Introduction
Nickel and Grice (Reference Nickel and Grice1998), in their definition of the guidelines followed by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA–CNMNC) for the definition of mineral species, discussed the status of biogenic substances, i.e. those “substances formed by the action of geological processes on organic material”. Echigo and Kimata (Reference Echigo and Kimata2010) reviewed the crystal chemistry and origin of organic minerals, dividing them into two groups: ionic organic minerals and molecular organic minerals. Among the latter, the most common are the so-called polycyclic aromatic hydrocarbon (PAH) minerals, currently represented by nine mineral species reported in the official IMA–CNMNC List of Mineral Names (updated November 2021, Pasero, Reference Pasero2021). Another molecular organic mineral, evenkite, can be considered as an alkane mineral (Echigo and Kimata, Reference Echigo and Kimata2010). The ten hydrocarbon minerals are listed in Table 1. An additional mineral, phylloretine, C18H18, is considered as questionable and is not shown in Table 1.
Table 1. Hydrocarbon minerals reported in the official IMA List of Mineral Names (updated November 2021).

References: [1] this work; [2] Echigo et al. (Reference Echigo, Kimata and Maruoka2007); [3] Franzini et al. (Reference Franzini, Pasero and Perchiazzi1991); [4] Koltenikova et al. (Reference Koltenikova, Filatov and Chukanov2004); [5] Mace and Peterson (Reference Mace and Peterson1995); [6] Strunz and Contag (Reference Strunz and Contag1965); [7] Burns and Iball (Reference Burns and Iball1955); [8] Petříček et al. (Reference Petříček, Císařová, Hummel, Kroupa and Březina1990); [9] Foresti and Riva di Sanseverino (Reference Foresti and Riva di1969); and [10] Mills et al. (Reference Mills, Kampf, Nestola, Williams, Leverett, Hejazi, Hibbs, Mrorsko, Alvaro and Kasatkin2017).
The natural occurrence of organic minerals has been known since the end of the 18th Century, when mellite, Al2C6(COO)6⋅16H2O, was described (Werner and Hoffmann, Reference Werner and Hoffmann1789; Gmelin, Reference Gmelin1793). However, their actual definition had been a difficult task for a long time, owing to experimental shortcomings, primarily the determination of the correct chemical composition. For this reason, whereas >100 different organic mineral species were reported at the beginning of the 20th Century, after the introduction of X-ray diffraction techniques and structure determination, fewer than 10 organic mineral species survived and were still considered valid in the 1950s (Mottana, Reference Mottana, Ballio and Paoloni1990). The compound C20H34 is one of these problematic organic minerals. The same species was described under different mineral names: for instance: ‘bombiccite’, ‘branchite’, ‘hartite’ and ‘hofmannite’. Hartite was considered as the grandfathered mineral name, whereas the other mineral names were discredited.
During the re-examination of the scientific activity of Prof. Paolo Savi (1798–1871), an important Italian geologist and zoologist of the 19th Century, carried out by one of us (S.F.), the original description of ‘branchite’ was found, along with the original samples studied by Prof. Savi himself. This promoted a reinvestigation of the actual status of ‘branchite’ and of its relationship with hartite.
As it will be discussed below, it became apparent that the names branchite and hartite were applied to the same mineral substance and that the former has priority over the latter. Notwithstanding the long history of the name hartite, first proposed by Haidinger (Reference Haidinger1841), this mineral has been reported from only a dozen localities worldwide and, considering its relative rarity, its name does not seem to be widely used by the Geoscience community. For this reason, a proposal for the reinstatement of the name branchite was submitted to the IMA–CNMNC (proposal 21-A, Miyawaki et al., Reference Miyawaki, Hatert, Pasero and Mills2021) and approved. The type locality of branchite is the Botro di Lavajano, Monte Vaso, near Chianni, Pisa, Tuscany, Italy; neotype material is the specimen #14426 kept in the mineralogical collection of the Natural History Museum of the Pisa University, Via Roma 79, Calci (Pisa), Italy.
In this paper the troubled history of branchite is discussed, along with new structural data confirming its identity with hartite.
Branchite and hartite, a troubled history
In Autumn 1838, Mr. Salvadore Arevalo found a cavity within a Miocenic lignite exploited in the Botro di Lavajano, Monte Vaso, near Chianni, Pisa, Tuscany, Italy. The cavity was filled by chalcedony, iron sulfides, and a brittle and colourless combustible compound. Mr. Arevalo gave a sample to Prof. Paolo Savi, who asked Prof. Giuseppe Branchi, chemist at the Pisa University, to collect chemical data. In September 1839, Branchi wrote a letter to Prof. Savi, reporting the results of his qualitative analyses. In October 1839, Savi gave a report of his results at the first meeting of Italian scientists, held in Pisa (Savi, Reference Savi1840, pp. 67–68 and 73). Moreover, Savi published a paper (Savi, Reference Savi1839) in which his data and the results of the qualitative analysis by Branchi are given; in that paper the new phase was given the name branchite. This is the first published report of this new hydrocarbon mineral. Similarities with other possible phases were discussed and discarded. Later on, Branchi (Reference Branchi1840) published the results of his qualitative chemical analyses in the Giornale Toscano di Scienze Mediche, Fisiche, e Naturali. Abstracts of these papers and of the communication given by Savi during the first meeting of Italian scientists were published in two foreign journals: Isis in 1841 (on pages 558–559) and Neues Jahrbuch für Mineralogie, Geognosie, Geologie und Petrefaktenkunde in 1842 (on page 459).
Shortly thereafter, Haidinger (Reference Haidinger1841) described hartite from Hart, Enzenreith, Neunkirchen District, Lower Austria, Austria. This marked the beginning of a troubled relationship between branchite and hartite. Indeed, some authors began to hypothesise that hartite and branchite were the same mineral species.
In the third and fourth editions of ‘A System of Mineralogy’, Dana (Reference Dana1850, Reference Dana1854) related branchite to ‘scheererite’, distinguishing it from hartite. As a reference for branchite, the abstract published in 1842 in the Neues Jahrbuch für Mineralogie, Geognosie, Geologie und Petrefaktenkunde was mentioned. Although the similarity between branchite and ‘scheererite’ was already discarded by Savi (Reference Savi1839), Dana (Reference Dana1854) wrote: “Branchite, Savi (Leonh. and Bronn, 1842, 459). A colorless translucent substance resembling Scheererite, from the brown coal of Mount Vaso in Tuscany. It fuses at 75°C. (167°F.) but does not crystallize on cooling. G = 1.00. It dissolves in alcohol.” Subsequently Savi (Reference Savi1855) published a new paper about branchite, providing new information about its occurrence, whereas Piria (Reference Piria1855) reported its chemical composition (in wt.%): C 87.02, H 13.40, total 100.42. On the basis of 20 C atoms per formula unit, this composition corresponds to the formula C20H36.7, close to the accepted formula for dinite, C20H36 (Franzini et al., Reference Franzini, Pasero and Perchiazzi1991). This discrepancy is not surprising, considering the analytical difficulties by chemists in the mid 19th Century.
To the best of our knowledge, Dana (Reference Dana1869) was the first scholar to consider hartite and branchite as the same mineral species. It is likely that – according to him – hartite had priority, having been described in 1841, whereas a paper of 1842 was reported for branchite, neglecting the previous 1839 paper. In the fifth edition of A System of Mineralogy, Dana (Reference Dana1869) also added a reference to the paper by Savi (Reference Savi1855). In the following years, other two phases were described, which were later recognised as identical to hartite: ‘bombiccite’ (Bechi, Reference Bechi1868; Bombicci, Reference Bombicci1869; Pellizzer, Reference Pellizzer1955a, Reference Pellizzer1955b), and ‘hofmannite’ (Boeris, Reference Boeris1921). In his Mineralogia della Toscana, D'Achiardi (Reference D'Achiardi1873) stressed that Dana grouped hartite and branchite together, but he pointed out that the first description was given by Savi (Reference Savi1839).
At the beginning of the 20th Century, D'Achiardi (Reference D'Achiardi1910), in the first edition of his Corso di Mineralogia, compared the chemistry of branchite and hartite, highlighting their similarity. It is worth noting that in the second edition of his book (D'Achiardi, Reference D'Achiardi1925), the author reported that branchite and hartite were the same mineral species, in agreement with other authors (e.g. Boeris, Reference Boeris1921). A review of the hydrocarbon minerals from Tuscany was reported by Carobbi and Rodolico (Reference Carobbi and Rodolico1976), who stressed the identity of branchite, ‘bombiccite’ and ‘hofmannite’ with hartite. More recently, the priority of branchite over hartite was also discussed by Mottana (Reference Mottana, Ballio and Paoloni1990).
Experimental
Specimen number 14426 (Fig. 1), belonging to the collection of the Natural History Museum of the Pisa University and collected by Prof. Paolo Savi, is labelled as ‘Branchite. M.te Vaso’. It is represented by cm-sized thin fragments of lignite (Fig. 1) covered by a thin crust of microcrystalline quartz and colourless masses with waxy lustre, up to some millimetres across, of branchite. These features correspond to those reported by Savi (Reference Savi1839, Reference Savi1855).

Fig. 1. Branchite, neotype material and original labels dating back to the 19th Century (upper right) and the beginning of the 20th Century (lower right).
Micro-Raman spectra of branchite were collected using a Horiba Jobin-Yvon XploRA Plus apparatus, equipped with a motorised x–y stage and an Olympus BX41 microscope with a 50× objective. Raman spectra were excited using a 532 nm line of a solid-state laser. The minimum lateral and depth resolution was set to a few micrometres. The system was calibrated using the 520.6 cm–1 Raman band of silicon before each experimental session. Spectra were collected through multiple acquisitions (3) with a counting time of 30 s. Back-scattered radiation was analysed with a 1200 gr/mm grating monochromator. Experimental precision can be estimated at ± 2 cm–1.
Single-crystal X-ray diffraction intensity data for branchite were collected using a Bruker Apex II diffractometer (50 kV, 30 mA) equipped with a Photon II CCD detector and graphite-monochromatised MoKα radiation. The detector-to-crystal distance was set at 50 mm. Data were collected using ω and φ scan modes in 0.5° slices, with an exposure time of 20 s per frame, and were corrected for Lorentz and polarisation factors as well as for absorption using the software package Apex3 (Bruker AXS Inc., 2016). The refined unit-cell parameters are a = 11.4116(7), b = 20.9688(12), c = 7.4100(4) Å, α = 93.947(2), β = 100.734(2), γ = 80.524(2)°, V = 1716.99(17) Å3 and Z = 4; space group P1. The crystal structure was refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015) starting from the C atomic coordinates given by Bouška et al. (Reference Bouška, Císařová, Skála, Dvořák, Zelinka and Zák1998); their same atom labels were used. Scattering curves for neutral atoms were taken from the International Tables for Crystallography (Wilson, Reference Wilson1992). All hydrogen atom positions were found through difference-Fourier maps. One C–H distance was restrained to an appropriate value. After several cycles of refinement, the structural model converged to R 1 = 0.0424 for 13512 unique reflections with F o > 4σ(F o), 1266 refined parameters and 3 restraints. Details of data collection and refinement are given in Table 2. Atomic coordinates and displacement parameters are available in the Crystallographic Information File (CIF) that has been deposited with the Principal Editor of Mineralogical Magazine and is available as supplementary material. Our structural analysis allows for an unambiguous determination of the chemical formula of branchite; no other chemical data were collected, which would have resulted in the destruction of a historical sample without adding any new information to the mineralogical knowledge of this phase.
Table 2. Summary of crystal data and parameters describing data collection and refinement for branchite.

Results and discussion
Micro-Raman spectroscopy
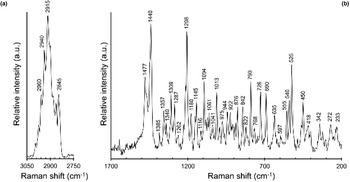
The micro-Raman spectrum of branchite is shown in Fig. 2. Band interpretation discussed below is after Jehlička et al. (Reference Jehlička, Edwards, Villar and Pokorný2005) who studied three samples of ‘hartite’ from Bílina, northern Bohemia, Czech Republic and Valdarno, Tuscany, Italy.

Fig. 2. Raman spectrum of branchite in the range 3050–2750 cm–1 (a) and 1700–200 cm–1 (b).
The Raman spectrum of branchite is characterised by a strong and broad band between 3000 and 2800 cm–1 (Fig. 2a), due to the convolution of several bands related to ν(CH2) and ν(CH3) modes.
In the region between 1700 and 200 cm–1 (Fig. 2b), two relatively strong bands at 1477 and 1440 cm–1, as well as a band at 1309 cm–1, are interpreted as due to δ(CH2) and δ(CH3) vibration modes; another relatively strong band occurs at 1208 cm–1 and can be interpreted as δ(CCH) modes. Bands at 1180 and 1145 cm–1 can be due, following Jehlička et al. (Reference Jehlička, Edwards, Villar and Pokorný2005), to the ν(CC) ring breathing. The ρ(CH3) rocking modes are probably at the origin of bands at 975 and 944 cm–1, whereas the bands at 922 and 876 cm–1 may be due to the ρ(CH2) modes. The band at 790 cm–1 is relatively strong and is attributed to a ν(CC) ring mode; the band at 726 cm–1 is related to a ν(CC) mode, whereas the band at 690 cm–1 may be due to ν(CC) or δ(CCC) modes. The latter interpretation is applied to the band at 635 cm–1. Bands between 580 and 480 cm–1 are attributed by Jehlička et al. (Reference Jehlička, Edwards, Villar and Pokorný2005) to δ(CCC), or to δ(CCO) and ν(COC). These authors discussed the discrepancy between these assignments and the absence of O in C20H34, suggesting the possibility of weathering of the studied sample. In branchite, the same spectral features occur, but no evidence of weathering or damages from the laser was observed. In accordance with that, at least for our sample, the attribution to δ(CCC) seems the most probable. Finally, Raman bands occurring at lower wavenumbers are attributed to ring deformations and torsional modes.
Micro-Raman spectroscopy was also useful for a quick distinction between branchite and the associated microcrystalline quartz.
Crystallographic data
Data collected on branchite are fully consistent with the results of Bouška et al. (Reference Bouška, Císařová, Skála, Dvořák, Zelinka and Zák1998). These authors reported the following unit-cell parameters: a = 11.407(1), b = 20.952(2), c = 7.4060(8) Å, α = 93.941(9), β = 100.750(8), γ = 80.499(9)°, V = 1713.8(3) Å3 and Z = 4; space group P1. These data can also be compared with the unit-cell parameters of ‘bombiccite’: a = 11.39(7), b = 21.29(5), c = 7.45(1) Å, α = 94.6(1), β = 101.8(2), γ = 81.5(1)°, V = 1707.7 Å3 and Z = 4; space group P1 (Foresti Serantoni et al., Reference Foresti, Krajewski, Mongiorgi, Riva di and Sheldrick1978).
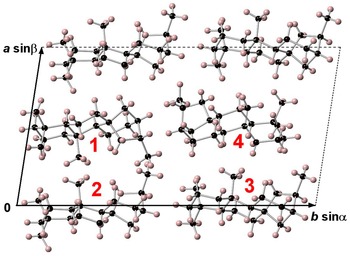
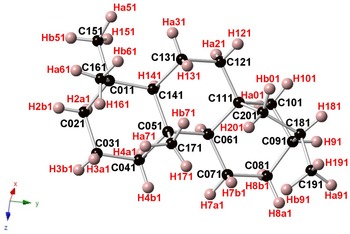
Branchite is a molecular solid (the structural formula of the C20H34 molecule is reported in Fig. 3) and its structural features fully agree with those described by previous authors involved in the study of 16α(H)-phyllocladane (e.g. Foresti Serantoni et al., Reference Foresti, Krajewski, Mongiorgi, Riva di and Sheldrick1978; Bouška et al., Reference Bouška, Císařová, Skála, Dvořák, Zelinka and Zák1998). The crystal structure is formed by four independent molecules (Fig. 4). Supplementary Table S1 reports selected distances observed in branchite. The C–C distances range between 1.506 and 1.567 Å, to be compared with the distance range reported in Bouška et al. (Reference Bouška, Císařová, Skála, Dvořák, Zelinka and Zák1998), i.e. 1.499–1.563 Å. Such distances are also similar to those reported by Franzini et al. (Reference Franzini, Pasero and Perchiazzi1991) for dinite, C20H36, ranging between 1.512 and 1.608 Å. Carbon atoms can be bonded to four other C (forming the so-called quaternary C), or they are also involved in C–H bonds. In each molecule there are four methyls –CH3 groups, nine methylenes –CH2–, four methines, and three quaternary C (Fig. 5). Hydrogen atoms are bonded to C atoms with bond distances ranging between 0.87 and 1.11 Å. The determination of the absolute configuration of this non-centrosymmetric structure is not straightforward due to the absence of any atom heavier than carbon. No conclusions about the absolute structure are available from our diffraction data, due to the high calculated standard deviation of the Flack index (Flack, Reference Flack1983), i.e. 1(4).

Fig. 3. Structural formula of branchite.

Fig. 4. Crystal structure of branchite as seen down c. Numbers 1–4 indicate the four independent molecules occurring in the unit cell (dashed lines). Black and pink spheres represent C and H atoms, respectively.

Fig. 5. One of the four C20H34 molecules occurring in the crystal structure of branchite. Same symbols as in Fig. 4.
Genesis of branchite
Branchite is a natural phyllocladane and some authors have proposed that these organic compounds can be considered as biomarkers for the order Pinales (e.g. Otto et al., Reference Otto, Walther and Puttmann1997; Zubrik et al., Reference Zubrik, Šaman, Vašíčková, Simoneit, Turčániová, Lovás and Cvačka2009), which comprises all the extant conifers. Indeed, Mio-pliocenic lignite deposits from Tuscany are characterised by the occurrence of remains of Taxodium and Glyptostrobus (e.g. De Stefani, Reference De Stefani1887; Sammartino et al., Reference Sammartino, von Löwenstern A. and Grassi2011–2012).
Echigo and Kimata (Reference Echigo and Kimata2010) proposed that ‘hartite’ from Bílina (Czech Republic), occurring in siderite nodules within lignite bodies (Bouška et al., Reference Bouška, Císařová, Skála, Dvořák, Zelinka and Zák1998), was formed as a result of the concentration of phyllocladane molecules by hydrothermal fluids. Indeed, Echigo et al. (Reference Echigo, Kimata and Maruoka2007, Reference Echigo, Kimata, Maruoka, Shimizu and Nishida2009) discussed similar genesis for carpathite, C24H12, and idrialite, C22H14. These two organic minerals occur in Hg hydrothermal ores and their formation seems to be related to hydrothermal activity. For instance, crystals of carpathite are crystallised on euhedral quartz, supporting the hypothesis that the C24H12 molecules were transported by hydrothermal fluids. The finding of branchite at Monte Vaso as colourless masses with waxy lustre, associated intimately with and grown on microcrystalline quartz supports the hypothesis that its crystallisation could be a consequence of the migration and concentration of phyllocladane molecules by low-temperature hydrothermal fluids that were also responsible for silicification phenomena.
Conclusions
The study of historical samples kept in the Natural History Museum of Pisa University, coupled with the re-examination of relevant papers published by naturalists of the 19th Century, has shown that hartite should be renamed branchite, due to historical precedence. This study triggered the structural investigation of this polycyclic aromatic hydrocarbon, which has improved our knowledge about this rare group of mineral substances. Finally, the role of hydrothermal fluids in the crystallisation of some molecular organic minerals is supported by the close association of branchite with microcrystalline quartz within lignite.
Acknowledgements
Fabio Marchetti is acknowledged for useful discussion. The suggestions of the anonymous referees and of Mike Rumsey greatly improved the readability of the text.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.48
Competing interests
The authors declare none