Many countries have implemented folic acid supplementation (FAS) pre-pregnancy and during the first trimester of pregnancy, due to the unequivocal evidence for its protection against neural tube defects (NTD)( 1 , Reference Gomes, Lopes and Pinto 2 ). However, adherence with this policy is often poor, with use of folic acid supplements (FAS) being reported for <50 % of pregnant women in several countries, including Australia, Canada, the UK, the USA, Pakistan, Indonesia and China( Reference Ray, Singh and Burrows 3 – Reference Titaley and Dibley 6 ). Factors that have been associated with inadequate use of FAS among women include younger age, belonging to particular ethnic groups, single relationship status, multiparity, unplanned pregnancy, a less healthy lifestyle and poorer socio-economic conditions( Reference Ray, Singh and Burrows 3 , Reference Stockley and Lund 7 , Reference Morton, Grant and Carr 8 ).
In addition to the prevention of NTD, maternal FAS in the pre-pregnancy and/or early pregnancy period is associated with a reduced risk of other disorders, including congenital heart defects( Reference Feng, Wang and Chen 9 ), small-for-gestational-age birth( Reference Hodgetts, Morris and Francis 10 ), language delay( Reference Roth, Magnus and Schjølberg 11 ), behavioural problems( Reference Roza, van Batenburg-Eddes and Steegers 12 ) and autistic spectrum disorder( Reference Barua, Kuizon and Brown 13 – Reference Surén, Roth and Bresnahan 16 ). However, emerging evidence suggests a U-shaped relationship of maternal folate status during pregnancy with birth and childhood health outcomes. Studies have reported associations of increased exposure to folic acid (in duration and/or dose) with an increased risk of several adverse health outcomes( Reference Choi, Yates and Veysey 17 , Reference Patel and Sobczyńska-Malefora 18 ), including large-for-gestational-age birth( Reference Wang, Ge and Zhu 19 ), insulin resistance( Reference Keating, Correia-Branco and Araujo 20 – Reference Yajnik, Deshpande and Jackson 22 ), increased adiposity( Reference Krishnaveni, Veena and Karat 21 ), lower psychomotor scores( Reference Valera-Gran, García de la Hera and Navarrete-Muñoz 23 ) and asthma( Reference Whitrow, Moore and Rumbold 24 ).
It is now recognized that there is the need to identify groups within populations at increased risk of both insufficient and excessive periconceptional FAS use( Reference Choi, Yates and Veysey 17 ). The opportunity to determine the prevalence of both insufficient and excessive use of FAS and the factors associated with each in New Zealand (NZ) was created by the establishment of a large cohort study into which enrolment occurred during the last trimester of pregnancy. We have previously used the data collected in this cohort study to show that only 39 % of women reported starting FAS before pregnancy( Reference Morton, Grant and Carr 8 ). To date though, there has been no investigation describing the adherence to the NZ Ministry of Health (MoH) recommendations for FAS during pregnancy that considers both insufficient and excessive use.
Our aim was to describe the use of FAS during pregnancy and to identify the sociodemographic and lifestyle factors associated with insufficient and excessive use of FAS in a nationally generalizable birth cohort study( Reference Morton, Ramke and Kinloch 25 ).
Methods
The Growing Up in New Zealand study
Growing Up in New Zealand (GUiNZ) is a multi-ethnic nationally representative longitudinal birth cohort study, consisting of 6853 children born to 6822 women enrolled while pregnant( Reference Morton, Ramke and Kinloch 25 , Reference Morton, Atatoa Carr and Grant 26 ). As described previously, pregnant women were eligible if they had an estimated delivery date between 25 April 2009 and 25 March 2010 and were living in a geographical region defined by the three contiguous District Health Boards of Auckland, Counties-Manukau and Waikato( Reference Morton, Atatoa Carr and Grant 26 ). For the present study, we utilized data from the first collection wave (antenatal period; completed in 2010). Data were collected at a face-to-face home computer-assisted personal interview conducted with each pregnant woman, most often in the last trimester of her pregnancy. Linkage was established to perinatal health records, providing information about the latter stages of pregnancy, the birth and the immediate neonatal period.
Assessment of folic acid supplement use
Subsidized folic acid supplements dispensed from community pharmacies
Folic acid tablets are available over the counter from pharmacies in NZ and are also dispensed, at a lower cost, to pregnant women with a prescription. All the prescribed dispensing from community pharmacies is recorded in the NZ MoH Pharmaceutical Collection database( Reference Horsburgh, Malik and Pauline 27 ). Data linkage with the Pharmaceutical Collection database identified FAS dispensed by pharmacists to the women participating in the GUiNZ cohort study from when their pregnancy was confirmed (mostly in the first trimester of pregnancy). The GUiNZ study obtained consent to access women’s pharmaceutical information only for their pregnancy with the cohort child. For this reason, the data on pharmacy dispensing in the pre-pregnancy period were not available for use in the present study. FAS dispensed from pharmacies were described according to the tablets’ folic acid content (folic acid 5 mg, folic acid 0·8 mg, folic acid 0·35 mg with a ferrous compound).
Using the infant’s gestational age at birth we estimated the gestational age of each woman when first dispensed FAS and simulated the total number of weeks of FAS using the pharmacy dispensing date and number of tablets dispensed (assuming the use of 1 tablet/d). Information on use, duration of use and dose of FAS was available for 6044/6822 (89 %) of the women. After additional exclusions from missing Pharmaceutical Collection data, the final sample was 5857/6822 (86 %) women.
Maternal report of folic acid supplement use
The use of FAS was evaluated in three time periods: 3 months before pregnancy, during the first trimester of pregnancy and after the first trimester of pregnancy. For each of those time intervals the following questions were asked: ‘Have you taken folate or folic acid, even as part of a multivitamin?’, ‘How many days per week on average?’ and ‘For how many weeks?’.
The current NZ MoH policy on use of FAS for reducing NTD in NZ is the same as that in place at the time of the present study. In NZ it is recommended that women at low risk of an NTD-affected pregnancy and who plan to become pregnant take 0·8 mg of folic acid daily for 4 weeks before until 12 weeks after conception( 28 ). For women at high risk of an NTD-affected pregnancy the recommendation is to take a higher dosage (5 mg of folic acid daily) during the same time period. The NZ MoH considers women at high risk as those: with a previous NTD-affected pregnancy; or with a close family member who has had an NTD; or who are on insulin for diabetes, or who are taking medications known to affect folate metabolism; or whose partner is affected or has a family history of NTD( 28 ).
Based on the reported FAS, the study population was divided in five categories. The category ‘no use’ refers to the women who did not use FAS either before or during the pregnancy. The category ‘recommended use’ refers to women who used FAS 6–7 times/week in at least 4 weeks pre-pregnancy and for 12 weeks after conception. The category ‘recommended and extended use’ refers to the women who used FAS according to recommendation but then also extended its use after the first trimester. The category ‘insufficient use’ refers to the women who did not take FAS as recommended in early pregnancy. The category ‘insufficient and extended use’ refers to the women who did not take enough FAS in early pregnancy but extended its use after the first trimester of pregnancy.
Assessment of covariates
Variables describing maternal self-prioritized ethnicity, age, education, relationship status, employment, parity, alcohol consumption, smoking patterns, pregnancy planning, and sources of information about vitamins and minerals were collected during the antenatal interview.
Maternal self-prioritized ethnicities were self-reported. Response options provided a list of thirty-three ethnicities, with the ability for participants to indicate alternative ‘other’ ethnicities in addition to those listed. Ethnicity was then coded into six Level 1 categories following the Statistics NZ coding criteria: (i) European, (ii) Māori, (iii) Pacific Peoples, (iv) Asian, (v) Middle Eastern, Latin American and African (MELAA) and (vi) other, with MELAA and other then combined for analysis purposes because of the smaller sizes of these two groups.
Socio-economic deprivation was described using the 2006 NZ Index of Deprivation (NZDep06), grouped as deciles. NZDep06, derived from 2006 census data on nine socio-economic characteristics, is a well-validated measure of small area socio-economic deprivation in NZ( 29 ).
Maternal self-reported weight (in kilograms) and height (in centimetres) during pregnancy were collected and BMI calculated and categorized according to WHO criteria( 30 ). During pregnancy, self-reported height and weight have been shown to classify the majority (84 %) of women into appropriate BMI categories( Reference Brunner Huber 31 ).
Statistical analyses
Proportions, means, medians and interquartile ranges were used to describe the population and the FAS use. Proportions were compared with the χ 2 test. Associations between the categories of FAS use and maternal sociodemographic and lifestyle characteristics were described using relative risk ratios (RRR) and 95 % CI obtained from multivariate multinomial logistic regression models. The women who used FAS during pregnancy as recommended formed the reference group for these analyses. Participants with missing values for any of the covariates were excluded from the final multivariate model.
All analyses were performed using the Stata statistical software package release 12 (2011). Two-sided significance was determined at P<0·05.
Results
Folic acid dispensed from pharmacies to cohort women
For 29·6 % (1791/6044) of the women, FAS were reported to have been used during the pregnancy and were also dispensed from a pharmacy. Additionally, 3·1 % (188/6044) of the women were dispensed folic acid tablets but did not report using FAS. Overall, a mean of eighty-nine tablets containing folic acid were dispensed per woman. Of the tablets dispensed, 71 % contained 0·8 mg of folic acid, 15 % contained 5 mg of folic acid and 14 % contained 0·35 mg with a ferrous compound (Table 1).
Table 1 Number and proportion of women according to category of folic acid supplement use, pharmacy dispensing and type of supplement dispensed in New Zealand, 2008–2010
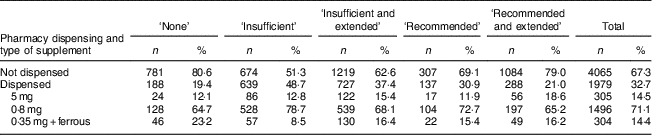
Figure 1 illustrates the distribution of gestational age of the women during the time they were dispensed folic acid tablets. More than 75 % of the women who were dispensed folic acid tablets, in its different formulations, potentially extended its use beyond the first trimester of pregnancy.
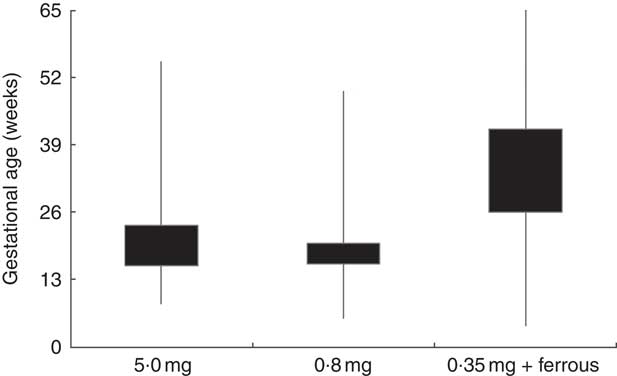
Fig. 1 Box-and whisker plot showing the potential duration* of use of subsidized folic acid supplements according to the type of tablet dispensed in New Zealand, 2008–2010. The bottom and top edge of the boxes represent the 25th and 75th percentile (interquartile range), respectively; and the ends of the bottom and top whiskers represent the minimum and maximum values, respectively, of gestational age in weeks. *Potential duration of use of subsidized tablets was estimated using infant’s gestational age at birth, gestational age when the woman was first dispensed folic acid tablets and the number of tablets dispensed (assuming the use of 1 tablet/d)
Maternal report of folic acid supplement use
Only a small proportion of women (7·6 %) reported the use of FAS as recommended, with another 23·4 % reporting using FAS according to the recommendation but with extension into the second trimester of pregnancy. Thirteen per cent of the women reported no use of FAS either before or during pregnancy. Almost one-quarter of the women (22·4 %) had insufficient use of FAS in early pregnancy and 33·3 % had insufficient use of FAS in early pregnancy but extended its use beyond the first trimester.
The majority of women in the ‘insufficient and extended’ use group did not use FAS during the pre-pregnancy period but used 6–7 folic acid tablets/week from the first trimester of pregnancy. Ninety-two per cent of the women in the ‘recommended and extended use’ group continued using 6–7 folic acid tablets/week after the first trimester of pregnancy, for a median of an additional 20 weeks (interquartile range=15–26 weeks; Table 2).
Table 2 Distribution of women by category of folic acid supplement (FAS) use, reported dose of FAS used per week and reported duration of FAS use (weeks) in the pre-pregnancy period, first trimester and after the first trimester of pregnancy in New Zealand, 2008–2010
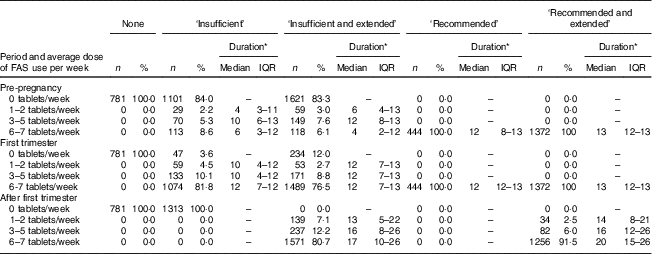
IQR, interquartile range.
* Duration in weeks.
Maternal sociodemographic and lifestyle characteristics
Over one-half (55·2 %) of the women identified themselves as European, 30·6 % had completed at least a diploma/trade certificate, for 61·3 % the current pregnancy was planned and 37·2 % lived in the most deprived households. Higher level of education, more social support (relationship status), employment, having a planned pregnancy and living in a less deprived household were more prevalent when FAS was used according to the recommendation, as identified by the ‘recommended use’ and ‘recommended and extended use’ categories when compared with the ‘no use’, ‘insufficient use’ and ‘insufficient and extended use’ (Table 3).
Table 3 Sociodemographic and lifestyle characteristics of women according to the category of folic acid supplement use in New Zealand, 2008–2010
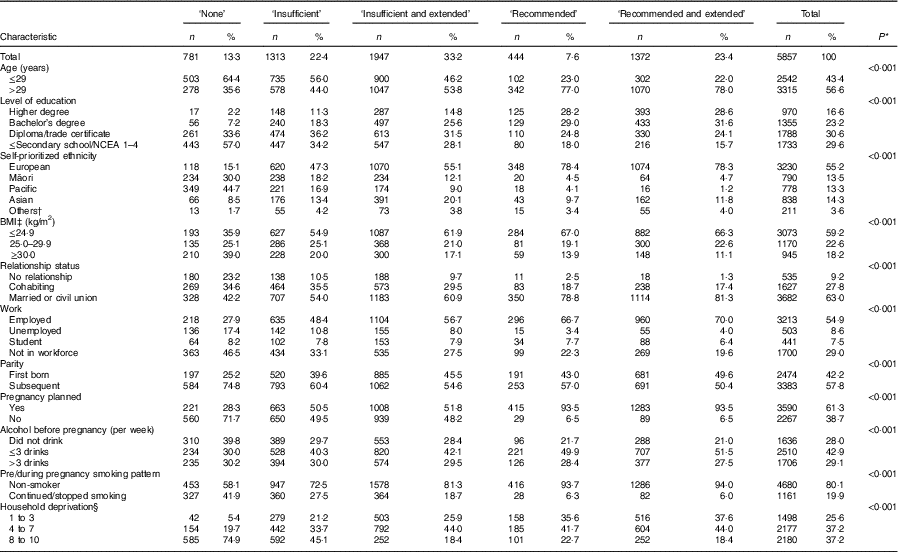
NCEA, National Certificate of Educational Achievement.
* The χ 2 test was used to determine if any significant differences existed among the groups according to maternal characteristics (P<0·05 indicates there is difference).
† Others include Middle Eastern, Latin American, African and others.
‡ BMI: underweight and eutrophic, ≤24·9 kg/m2; overweight, 25·0–29·9 kg/m2; and obese, ≥30·0 kg/m2.
§ Area-level socio-economic deprivation was measured using the NZ Index of Deprivation: decile 1, 2 and 3=least deprived households; decile 8, 9 and 10=most deprived households.
Determinants of folic acid supplement use
In the univariate analyses, all the variables describing women’s sociodemographic and lifestyle characteristics were associated with the categories of FAS use (see online supplementary material, Supplemental Table 1).
The results of the multivariate analysis with the independent associations of maternal characteristics with the categories of FAS use are presented in Table 4. The variables nutritional status, relationship status and alcohol consumption before pregnancy were excluded from the multivariate analyses because they were not associated with any category of FAS use. In the multivariate analysis, the two factors independently associated with all FAS categories of use, when compared with the ‘recommended’ group, were self-prioritized ethnicity and parity. Pregnancy planning, education and age were independently associated with the ‘none’, ‘insufficient use’ or ‘insufficient and extended use’ groups. Smoking was independently associated with the ‘none’ or ‘insufficient use’ group. Work status and household deprivation were factors independently associated with the ‘none’ group (Table 4).
Table 4 Adjusted relative risk ratios (RRR) and 95 % CI for the association of maternal sociodemographic and lifestyle characteristics with category of folic acid supplement use in New Zealand, 2008–2010
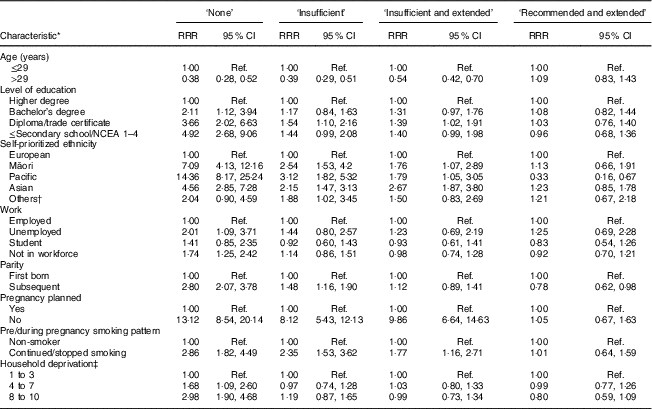
NCEA, National Certificate of Educational Achievement; Ref., reference category.
* Multivariate multinomial logistic regression models included all variables presented in the table. The group of women who reached the recommendation for folic acid use was the reference in this analysis (two-sided significance was determined at P<0·05).
† Others include Middle Eastern, Latin American, African, and others.
‡ Area-level socio-economic deprivation was measured using the NZ Index of Deprivation: decile 1, 2 and 3=least deprived households; decile 8, 9 and 10=most deprived households.
Sources of information about vitamins and minerals during pregnancy
The main sources of information about vitamins and minerals during pregnancy were health-care professionals (83·3 %), family and friends (31·4 %) and media (31·3 %; Table 5).
Table 5 Number and proportion of women, according to category of use of folic acid supplements, using different sources of information about vitamins and minerals during pregnancy in New Zealand, 2008–2010
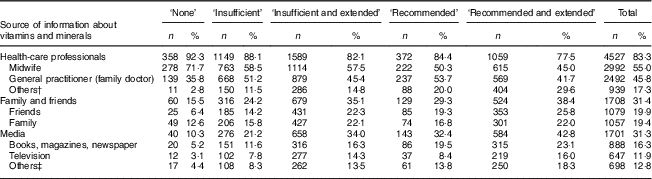
† Other health-care professionals include obstetrician, alternative health practitioner and dietitian/nutritionist.
‡ Other media sources include Internet, pharmacy/retailer and radio.
Discussion
Ninety-two per cent of pregnant women from this nationally generalizable birth cohort study in NZ were not taking FAS as recommended. Thirteen per cent of pregnant women did not use FAS at any time, with non-use associated with age, education, ethnicity, employment, parity, pregnancy planning, smoking and household deprivation. One-quarter of the women had insufficient use of FAS during pregnancy and this insufficient use was associated with age, education, ethnicity, parity, pregnancy planning and smoking. One-third of the women had insufficient use of FAS in early pregnancy and then extended its use beyond the first trimester. This pattern of FAS use was associated with age, education, ethnicity, parity and pregnancy planning. The remaining quarter of women initially achieved the recommendation but then extended FAS use beyond the first trimester of pregnancy, and this pattern was associated with ethnicity and parity. Health-care professionals were the main sources of information about vitamins and minerals, followed by family and friends. For only 35 % of the pregnant women was FAS use during pregnancy both self-reported and confirmed by receipt of subsidized folic acid tablets dispensed from a pharmacy.
Improving adherence of pregnant women with national recommendations for FAS is a challenge faced by many countries. In most European countries, a minority of women take FAS during the entire period recommended (4 weeks before conception until 8 weeks after), ranging from 3 % in Italy to 51 % in the Netherlands (where 85 % of pregnancies are believed to be planned)( 32 , Reference de Walle and de Jong-van den Berg 33 ). In Australia, from 28 to 46 % of women use FAS periconceptionally( Reference Stockley and Lund 7 ). Approximately 39 % of NZ women in the GUiNZ cohort started using FAS before pregnancy( Reference Morton, Grant and Carr 8 ), but only 8 % used it as recommended both pre- and post-conception. Lack of pregnancy planning is likely to be one of the main reasons for the proportion of women who used FAS as directed being as low as it was in our cohort. We have previously shown that the odds of starting FAS prior to the pregnancy were approximately ten times lower for women whose pregnancy was not planned compared with women whose pregnancy was planned( Reference Morton, Grant and Carr 8 ). Another potential reason for the high prevalence of non-use of FAS within the GUiNZ cohort could be the lack of adequate and complete information about the importance of taking FAS during pregnancy. While almost all women in the cohort had engaged with a lead maternity carer for their antenatal care (98 %), for 8 to 14 % of them engagement with a lead maternity carer was delayed past 10 weeks of gestation( Reference Bartholomew, Morton and Atatoa Carr 34 ).
The determinants of inadequate FAS use during pregnancy have been reported in other studies( Reference Ray, Singh and Burrows 3 – Reference Stockley and Lund 7 , Reference Ibrahim, El-Hamid and Mikhail 35 ). Those women who are younger, from lower incomes and educational levels, with less social support, or of a minority ethnic group are less likely to take FAS( Reference Stockley and Lund 7 , Reference Manniën, de Jonge and Cornel 36 ). These associations were also evident in our study and highlight that the current policy provides the poorest protection against NTD for women who have the least resources available to care for a child with an NTD( Reference Yi, Lindemann and Colligs 37 ).
In contemporary society where information is available from many sources and where folic acid is easily accessed (via supplements and fortified food), excessive intake becomes a potentially important issue( Reference Patel and Sobczyńska-Malefora 18 ). Despite the limited number of published data on the prevalence of FAS use which exceeds recommendations, recent studies have suggested that excessive maternal folic acid can influence fetal programming, with this being of relevance to the subsequent development of diabetes( Reference Keating, Correia-Branco and Araujo 20 – Reference Yajnik, Deshpande and Jackson 22 ), asthma( Reference Whitrow, Moore and Rumbold 24 ), and neurological and psychiatric diseases( Reference Barua, Kuizon and Brown 13 , Reference Valera-Gran, García de la Hera and Navarrete-Muñoz 23 , Reference Girotto, Scott and Avchalumov 38 ). In an Indian cohort of 533 pregnant women and their children, higher maternal folate concentrations (plasma level assessed at 30 weeks’ gestation) were associated with higher homeostatic model assessment of insulin resistance in the children at 9·5 and 13·5 years of age( Reference Krishnaveni, Veena and Karat 21 ). The Generation 1 cohort study of Australian families (n 557), where mothers reported retrospectively about consumption of FAS, showed that FAS in late pregnancy (30–34 weeks) was associated with an increased odds of asthma when the cohort children were 3·5 years of age( Reference Whitrow, Moore and Rumbold 24 ). In the same study, 16 % of women used FAS in pre-pregnancy, early and late pregnancy and 12 % used in early and late pregnancy, compared with 23 % (‘recommended and extended use’) and 33 % (‘insufficient and extended use’), respectively, in the GUiNZ study( Reference Whitrow, Moore and Rumbold 24 ). A population-based cohort study in a Chinese province found that among those who took FAS in the first trimester, 18 % extended supplement use for the second and/or third trimester of pregnancy( Reference Wang, Ge and Zhu 19 ).
The prevalence of extended use of FAS in NZ is high and it will be important to determine if such use is associated with any adverse health outcomes, for example increased adiposity, insulin resistance and asthma. Two distinct groups of extended use were apparent. In common, a larger proportion of both groups reported accessing information about vitamin and mineral use during pregnancy from family, friends and television than did women in other categories of FAS use. The women in the ‘recommended and extended use’ group were comparable to those in the ‘recommended use group’. They were older, had better sociodemographic indicators and, for a larger proportion of them, the pregnancy was planned. Other studies have reported similar demographic characteristics among those with extended use of folic acid( Reference Wang, Ge and Zhu 19 ). In comparison with the ‘recommended use’ group, for a larger proportion of those in the ‘recommended and extended use’ group this was their first pregnancy. During the first pregnancy fears for the well-being of the fetus are increased, enhancing the perception of risk to their baby that could potentially make extended use of folic acid more likely to occur( Reference Phelan 39 , Reference Atkinson, Shaw and French 40 ).
In NZ, there is no pre-approval process by the NZ Medicines and Medical Devices Safety Authority for dietary supplements to be sold and the sponsor is responsible to ensure the product is safe and complies with the Dietary Supplements Regulations 1985( 41 ). Between 2008 and 2010, there were thirty-three different dietary supplements available in NZ that contained folic acid, seventeen of which were sold in pharmacies only( 42 ). More than 60 % of pregnant women who reported using FAS in the GUiNZ study had not been dispensed subsidized tablets, which implies they were buying these supplements from pharmacies or other retail stores. Yet, within the subsidized dispensation system, there should be a mechanism to control FAS use and to prevent the potential for extended use of this supplement (Fig. 1). We hypothesize that the potential for extended use arises because of the lack of precise calculation of the number of tablets dispensed and that prescriptions remain valid for 3 months after the date they are written. Thus, health-care professionals’ prescribing appears to contribute to at least some of this extended FAS use( 43 ).
Subsidized folic acid tablets with dosages of 0·8 mg and 5 mg are the only registered folic acid preparations available over the counter from pharmacies in NZ( 28 ). The NZ policy recommends a dosage of folic acid for women at low risk of an NTD-affected pregnancy that is twice as high as that recommended by the WHO and adopted by several countries( Reference Gomes, Lopes and Pinto 2 ). To avoid or minimize its excessive use, the available dose of folic acid in supplements and multivitamins in NZ could be reduced to 0·4 mg/d for women at low risk of an NTD-affected pregnancy( Reference Hoffman and Klein 44 ).
Among the GUiNZ participants who were dispensed subsidized FAS from pharmacies, 15 % (n 305) took tablets containing 5 mg of folic acid. The frequency of this higher-dose prescription seems higher than the prevalence of women with increased risk of NTD (family history of NTD, use of medications known to affect folate metabolism and/or insulin treatment for diabetes)( 28 ). For example, the self-reported prevalence of diabetes (before and/or during the current pregnancy) was 4·6 % among those who were dispensed FAS in the GUiNZ study (data not shown). A Spanish multicentre mother and child cohort study (n 2226) suggested that high dose of FAS (5 mg/d) during pregnancy, used by almost 3·5 % of mothers, was associated with lower psychomotor development of infants at 1 year of age( Reference Valera-Gran, García de la Hera and Navarrete-Muñoz 23 ). These findings suggest the need for better education of health-care providers regarding folic acid prescribing practices.
Our study findings provide an important foundation upon which policy makers could develop strategies to ensure that neither insufficient nor excessive use of FAS increases the risk of poor outcomes for the mother and/or her child. Some strategies have been described to be effective interventions for improving FAS use mainly among disadvantaged women( Reference Stockley and Lund 7 ). As a potential short-term intervention, the importance of FAS could be addressed during routine family doctor visits, for example during routine cervical screening with women who are less likely to use FAS. NZ is in the highest five of OECD (Organisation for Economic Co-operation and Development) countries in terms of cervical screening rates (76·7 % of total coverage), although with lower coverage rates among Māori (65·5 %) and Asian (64·8 %) populations( Reference Stockley and Lund 7 , 45 ). During antenatal care appointments health professionals should discuss with women, more likely to use FAS beyond the recommendation, the possible risks associated with this behaviour. As a longer-term intervention, several studies have reported that more effective and safer prevention of NTD can be achieved with fortification of food with folic acid( Reference Williams, Mai and Mulinare 46 , Reference Atta, Fiest and Frolkis 47 ). Since 1996, NZ has allowed the voluntary fortification of bread with folic acid, but the majority of breads remain unfortified with folic acid( 48 ). In 2009, legislation for the mandatory fortification of wheat flour or bread with folic acid was introduced in Australia and with the intention that it would also be introduced in NZ, where the government instead decided to encourage increased voluntary fortification by the baking industry. That this legislation in Australia was effective in reducing the incidence of NTD-affected pregnancies and safe was confirmed in a 2016 report by the Australian Government( 49 , Reference Hilder 50 ). NZ does need to reconsider the decision not to mandate for folic acid fortification. The folate status of women of childbearing age in NZ was assessed in a population-based health survey conducted in 2014–2015( 51 ). Sixteen per cent of the women aged 15–49 years who were included in the survey had erythrocyte folate levels accepted internationally to confer minimal risk of an NTD-affected pregnancy( 51 ). These data add additional support to the implementation of the fortification programme.
Our study has a number of potential limitations. The data on FAS use could be biased since this was reported retrospectively by women, most often in the last trimester of pregnancy. Such data collection methods tend to result in an overestimation of FAS use due to the social desirability of particular responses to such questions( Reference Althubaiti 52 ). As part of the baseline and monitoring assessment, only updated national nutrition surveys, ideally with assessment of erythrocyte folate levels( Reference Green, Newton and Bourn 53 ), will allow for estimation of the prevalence of inadequate and excess folic acid intake across life-cycle groups( Reference Evans, Mygind and Peddie 54 – Reference Lawrence 59 ). We have conducted a cross-sectional analysis within the GUiNZ cohort and longitudinal analyses, with cohort sizes larger than ours, are needed in this field. A major strength of the present study is the size of the cohort, its diversity and generalizability to the current population of NZ births( Reference Morton, Ramke and Kinloch 25 , Reference Morton, Atatoa Carr and Grant 26 ). We add important findings to this field of research, given that we were able to characterize in detail the use of FAS throughout pregnancy in a nationally generalizable sample. In addition, the use of data linkage to Pharmaceutical Collection database permitted more accurate evaluation of the use of FAS( Reference Horsburgh, Malik and Pauline 27 ).
Conclusions
A very large proportion of NZ pregnant women were not taking FAS according to the MoH recommendation, with most of them extending its use beyond the recommended duration. In NZ, the public health messaging and education on FAS during pregnancy needs to be addressed since the current strategy appears to be inadequate, creates inequity and has a potential for harm to be caused by its inappropriate implementation. The case for revising the public policy for the prevention of NTD by folic acid fortification of foods needs to be raised again in NZ. This appears to be the only policy option that would ensure greater coverage to those at risk of insufficient intake.
Acknowledgements
Acknowledgements: The authors would like to acknowledge the participants and all members of the GUiNZ study. They would also like to acknowledge the funders, the New Zealand Ministry of Social Development, supported by the Health Research Council, as well as the ongoing support from Auckland UniServices and The University of Auckland. Additionally, they acknowledge the São Paulo Research Foundation (FAPESP, Brazil) for supporting J.A.T. (grant number 2016/15356-3). The contributions of Dr Mark Robbs to the pharmacy data analysis presented in this manuscript are gratefully acknowledged. Financial support: GUiNZ has been funded by the New Zealand Ministries of Social Development, Health, Education, Justice and Pacific Island Affairs; the former Ministry of Science Innovation and the former Department of Labour (now both part of the Ministry of Business, Innovation and Employment); the former Ministry of Women’s Affairs (now the Ministry for Women); the Department of Corrections; the Families Commission (now known as the Social Policy Evaluation and Research Unit); Te Puni Kokiri; New Zealand Police; Sport New Zealand; the Housing New Zealand Corporation; and the former Mental Health Commission, The University of Auckland and Auckland UniServices Limited. Other support for the study has been provided by the NZ Health Research Council, Statistics New Zealand, the Office of the Children’s Commissioner and the Office of Ethnic Affairs. J.A.T. is supported by FAPESP, Brazil (grant number 2016/15356-3). Conflict of interest: None. Authorship: S.M.B.M., C.C.G., C.R.W. and S.B. were involved in the conception and design of the study; J.A.T. conducted the data analysis; J.A.T., T.G.C., C.R.W., C.C.G. and D.M.M. contributed to the interpretation of data and manuscript development. All authors participated in critically revising the manuscript and approved the final version. Ethics of human subject participation: The approval for this human subjects research was granted by Ministry of Health Northern Y Regional Ethics Committee. Written informed consent was obtained from all participating women.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980018000836








