Gallstone disease (GSD) is the most common biliary tract disease and its prevalence varies from 5 to 25 % worldwide. Although over two thirds of patients with gallstones remain asymptomatic, GSD represents a significant medical burden because of the associated risks of cholecystitis, pancreatitis, gallbladder cancer and the development of major cardiometabolic diseases( Reference Stinton, Myers and Shaffer 1 – Reference Chen, Huang and Yang 3 ).
Epidemiological studies report that advanced age, the female sex, race, oral contraceptive use, diabetes mellitus, family history and obesity are commonly reported risk factors for GSD development( Reference Chen, Huang and Yang 3 , Reference Zamani, Sohrabi and Alipour 4 ). Although the risk factors for GSD vary by geographic area and ethnicity, obesity remains a traditional risk factor for GSD( Reference Cruz-Monserrate, Conwell and Krishna 5 – Reference Premkumar and Sable 7 ). Only a few studies demonstrated the association between obesity and GSD development using different standards to define obesity. Tsai et al. ( Reference Tsai, Leitzmann and Willett 8 ), in Kentucky, reported that central obesity and regional fat distribution are independent risk factors for GSD-related cholecystectomy in women. Radmard et al. ( Reference Radmard, Merat and Kooraki 9 ) demonstrated that the best anthropometric parameter for GSD risk in men is the waist:hip ratio. Another study reported that multiple obesity indicators are associated with the risk of GSD. However, it is unclear which obesity indicator provides the strongest association with GSD risk. Therefore, we evaluated three obesity indicators in the current study: BMI as an indicator for general obesity, waist circumference (WC) for the estimation of central obesity, and percentage body fat mass (%FM) to evaluate general fat mass. We examined the relationship of BMI, WC and %FM with GSD, aiming to determine which obesity index has the strongest association with the risk of GSD in men and women.
Methods
Study population
A total of 15 671 middle-aged Taiwanese adults undergoing a physical check-up at a Northern Taiwan health examination centre were recruited for the study. We excluded those with viral hepatitis, heavy alcohol consumption, age more than 65 years, those with a history of cholecystectomy or those who did not undergo abdominal ultrasonographic examination or fasting blood testing. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by Mackay Memorial Hospital, Taipei, Taiwan (approval number 12MMHIS092). Verbal consent was witnessed and formally recorded.
Study methods
This was a cross-sectional study. The data gathered included age, sex, height, weight, obesity indicators (BMI, WC and %FM measured by bioelectric impedance analysis: Tanita, BC-418), exercise habit (regular exercise defined as >3 times/week) and history of hypertension or diabetes mellitus (defined as ever being diagnosed with hypertension or diabetes mellitus, regardless of the prescription of medication). We also collected standard biochemical data including fasting blood glucose, lipid profile and renal and liver function tests. According to previous studies, the positive and negative predictive values of abdominal sonography for GSD are approximately 99–100 and 90–96 %( Reference Jensen and Jorgensen 10 ), respectively. GSD was diagnosed according to the presence of gallstones on abdominal sonography after fasting for at least 8 h. Obesity was defined as BMI ≥27 kg/m2, WC ≥80 cm in women and ≥90 cm in men or body fat ≥7 % in women and ≥23 % in men, using the criteria defined by the Health Promotion Administration, Ministry of Health and Welfare in Taiwan.
Statistical analyses
SPSS for Windows version 24.0 (IBM Corp.) and R Studio version 1.1.45 were used for all statistical analyses. Continuous variables, such as age, BMI, WC and %FM, are expressed as means and standard deviations. Categorical variables included obesity (by each obesity standard), exercise and history of hypertension and diabetes mellitus. The χ 2 test was used to analyse the difference in the GSD rate among each of these categorical variables. Univariate analyses were used to select variables that predicted the risk of GSD. All variables that predicted the risk of GSD in the univariate analyses were entered into a multiple logistic regression analysis, controlling for potential confounders, including age and sex. Multiple logistic regression analysis was conducted to identify the association between BMI, WC and %FM and the presence of GSD. AUC of the receiver–operating characteristic (ROC) curves were calculated for BMI, WC and %FM to compare their relative ability to correctly identify patients with GSD using the methodology of DeLong et al. ( Reference DeLong, DeLong and Clarke-Pearson 11 ). Results are presented as OR with 95 % CI. Statistical significance was set at P<0·05.
Results
The 15 671 included participants were stratified by sex (men: 8146, 52·0 % and women: 7525, 48·0 %) (Table 1). The male group was older than the female group (43·1 v. 40·8 years, P<0·001) and had a significantly higher rate of comorbid hypertension or diabetes mellitus. Higher biochemical parameter levels including fasting plasma glucose, total cholesterol level and TAG were found in the male group. Obesity parameters (BMI, WC and %FM) were all significantly higher in men than in women. Men had a significantly higher rate of a regular exercise habit. Men also had a significantly higher rate of GSD than women (8·3 v. 6·3 %, P<0·001).
Table 1 Demographic and basic characteristics of the study populationFootnote * (Mean values and standard deviations; numbers and percentages)
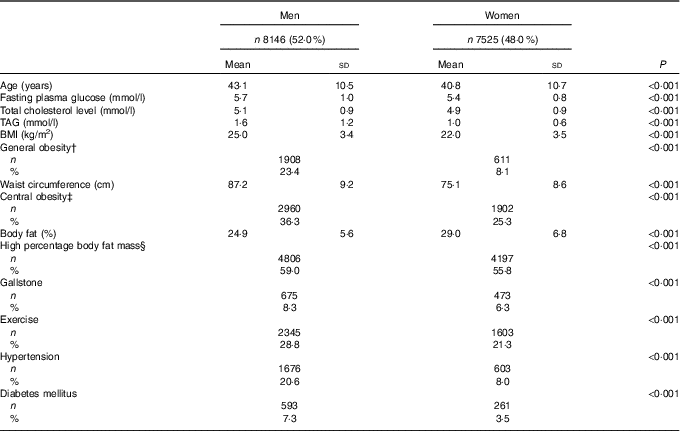
* Continuous variables are shown as means and standard deviations; categorical variables are shown as numbers and percentages.
† General obesity indicated by BMI ≥27 kg/m2.
‡ Central obesity indicated by waist circumference ≥80 cm in women and ≥90 cm in men.
§ Higher percentage body fat mass indicated by body fat ≥27 % in women and ≥23 % in men.
Table 2 compares parameters according to the presence or absence of GSD between men and women. Among men, 675 patients (8·3 %) had GSD; men with GSD were older than the men without GSD (48·9 v. 42·5 years, P<0·001). Men with GSD had a higher rate of obesity (29·1 v. 23·6 %, P<0·001) based on BMI ≥27 kg/m2 and the presence of central obesity (45·5 v. 35·5 %, P<0·001) based on WC ≥90 cm. However, there was no statistical difference in the rate of a higher %FM (≥23 %) between men with and without GSD (62·1 v. 58·7 %, P<0·100). The rate of regular exercise was similar between men with and without GSD. Men with GSD had a higher rate of comorbid hypertension (31·3 v. 19·6 %, P<0·001) and diabetes mellitus (12·9 v. 6·9 %, P<0·001) than men without GSD. In the female group, 473 patients (6·3 %) had GSD; women with GSD were older than women without GSD (46·1 v. 40·4 years, P<0·001). The three obesity parameters were all significantly higher in women with GSD than in women without GSD (BMI ≥27 kg/m2, 16·1 v. 7·6 %, P<0·001; WC ≥80 cm, 45·5 v. 23·9 %, P<0·001; %FM ≥7 %, 74·8 v. 54·6 %, P<0·001). The rate of regular exercise was similar between women with and without GSD. Women with GSD had a higher rate of hypertension (15·9 v. 7·5 %, P<0·001) and diabetes mellitus (7·6 v. 3·2 %, P<0·001) compared to women without GSD.
Table 2 Demographic and obesity parameters according to the presence of gallstone disease by sex (Numbers and percentages; mean values and standard deviations)
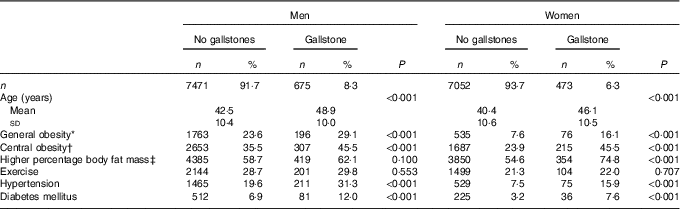
* General obesity indicated by BMI ≥27 kg/m2.
† Central obesity indicated by waist circumference ≥80 cm in women and ≥90 cm in men.
‡ Higher percentage body fat mass indicated by body fat ≥27 % in women and ≥23 % in men.
Multiple logistic regression was performed to analyse the association between obesity parameters and GSD by sex (Table 3); the OR was adjusted by age and the presence of hypertension and diabetes mellitus. In the male group, %FM had no statistically significant association with GSD. Independent predictors of GSD in men were BMI (OR=1·33, P=0·003), central obesity (OR=1·26, P=0·007) and the combination of the three obesity parameters (OR=1·47, P<0·001). In the female group, the independent predictors of GSD were %FM (OR=1·94, P<0·001), BMI (OR=1·83, P<0·001), central obesity (OR=2·00, P<0·001) and the combination of the three obesity parameters (OR=2·76, P<0·001) (Fig. 1).
Table 3 Multiple logistic regression analysis of the association between obesity parameters and gallstone disease by sexFootnote * (Odds ratios and 95 % confidence intervals)
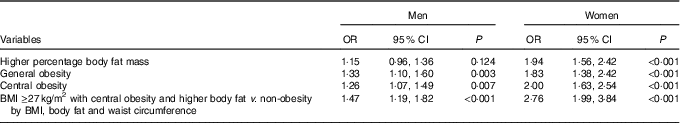
* OR was adjusted by age and the presence of hypertension and diabetes.
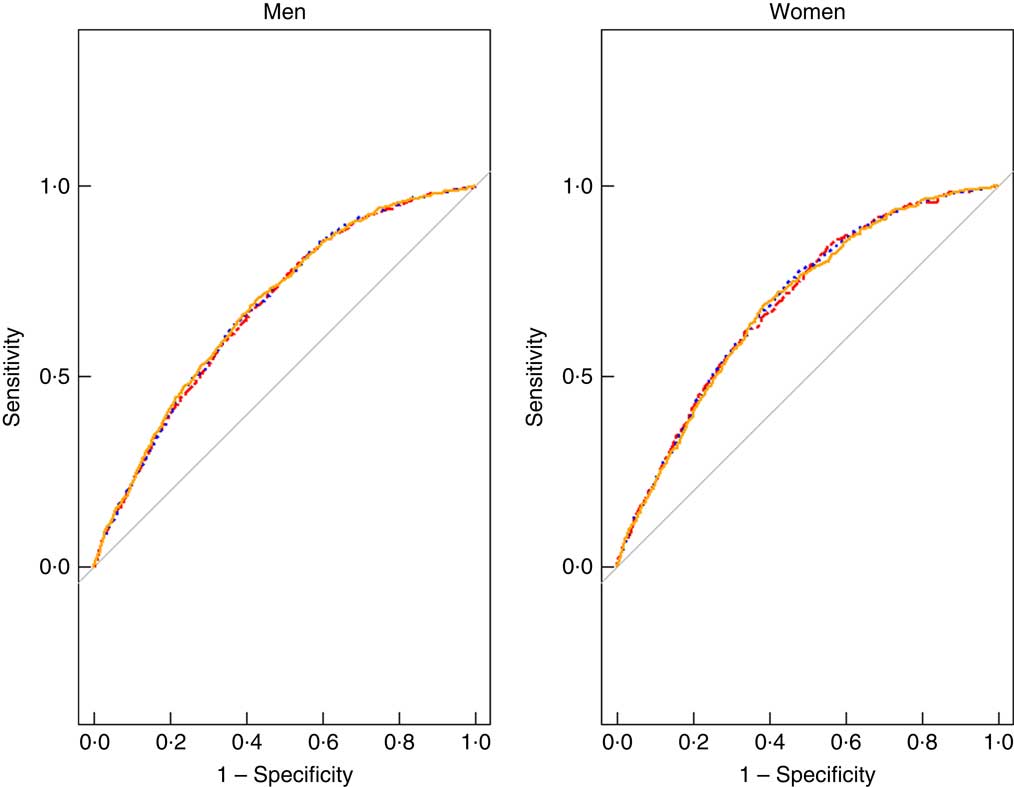
Fig. 1 Receiver–operating characteristic analysis result for the association of BMI (![]() ), waist circumference (WC,
), waist circumference (WC, ![]() ) and percentage body fat (
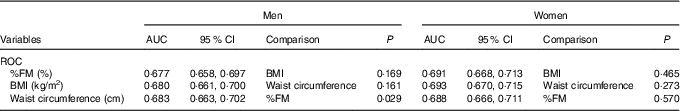
) and percentage body fat (![]() ) mass with gallstone disease in both sexes. For men, the AUC for WC was largest (AUC: 0·683). For women, the AUC was largest for BMI (AUC: 0·693).
) mass with gallstone disease in both sexes. For men, the AUC for WC was largest (AUC: 0·683). For women, the AUC was largest for BMI (AUC: 0·693).
Table 4 shows the ROC analysis for the association of BMI, WC and %FM with GSD. The results showed that, in men, the AUC for WC was largest (AUC: 0·683). For women, the AUC was largest for BMI (AUC: 0·693). The AUC for each obesity indicator was slightly larger in women than in men.
Table 4 Comparison of the receiver-operating characteristic (ROC) curves of BMI, waist circumference and percentage body fat mass (%FM) by sex
Discussion
Our study revealed that the use of different obesity indicators leads to a variable distribution of obesity among men and women in Taiwan. We found that, compared with central obesity and BMI, obesity defined as a higher %FM resulted in the largest proportion of patients diagnosed with obesity in both men and women. The discrepancy in the percentage of participants described as obese between these obesity indicators might be because BMI focuses only on the ratio between weight and square of height and ignores the importance of adiposity; thus, potentially leading to misclassification of non-obese individuals who do not have a high %FM or regional obesity. In previous studies, Peltz et al. ( Reference Peltz, Aguirre and Sanderson 12 ) reported that %FM could more accurately assess obesity in Mexican Americans compared with BMI. Considering the differences in obesity diagnosis between %FM and WC, we found that most patients with central obesity were included among those with a higher %FM.
The prevalence of GSD varies according to ethnicity and geographic location. Studies have shown that Western countries have the highest prevalence of GSD (10–25 %), followed by Asian regions; African countries show the lowest prevalence of GSD( Reference Stinton, Myers and Shaffer 1 , Reference Stinton and Shaffer 2 , Reference Zamani, Sohrabi and Alipour 4 , Reference Zhu, Aili and Zhang 13 ). Most patients with GSD remain asymptomatic( Reference Stinton, Myers and Shaffer 1 , Reference You, Huang and Ow 14 ), potentially leading to an underestimation of the prevalence of GSD, especially among individuals living in areas where routine physical examinations are uncommon. In our studies, the prevalence of GSD among women and men was 8·3 and 6·3 %, respectively, which is lower than the prevalence reported in other Asian populations( Reference Stinton, Myers and Shaffer 1 – Reference Chen, Huang and Yang 3 , Reference Zhu, Aili and Zhang 13 ). This may be due to the fact that the rate of GSD increases with age( Reference Lee, Wu and Yang 15 ), while we only enrolled participants aged between 18 and 64 years. In addition, studies in Western countries reveal that the female sex is a traditional risk factor for GSD. The hypothesis that women are prone to GSD is based on the potential ability of oestrogen to increase the cholesterol concentration in bile, increasing the possibility of cholesterol gallstone formation( Reference Jensen and Jorgensen 10 , Reference You, Huang and Ow 14 ). This is consistent with the high prevalence of cholesterol gallstones in the western population. However, this study found that GSD is slightly more prevalent among men than among women. Stringer et al. ( Reference Stringer, Fraser and Gordon 16 ) studied the composition of gallstones according to ethnicity and reported that black pigment gallstones (predominantly calcium bilirubinate), often associated with liver disease, bacterial infection and other causes and least commonly associated with obesity, accounted for approximately 40 % of gallstones among Asian patients. Thus, the interaction of various risk factors for GSD may contribute to the different distribution of GSD between sexes in the Asian population compared with western populations( Reference Chen, Huang and Yang 3 , Reference Zamani, Sohrabi and Alipour 4 , Reference Zhu, Aili and Zhang 13 , Reference Stringer, Fraser and Gordon 16 ). Studies elucidating the relationship between obesity and GSD in the Asian population are scarce. As the diet habits among Asians become westernised, there is increasing evidence demonstrating the impact of obesity on GSD risk in Asian people. However, few studies clarified the strength of the association between different obesity indicators and GSD risk.
Obesity is a major health issue because of its close association with many important chronic diseases, such as diabetes mellitus, CVD, etc.( Reference Guh, Zhang and Bansback 17 ). Obesity has long been recognised as an important risk factor for GSD, possibly due to hyper-saturated fat depositions releasing cytokines via the inflammatory cascade, leading to gallbladder dysfunction( Reference Radmard, Merat and Kooraki 9 , Reference Lee, Wu and Yang 15 , Reference Shabanzadeh, Skaaby and Sorensen 18 , Reference Sodhi, Zargar and Khateeb 19 ). Many epidemiological studies demonstrated that obesity increases the risk of GSD( Reference Stinton, Myers and Shaffer 1 , Reference Chen, Huang and Yang 3 – Reference Radmard, Merat and Kooraki 9 ), but few of them clarified the difference between obesity indicators, such as BMI, WC and %FM. BMI as an overall obesity parameter does not reflect adiposity related to abdominal obesity. Abdominal obesity has been shown to be closely related to the risk of GSD. Thus, we included other obesity indicators – WC and %FM – to assess the risk of GSD( Reference Tsai, Leitzmann and Willett 8 , Reference Sekine, Nagata and Sakamoto 20 , Reference Aune, Norat and Vatten 21 ). Our results show that obesity, defined based on BMI, WC or %FM, significantly increased the risk of GSD among women, even after adjusting for potential confounding factors. However, only BMI- and WC-defined obesity significantly increased the risk of GSD among men. The combination of all markedly increased obesity indicators was associated with a higher risk of GSD in both men and women. Among different obesity indicators, our study demonstrates that the best obesity indicator differed between men and women. Obesity, when defined based on WC, showed the strongest association with GSD, significantly greater than that %FM-defined obesity, in men. In contrast, among women, obesity, when defined based on BMI, showed the strongest association with the presence of GSD. BMI is generally viewed as a parameter of general obesity, %FM is considered to reflect the general adiposity of the body and WC is usually considered to estimate regional abdominal adiposity( Reference Tsai, Leitzmann and Willett 8 , Reference Radmard, Merat and Kooraki 9 , Reference Sekine, Nagata and Sakamoto 20 – Reference Tsai, Leitzmann and Willett 22 ). The weaker association between BMI and GSD in men than in women has been reported in previous studies and is considered to be due to the fact that men with a high BMI are more likely to have more lean body mass compared to women with high BMI( Reference Tsai, Leitzmann and Willett 22 ). As shown in Table 4, we found that obesity, when defined by all obesity indicators, had a stronger relationship with the risk of GSD in women than in men. Few large studies demonstrated a different impact of obesity on health between men and women. Some studies showed that obesity-related metabolic syndrome better predicts early carotid atherosclerosis in women than in men( Reference Iglseder, Cip and Malaimare 23 , Reference Kawamoto, Tomita and Inoue 24 ). However, further studies are needed to identify the different health effects of obesity according to sex.
There are some limitations to our study. First, it was cross-sectional and we can only report the strength of the relationship between the obesity indicators and GSD risk. Prospective studies are needed to clarify the causal relationship between obesity indicators and GSD risk. Second, we used a lenient method to describe the presence of GSD. Further studies are needed to evaluate the possibility of a dose–response relationship between GSD severity, complications and each obesity indicator.
In conclusion, all obesity indicators predicted the risk of GSD in women but only BMI and WC were associated with GSD risk in men. A combination of all obesity indicators was related to the risk of GSD in both men and women. Obesity indicators are better predictors of the presence of GSD in women than in men. The best obesity indicator for GSD risk may differ by sex. In addition to body weight control, fat and central obesity are associated with the risk of GSD in women. However, in men, central obesity might play a more important role in the prediction of GSD risk.
Acknowledgements
The authors thank all members of the Department of Family Medicine, MacKay Memorial Hospital, Taipei, for help rendered for this study. Special thanks to Dr Lee-Ching Hwang who contributed to revising drafts of the manuscript. The authors also thank Mr Jing-Rong Jhuang for statistical technique consultation.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
H.-Y. H., L.-C. H. participated in the design of the study, performed statistical analysis and interpretation of the data. H.-Y. H., L.-C. H. helped to draft the manuscript. H.-Y. H., C.-Y. H. and L.-C. H. contributed to revising drafts of the manuscript and all authors had the approval of the final manuscript.
The authors declare that there are no conflicts of interest.








