Introduction
Sexual transmission of the human immunodeficiency virus (HIV) depends on various factors [Reference Patel1], of which viral load [Reference Quinn2, Reference Blaser3] and stage of infection [Reference Wawer4, Reference Hollingsworth, Anderson and Fraser5] are of utmost importance. Use of condoms is the standard procedure for protection against exposure to and transmission of HIV as well as other sexually transmitted infections (STIs). A Cochrane review [Reference Weller and Davis6] has estimated the effectiveness of condoms in reducing heterosexual HIV transmission at 80% with a 95% confidence interval of 35.4% to 94.2%. More recent assessments consider the protection rate of condoms to be slightly lower but in a similar range of more than 70% [Reference Giannou7]. Corresponding meta-analyses are missing for men having sex with men (MSM).
In spite of the undeniable protective effect of condoms in the reduction of HIV exposure risks, the effects of governmental and non-governmental efforts to promote this strategy are unsatisfying. UNAIDS reports that considerable proportions of people still favour non-protected risky sex [8].
As HIV infections are not curable and with no vaccine available, prevention remains important, especially for individuals favouring risky condom-free sex. Pre-exposure prophylaxis (PrEP) of HIV infection is currently the most widely discussed achievement in this field. PrEP, usually with a combination of tenofovir disoproxil fumarate and emtricitabine, is used either continuously [Reference McCormack9] or shortly before and/or after a risky sexual encounter (‘PrEP on demand’) [Reference Molina10]. HIV transmission reduction due to both forms of PrEP application is estimated to be 86% [Reference McCormack9, Reference Molina10] and is accordingly similar to transmission reduction due to condom use [Reference Weller and Davis6]. Latest approaches suggest that injectable depot drugs like carbotegravir can reduce HIV infection risks that result from poor adherence to tablet-based PrEP [Reference Whitfield11]. The risks of acquiring other but curable STIs such as gonorrhoeal, chlamydial and Trichomonas-associated urethritis or syphilis as well as hepatitis B, a vaccine-preventable disease, are not influenced.
HIV PrEP is not the only approach applied by groups at risk that avoid condom use to reduce HIV transmission after the introduction of easy-to-use rapid HIV-1/2 diagnostic test (RDT) kits. Such a test-based approach could lead to an agreement to having unprotected intercourse only if both of the potential sexual partners agree to perform such tests prior to having intercourse, with the intention of ensuring one another's concordant HIV-status. However, systematic scientific assessment of this testing-based preventive strategy is still missing.
A major concern of the reliability of on-the-spot testing and subsequent decision-making for having unprotected sex is the diagnostic gap in the early stage of an HIV infection, or, more precisely, during the early seroconversion period. Within this time, HIV copy numbers in blood and other body fluids are very high and, consequently, the transmission risk is increased by a factor of 10–100 compared with later stages of an HIV infection, when the immune system is still capable of keeping viral replication in check [Reference Wawer4, Reference Hollingsworth, Anderson and Fraser5]. In particular, the average viraemia in the various stages of very early HIV infection [Reference Fiebig12, Reference Fiebig13] is likely to have a major impact on the specific transmission risk [Reference Blaser3]. Increases in the transmission risk by a factor of up to 7 in late-stage infections compared with asymptomatic infections have been described [Reference Hollingsworth, Anderson and Fraser5].
Different diagnostic markers appear at different stages of early infection. HIV RNA in the blood becomes detectable between the first and second weeks after infection. With currently applied laboratory detection systems, the main viral protein p24 is earliest traceable between the second and third weeks after infection, while latest systems that decrease this gap to 1 week remain experimental [Reference Kosaka14]. HIV-specific antibodies with sufficient affinity and above the detection limit of standard diagnostic test systems usually appear only after 3 weeks [Reference Dewar, Goldstein, Maldarelli, Mandell, Bennett, Dolin and Mandell15].
The sequence of events during primary HIV infection, consisting of initial rapid virus replication, followed by antigen release and the primary immune response with antibody production, has consequences for the diagnostic gap. Sexual transmissibility of HIV is linked to the viral load as detailed in the Swiss consensus statement [Reference Vernazza16]. It is accepted that the risk of an individual without detectable HIV RNA sexually spreading HIV is extremely low, but still not zero [Reference Wilson17]. However, traditional RDTs detect antigen–antibody interactions rather than viral RNA or proviral DNA. To tackle the problem of the delayed formation of anti-HIV antibodies, only 4th-generation RDTs that identify both HIV-specific antibodies and viral p24 can be used to narrow the diagnostic gap.
The second concern about the validity of RDTs is the poor sensitivity even of 4th-generation systems in early infection stages as observed in seroconversion panels. The ‘Determine HIV1/2 Ag/Ab Combo’ test (Alere Inc., Waltham, MA, USA) is a thoroughly assessed 4th-generation HIV RDT system and the only one for which a meta-analysis has been performed [Reference Smallwood18]. Its pooled specificity is 99.1% (95% confidence interval (CI) 97.3–99.8) and the pooled sensitivity reaches 88.5% (CI 80.1–93.4). Regarding the test's antibody detection component, the specificity is 99.6% (CI 99.0–99.8) and the sensitivity 97.3% (CI 60.7–99.9). Finally, regarding the p24 antigen component, the specificity is 99.7% (CI 96.8–100) and the sensitivity 12.3% (CI 1.1–44.2), indicating a poor sensitivity for p24 detection. A slightly increased sensitivity in acute infections was observed for the successor of the Determine HIV1/2 Ag/Ab Combo test, a 4th-generation RDT (HIV Combo, Alere Inc.) [Reference Meyer19].
To simplify the complex situation: HIV RDTs react positively approximately 1 week after the first positive results assayed in serological 4th-generation bench-top device-driven examinations in diagnostic laboratories, as estimated by the Robert Koch Institute (RKI, the German National Reference Center for Infectious Diseases) based on test results obtained with a seroconverter panel [Reference Bremer20]. Polymerase chain reaction (PCR), in comparison, is about 2 weeks faster than 4th-generation devices [Reference Rabenau21]. Positive RDT results can be expected between 10 and 19 days after the first positive PCR result [Reference Scheiblauer22]. Accordingly, between the first detection of viral RNA as an indicator of transmissibility and a positive HIV RDT, there remains a diagnostic gap of approximately 3 weeks. The relevance of this 3-week gap for real exposure risks depends on the incidence of new HIV infections in sexual partners in a given population.
Apart from traditional RDTs, nucleic acid amplification-based molecular RDTs for HIV testing comprise detection of HIV-1 by the Liat HIV Quant system (Roche, Zurich, Switzerland) [Reference Scott23] and by the Xpert HIV-1 VL system (Cepheid Inc., Sunnyvale, CA, USA) [Reference Mor24–Reference Kulkarni28]; detection of HIV-1 and -2 through the Alere q HIV 1/2 Detect system (Alere Inc.) [Reference Jani29]; and isothermal-amplification-based HIV-1 detection by the SAMBA semi-Q system (Diagnostics for the Real World Ltd., Sunnyvale, CA, USA) [Reference Ritchie30]. Results are usually available within less than 2 h and the detection limits cover viral loads with relevance for sexual transmission, i.e. viral loads ⩾1000 copies. Results comparable with bench-top systems were obtained using the Xpert HIV-1 VL test [Reference Ceffa25]. Presently, the GeneXpert system is among the best-characterised rapid molecular systems [Reference Mor24–Reference Kulkarni28] and was therefore chosen for this assessment.
In this study, we modelled the HIV transmission risks for use of traditional or molecular RDT systems and exclusion from unprotected sex of sexual partners with a correctly or falsely positive test. This unconventional preventive strategy was compared with the transmission risks of condom use without knowledge about the HIV status of the sexual partners. Both strategies were assessed with and without use of HIV PrEP in a bio-statistical model considering effects of different HIV prevalences and incidences.
Transmission risk under PrEP intake has been modelled in several studies today. Concerns over declining condom use with the implementation of PrEP have been addressed by a South African modelling approach that led to the conclusion that the beneficial effect of PrEP could even make up for reduced condom use in female sex workers (FSW) [Reference Grant31]. Another South African group modelled the benefits of PrEP in different target populations. The model led to 75% risk reduction for HIV acquisition through PrEP in high-risk populations (FSW and MSM) with 50% coverage and in low-risk populations with 25% coverage. PrEP thus achieved the highest risk reduction with the lowest coverage requirements of all methods considered. Methods model comprised voluntary medical male circumcision, behaviour change communication, early anti-retroviral therapy (ART) and PrEP [Reference McGillen, Anderson and Hallett32]. However, previous modelling approaches to HIV risk assessments were focused on virus transmission [Reference Wilson17]. In the model presented here, we focus on exposure as the prerequisite of transmission and plus exposure modelling with traditional transmission modelling [Reference Wilson17] in a new combined approach.
Methods
If an individual is exposed to a sexual contact, the probability of transmission determines the risk of becoming infected. Since the risk of infection is determined by the risk of transmission and the risk of exposure, in the following we present separate subsections and models for the infection risk, exposure risk and transmission risk.
The risk of HIV infection
The aim of this modelling is the determination of the HIV infection risk per sexual contact. Various factors are relevant for the estimation of the infection risk:
• prevalence and incidence of HIV among potential sexual partners,
• preventive effects of precaution/protection measures,
• risk of transmission per individual sexual contact,
• stage of infection of the sexual partner,
• frequency/percentage of successfully treated infected potential sexual contacts.
The first condition that must be met for infection to occur is sexual contact with an HIV-infected individual. In this case, the uninfected individual is exposed. Through transmission of HIV, the exposed individual becomes infected. This risk can be described as the probability of infection under the condition of exposure:
The more general Infection Risk IR can be expressed as the probability of the combination of exposure and transmission:
The general risk of becoming infected within n sexual contacts (with n individual infection risks) is
 $$IR^n = 1 - \prod\limits_{i = 1}^n {(1 - IR_i} )$$
$$IR^n = 1 - \prod\limits_{i = 1}^n {(1 - IR_i} )$$Fiebig et al. have defined six stages of HIV infection with corresponding viral loads [Reference Fiebig12]. Since the viral load is related to the transmission risk [Reference Wilson17], the risk of transmission and so the risk of infection, is different over the various stages of infection and the ‘stage under successful treatment’ with reduced viral load as described by Wilson et al. [Reference Wilson17]. Thus, the relative frequency of seven stages – i.e. five stages of early infection [Reference Fiebig12], the stage of chronic infection [Reference Wilson17] and the stage under successful treatment – determine the risk of infection:
 $$IR = \sum\limits_{i = 1}^7 {TR_i\,ER_i} $$
$$IR = \sum\limits_{i = 1}^7 {TR_i\,ER_i} $$In the following, we introduce the risk of transmission and the risk of exposure.
The risk of HIV exposure
The basic prerequisite for HIV transmission through sexual contact is sexual contact with HIV-infected individuals and their infectious body fluids. This contact with infectious body fluids is defined as exposure. There are several strategies to prevent this contact. The most frequently advised strategy is the use of condoms. As reported [Reference Weller and Davis6], condoms reduce the exposure risk by about 80% per heterosexual contact. In high-risk populations, however, the use of condoms is often unpopular for various reasons that are beyond the scope of this report but are described by UNAIDS [8]. Unprotected sexual contact increases the infection risks. If condom use is not accepted as an option, applying RDTs for HIV could be an alternative to reduce the risk if a positive test result leads to exclusion of the positively tested individual from unprotected sex.
Since sexual contact with an HIV-infected individual can lead to transmission at any stage of the infection with varying risks of transmission, infection with HIV at any stage constitutes a basic risk of exposure.
As was discussed above, the risk of transmission varies over the stages of infection. To quantify an infection risk, the risk of exposure for each stage has to be estimated. To do this, we use the estimated duration of stages in Table 1 [Reference Fiebig12] and assume that a newly infected individual reaches the ‘under treatment’ stage after stage 5 of early infection or otherwise after the chronic stage of infection. It is assumed that eventually, virtually every HIV-infected individual gets treatment at least in very late symptomatic infection stages, an assumption that seems realistic at least in resource-rich settings. Additionally, the frequency of successfully treated infectious individuals has to be estimated. The risk of exposure per stage for an unprotected sexual contact ERu with known prevalence Prev and known cumulative incidence I is then given by
Table 1. Viral load by stage of infection as described by Fiebig et al. [Reference Fiebig12] & Wilson et al. [Reference Wilson17]

For i = 1 to 5 (early infection stages):
And for i = 6 (chronic infection):
Since the risk of exposure can be reduced with condom use by 80%, with a 95% confidence interval of 35.4% to 94.2% [Reference Weller and Davis6], this risk in case of condom use is given by
and
where c is the factor of risk reduction by condom use (in our model given by 0.8 with 95% CI of (0.354–0.942)).
Conducting diagnostic tests could be an alternative for those who reject condom use for prevention of infection. The most easily applicable systems are immunochromatographic antibody–antigen (Ab/Ag)-based RDTs. However, freshly infected individuals become infectious without having HIV-specific antibodies to be detected by common RDTs during the first weeks of their infection. The quality of p24 antigen detection by immunochromatographic RDT is poor [Reference Smallwood18] and, in line with that published meta-analysis, we assumed a sensitivity of 12.3% for antigen detection. About 1 month after infection, RDT-detectable antibodies circulate in the peripheral blood at levels above the detection limits of common RDTs. In line with the same meta-analysis, we assumed a sensitivity of 97.3% for antibody detection after day 31 [Reference Smallwood18]. Since the cumulative duration of the first four stages of HIV infection is 19.1 days, the exposure risk in stage 5 is separated into an Ag-only and an Ab/Ag period. The exposure risk for the first stage is the same as without protection since the Ag-sensitive RDT will not turn positive at this stage [Reference Fiebig12]:
For stage 5 then:
For stage 6:
Assuming, that an individual reaches the treatment stage on average about 1 year after getting infected, the exposure risk with prevention by RDT is given by
Prevention using PCR-based RDT systems is not yet available for personal use, but it may be in the future. For this prospective scenario, we assume that the performance of the PCR assays will be constant over the various stages of infection if the virus load is higher than the minimum detection limit. The exposure risk for the five early infection stages and the chronic infection stage is then given by
Since the viral load under successful treatment is very low, PCR-based prevention fails in the stage of successful treatment because the minimum detection limit is higher than the assumed 10 copies/ml. In this case, the PCR sensitivity is zero and prevention based on PCR testing is as effective as no prevention for this stage of infection. Under successful treatment, the exposure risk by PCR is therefore given by the unprotected exposure risk:
Exposure does not necessarily imply that an individual will become infected with HIV. Wilson et al. [Reference Wilson17] presented an exposure-based analysis of HIV transmission risks, which we used as the basis for our analysis. In the following, we present this model, which – in combination with our model of the exposure risk – leads to a model of the risk of infection for an uninfected individual who has a sexual contact with another of unknown HIV status.
The risk of HIV transmission
Viral load is strongly related to the transmission risk [Reference Quinn2, Reference Blaser3, Reference Wilson17]. The groups around Quinn and Wilson [Reference Quinn2, Reference Wilson17] have expressed the transmission probability based on exposure as
where r is the risk increase for each 10-fold increment in viral load, TR 0 is the baseline probability of transmission per sexual contact with an infected individual with a baseline viral load of v 0 and v 1 is any other viral load. The risk increase r was given as 2.45 with a 95% confidence interval of (1.85–3.21).
The baseline viral load as suggested by the Wilson group [Reference Wilson17] was given as 31 623 copies/ml (4.5 log10). This viral load represents the chronic stage of infection and was identically reported by others [Reference Kundro33] for late presenters (median viral load of 31 145 copies/ml), although threefold higher median viral loads have been described in another study [Reference Jipa34]. Nevertheless, late presenters, whose percentage in risk populations may vary considerably [Reference Ndiaye35–Reference Wilson37] and is difficult to predict due to individual immunological factors during the period leading to this stage [Reference Wilson37–Reference Okoye and Picker39], were not considered as a distinct group in this assessment. Wilson's baseline viral load of 31 623 copies/ml [Reference Wilson17] corresponds to a baseline transmission risk per sexual contact of 0.0005 for female partners in heterosexual contacts, 0.001 for male partners in heterosexual contacts and 0.01 for male partners in homosexual contacts. Others reported viral loads for five stages of early HIV infection as well as the average duration of these stages [Reference Fiebig12]. Wilson's estimate [Reference Wilson17] of residual viral loads under treatment of 10 copies/ml as well as baseline viral load of 31 623 copies/ml as a surrogate for chronic HIV infection was also used for this assessment. Both the viral loads by stage of infection and loads under treatment as assumed here are given in Table 1.
We applied Wilson's formula for the viral loads presented in Table 1 to calculate transmission risks per sexual contact by stage of infection and under treatment. These risks are given in Table 2 according to mode of transmission.
Table 2. Transmission risk per sexual contact by stage of infection
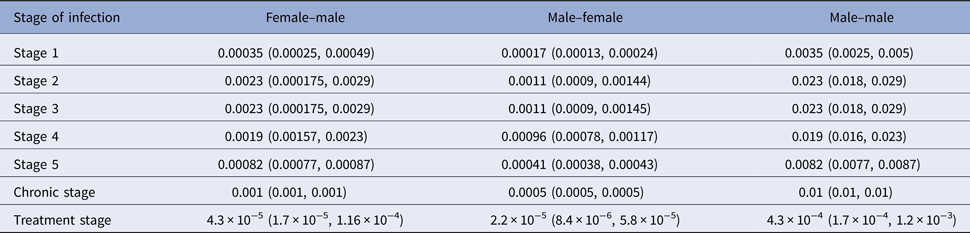
A strategy to further reduce the transmission risk is PrEP as detailed in the introduction. Reduction of HIV transmission due to PrEP on demand or continuous PrEP is estimated to be 86% under study conditions [Reference McCormack9, Reference Molina10] and is thus in the range of current condom effectiveness [Reference Weller and Davis6] or even better with a 95% confidence interval of 64% to 96% [Reference McCormack9]. Since PrEP is a strategy that actually works under exposure but by itself does not prevent exposure, it can be understood as an add-on for any prevention strategy as mentioned above and we will include this option in the Results section.
Results
The aim of our model was to evaluate the risk of acquiring an HIV infection through a casual sexual contact with and without use of one of the prevention strategies mentioned above, for example, in high-risk populations of persons not concerned about sexually transmitted infections.
Based on the prevalence and incidence of HIV in Germany in 2015 (Table 3) [40], we characterised the HIV exposure risk for females, heterosexual males and MSM by applying the following methods of exposure prevention:
• No prevention: this results in exposure risks equalling the prevalence.
• Ag/Ab- and PCR-based RDT: The exposure risk is largely determined by the sensitivity during Fiebig's five stages of early infection, the chronic stage, and the ‘under treatment stage’.
• Condom use: this prevention strategy results in an estimated reduction of the exposure risk of about 80% [Reference Weller and Davis6].
Table 3. Prevalence and incidence of HIV in Germany in 2015 as described by the Robert Koch Institute [40]

HET, heterosexual; MSM, men having sex with men.
For the MSM population, we assumed a frequency in Germany of 2%. Since reliable data on the MSM prevalence in Germany is essentially unavailable, as a basis for this assumption we used the LBGT (lesbians, bisexuals, gays, transsexuals) report by Gates [Reference Gates41], which states ranges of prevalence of gay/lesbian or bisexual activity ranging from 1.2% for Norway to over 1.5%, 1.9% and 2.1% for UK, Canada and Australia and up to 5.6% for the USA. The median prevalence of all reported surveys was about 2%.
Since the infectiousness of HIV depends on viral load [Reference Quinn2, Reference Blaser3, Reference Wilson17], which varies over the stages of infection [Reference Fiebig12], we estimated the exposure risk and, subsequently, the risk of infection weighted for infection stages. The overall stage-specific infection risks are given in Table 4 for females, in Table 5 for heterosexual males and in Table 6 for MSM. We assumed that, in general, a newly infected individual reaches the ‘under treatment stage’ or the chronic stage after 88.6 days – i.e. after Fiebig's stage 5 of early HIV infection. The frequency of HIV-infected individuals under treatment in Germany is reported as 71.7% [40]. The complementary population, which is not treated, is assumed to remain in the chronic stage of infection.
Table 4. Infection risk by stage and prevention strategy for females

Table 5. Infection risk by stage and prevention strategy for heterosexual males

HET, heterosexual.
Table 6. Infection risk by stage and prevention strategy for men having sex with men

MSM, men having sex with men.
Finally, we estimated infection risks after 100 random sexual contacts – a likely quantity in high-risk groups – within the same population (heterosexual, MSM) to simulate the risk of becoming infected within 1 year while applying one of the four prevention methods. These results are given in Table 7. The infection risk after 100 random sexual contacts in combination with PrEP is presented in Table 8.
Table 7. Infection risk depending on prevention strategy after 100 sexual contacts

HET, heterosexual; MSM, men having sex with men.
Table 8. Infection risk depending on prevention strategy in combination with PrEP after 100 sexual contacts

HET, heterosexual; MSM, men having sex with men.
All analyses indicate that condom use is an appropriate prevention method at all stages but that Ag/Ab-based RDTs reduce the overall risk of infection by about 80% compared with condom use and about 97% when compared with no use of prevention measures.
The PCR-based RDT approach is seen to be overall strongly inferior to the Ag/Ab-based RDT approach. Our analysis shows an overall infection risk reduction of about only 37% compared with condom use and about 87% compared with no prevention.
The results further demonstrate that the Ag/Ab-RDT approach is on the same level as PrEP. PCR-based prevention is inferior to PrEP and Ag/Ab-RDT prevention, but slightly more efficient than condom use alone in the absence of awareness of the HIV infection stage of the potential partner.
Discussion
Our estimates described above suggest that even following a traditional RDT-based approach to avoiding HIV infection during sexual interactions, the risk of exposure per risky sexual contact is lower than with condom use under most conditions. Nevertheless, condom use is a simple, inexpensive and rather secure strategy for preventing HIV infection and does not show the variation of prevention at any infection stage shown by both of the RDT-based preventive strategies assessed here.
Problems of the Ag/Ab-based RDT approaches result from the poor sensitivity of the antigen component as demonstrated for the Determine HIV1/2 Ag/Ab Combo test [Reference Smallwood18]. Accordingly, during seroconversion high transmission risks [Reference Wawer4, Reference Hollingsworth, Anderson and Fraser5] and a poor test sensitivity of traditional RDTs [Reference Smallwood18] coincide. Thus, primary HIV infection with high viral loads [Reference Blaser3] remains a stumbling block for such a prevention strategy and the main disadvantage of Ag/Ab-RDTs is their complete lack of sensitivity in the early stage of infection. Accordingly, condoms provide better protection against exposure than does testing with Ag/Ab-based RDT if high percentages of risky contacts involve individuals who are newly HIV-infected and still hatch the pathogen in the antigen-only phase. Although such a situation is unlikely because of the short period of time involved, the transmission may occur, for example, if HIV is newly introduced into a highly promiscuous community where HIV awareness is not established and HIV is not considered to be an immediate danger within such a circle.
Rapid PCR-based approaches with higher sensitivity in early infection stages [Reference Scott23–Reference Ceffa25, Reference Jani29, Reference Ritchie30] could resolve the problem if costs drop and such systems become suitable and more easily accessible, probably through a ‘fashion’ for testing in private environments. Accordingly, a PCR-based RDT that does not lack sensitivity in the first stage could be useful to detect early HIV infections, but it would lack sensitivity in the treatment stage. This might become an issue in the case of successfully treated individuals who deliberately conceal their infection in order to convince potential partners to have unprotected sex with them. In more detail: molecular RDT-based testing alone might fail due to the test's intrinsic sensitivity limits if RNA levels are below the detection limit. That the lack of sensitivity of the PCR-based approach is restricted to the treatment stage with very low viral loads resulting in a very low transmission risk per single sexual contact [Reference Wilson17] does not mean that this lack can be considered irrelevant. As Wilson et al. [Reference Wilson17] have already stated, the risk of transmission is expected to increase with repeated exposures. Particularly in health systems that provide ‘treatment-as-prevention’ with successful viral suppression in a high proportion of infected individuals below the detection threshold, the exclusive application of RNA-based RDTs for transmission prevention can be inferior to condom use as shown in our model.
The combination of a test-based prevention strategy plus condom use can further reduce HIV exposure compared with single strategies, with or without the use of HIV PrEP. As shown in the model, a combination of several of the strategies is to be recommended for high-risk populations. In a recent assessment, for example, the combination of HIV treatment of the infected partner and condom use was shown to reduce the sexual transmission risk by 99.2% [Reference Patel1]. The treatment approach [Reference Supervie42] was not included as an own-prevention approach in the present study because it requires adherence by the infected potential partner as well and not just by the one who seeks to avoid infection.
The risk reduction of 80% ascribed to condom use in our model applies just to HIV and heterosexual contacts [Reference Weller and Davis6, Reference Vera43]. While condoms show good protective effects against most STDs, protection against STDs transmitted via skin or mucous membrane contact (herpes simplex virus and human papilloma virus infection) is considerably lower [Reference Mindel and Sawleshwarkar44]. A risk reduction due to condom use has been reported for syphilis, but with concerns regarding available evidence [Reference Koss, Dunne and Warner45]. However, even in difficult-to-prevent infections such as human papilloma virus infection, regression of lesions has been suggested in case of reduced exposure due to condom use [Reference Holmes, Levine and Weaver46].
Studies on the effectiveness of condoms may fail to distinguish consistent from the inconsistent use or to identify incorrect usage and struggle with inconsistent risk exposure; they do not distinguish pre-existing from incident infections during the interpretation of the results and face inconsistent reporting of problems [Reference Warner47]. Regardless of these limitations, even low to moderate risk reduction of STD transmission due to condom use provides better protection than neglect of condom use in a test-only prevention strategy.
Of note, the combination of ‘recreational drugs’, usually prohibited ones such amphetamines, cocaine, etc. and drugs against erectile dysfunction [Reference Daskalopoulou48] can encourage extremely prolonged and intense, often anal intercourse. Such behaviour can severely interfere with condom safety, as regular over-the-counter condoms are not designed for excessive mechanical friction over long periods of time. Moreover, drug use interferes with awareness of the necessity to use condoms in situations where otherwise well-aware individuals tend to lose control over their regular conduct. A recent meta-analysis has shown that the probability of unprotected sexual intercourse is increased by 3% to 5% per 0.1‰ alcohol in blood [Reference Rehm49]. For such situations, an RDT-checked sex partner is certainly the safer alternative.
This assessment has a number of limitations. The underlying assessment assumption is that sexual activity is not influenced by the stage of HIV infection. Further, we assumed that the reported durations of stages of infection are representative as well as the cited model of transmission risk. As detailed in the Methods section, no group of late presenters was defined because the size of such a group may vary tremendously depending on the setting and is difficult to determine. Further, neither rare groups such as elite controllers – i.e. HIV-infected individuals remaining under the detection limit of PCR without treatment [Reference Crowell and Hatano50, Reference Gonzalo-Gil, Ikediobi and Sutton51] – with very low sexual transmission risks were considered nor was the frequent phenomenon of blips in treated patients [Reference Pernas52, Reference Sörstedt53]. It would have been beyond the scope of this modeling approach to include phenomena such as these that have been insufficiently analysed regarding their effects on sexual HIV transmission risks. In addition, we assumed that virtually every HIV-infected individual will eventually receive treatment, at least if AIDS (acquired immunodeficiency syndrome)-associated symptoms appear. Although this assumption is reasonable for resource-rich settings, it may be unrealistic in resource-poor areas. A further limitation is the assumed distribution of sexual orientation, especially the relative frequency of the MSM community in Germany since the evidence for this distribution is very scarce in spite of a recent questionnaire-based study [Reference Haversath54]. In addition to this, there might be specific differences in risk after exposure for different sexually active populations that could be independent of simple prevalence. In spite of comparable per-contact risks, MSM couples were at higher risk of HIV acquisition than heterosexuals in a recent assessment on HIV transmission risk through anal intercourse [Reference Baggaley, White and Boily55]. Sex-specific differences in the mode of sexual encounters, such as the likelihood of anal intercourse, have recently been shown for the German population [Reference Haversath54]. Although such differences might account for the previously observed [Reference Baggaley, White and Boily55] variation in HIV infection risks, lack of data on the quantitative distribution, frequency and intensity of certain sexual practices in populations of interest in general and in high-risk groups, in particular, did not allow the incorporation of such considerations in the model described here.
In conclusion, RDT-based pre-screening of potential sex partners prior to unprotected sexual contacts can substantially reduce HIV transmission risks. Most modelling assumptions lead to an even lower HIV transmission risk for RDT-based pre-screening prior to sexual contacts than for use of condoms. Nevertheless, additional condom use and the combination of several prevention strategies, including HIV-PrEP, are recommended for prevention of transmission of other STDs.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
None.
Ethical standards
Not applicable.
Disclaimers
Conflicts of interest and Source of Funding: None declared.











