknowledgements
The authors thank all participants of ‘Corona Immunitas Ticino’ for their essential contribution and the Swiss Federal Statistical Office for providing the randomized list of participants. The serological data used in this study were collected and analysed thanks to the collaboration of Ente Ospedaliero Cantonale, Centro Medico, Institute of Microbiology SUPSI, Centre Hospitalier Universitaire Vaudois (CHUV), and Università della Svizzera italiana (USI). We also thank the Swiss School of Public Health and the Ceresio Foundation for their support in funding this project.
Finally, the authors acknowledge the ‘Corona Immunitas Ticino’ study group: Emiliano Albanese, Rebecca Amati, Antonio Amendola, Anna Maria Annoni, Granit Baqaj, Kleona Bezani, Peter Buttaroni, Anne-Linda Camerini, Anna Paola Caminada, Elia Cattani, Alessandro Ceschi, Laurie Corna, Cristina Corti Fragoso, Luca Crivelli, Diana Sofia Da Costa Santos, Giorgio Dal Bo’, Gladys Delai Venturelli, Daniela Dordoni, Marta Fadda, Luca Faillace, Ilaria Falvo, Paolo Ferrari, Maddalena Fiordelli, Carolina Foglia, Giovanni Franscella, Sara Gamberoni, Roberta Gandolfi, Rosita Ghidossi, Daniele Giottonini, Paola Guglielmetti, Sandra Jovic, Franco Keller, Sara Levati, Isabella Martinelli, Federico Mele, Rosalba Morese, Anna Papis, Giovanni Piumatti, Greta Rizzi, Serena Sabatini, Federica Sallusto, Tatiana Terrot, and Mauro Tonolla.
Availability of data and materials
Data will be available upon reasonable request through a methodologically sound proposal directed to the corresponding author (Emiliano Albanese, [email protected]).
Author contributions
B. Bano and C. Sculco equally contributed to this manuscript, and they share co-first authorship.
Financial support
The Corona Immunitas Ticino study was funded by the Swiss School of Public Health through the national research program Corona Immunitas, which is a public-private partnership supported by the Swiss Federal Office of Public Health, various cantons, and private funders, and in part by Ceresio Foundation.
Ethical standards
The Corona Immunitas Ticino study was approved by the Ethics Committee of Ticino (part of SwissEthics) (BASEC 2020-01514) on June 23, 2020.
The authors assert that all procedures contributing to this study comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Competing interests
The authors declare that they have no competing interests.






