Pregnancy had been described as a special physiological and metabolic processes because of significant changes in sow nutritional metabolism(Reference Weissgerber, Wolfe and Davies1). Glucose derived from maternal blood is the most significant energy source for fetal growth and development, and late pregnancy is the rapid growth stage of the fetus(Reference Henrichs, Schenk and Barendregt2,Reference Van Assche, Dallequin and Holemans3) . The fetus’s demand for glucose from the mother has increased dramatically, so maternal glucose metabolism will be abnormal in the late pregnancy. Remarkably, the abnormal glucose metabolism of sows in the late pregnancy is characterised by large fluctuations in blood glucose levels(Reference Jezková and Smrcková4). The maternal insulin sensitivity is particularly reduced in the late pregnancy, and the postprandial blood glucose concentration remains high, allowing more glucose to transfer to the fetus. Additionally, maternal fasting blood glucose level is low due to increased energy requirements for rapid fetal growth and development in the late pregnancy(Reference Van Assche, Dallequin and Holemans3,Reference Rogne and Jacobsen5) . Abnormal glucose metabolism of sows in the late pregnancy will cause sows to have infertility and even reproductive disorders such as abortion and return to oestrus. It also can cause hypoglycaemia in newborn piglets and affect growth potential(Reference Pere, Etienne and Dourmad6,Reference Kim, Yang and Baidoo7) . Therefore, improvement of abnormal glucose metabolism in the late pregnancy is important for sow reproductive performance and newborn piglet health.
The liver plays a decisive role in metabolism, as it controls the regulation of both lipid and glucose homoeostasis in mammals. Gluconeogenesis generates glucose from non-carbohydrate carbon substrates such as pyruvate, glycerol and lactate. Champigny et al. found that lactate and pyruvate accumulated in the liver of rats during late pregnancy, suggesting that the liver suffered from metabolic disorder due to pregnancy stress, greatly reduced gluconeogenesis and impaired lactate clearance(Reference Champigny, Hitier and Bourdel8). Wang et al.(Reference Wang, Yao and Xia9) showed that the rate of glycolysis was promoted and hepatic gluconeogenesis was inhibited in the late pregnancy of sows, which resulted in the systemic accumulation of lactate and thus adversely affected maternal health and offspring development. However, the regulation of gluconeogenesis in the liver of pregnant sows by nutrients has not been reported.
Glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK) are the key enzymes of gluconeogenesis. The expression of G6Pase and PEPCK is controlled by various transcription factors and signalling pathways that reply to changes of nutrient availability. The decreased activity of the target proteins of Forkhead box O1 (FOXO1), such as PEPCK and G6Pase, is responsible for decreased gluconeogenesis during pregnancy(Reference Valenti, Rametta and Dongiovanni10,Reference Deng, Shoji and Ogawa11) . In addition, PPAR-γ co-activator 1α (PGC-1α) is also a pivotal transcription factor, which is a determinant for gluconeogenesis. Expression of PGC-1α mediated by adenovirus in the hepatocytes increases glucose output and highly activates the whole process of gluconeogenesis, which proved that PGC-1α is a pivotal transcription coactivator of gluconeogenesis in the liver(Reference Yoon, Puigserver and Chen12). FOXO1 and PGC-1α were recently found to be acetylated by P300/CBP-associating factor (PCAF) for attenuating its activity(Reference Sun, Wang and Liu13,Reference Yoshimochi, Daitoku and Fukamizu14) . Remarkably, Erina et al. found that PCAF is required for oestrogen-dependent normal growth of the uterus via oestrogen receptor-mediated transcriptional regulations(Reference Inoue, Hanai and Yamada15,Reference Yao, Wang and Xia16) . The maternal oestrogen level was significantly increased in the late pregnancy, so the PCAF level could be predicted to be increased. Thus, it can be assumed that the increased PCAF level in sows liver may result in decreased glucose levels by decreasing the processes of gluconeogenesis in the late pregnancy.
Accumulated evidences demonstrated that garcinol from Garcinia indica exerts beneficial effects on hepatocellular carcinoma, hepatotoxicity, non-alcoholic fatty liver disease and viral hepatitis, which indicated that liver is the important target organ of the garcinol in the plant of Garcinia indica (Reference Balasubramanyam, Altaf and Varier17,Reference Yamaguchi, Ariga and Yoshimura18) . Recently, garcinol has been described a natural inhibitor of PCAF(Reference Stimson, Rowlands and Newbatt19). The purpose of the present study was to investigate the changes in hepatic PCAF activity during pregnancy and further analyse the promotion of hepatic gluconeogenesis by garcinol-inhibited PCAF to elucidate the molecular mechanisms of hepatic glucose metabolism in the late pregnancy of mammals.
Methods
Animals, treatments and sample collection
The experiment had been approved by the Animal Care and Use Committee of Huazhong Agricultural University and conformed to the guidelines of the National Research Council for the Care and Use of Laboratory Animals. Ten non-pregnant sows (Duroc × Yorkshire × Landrace) were fed a basal diet. Thirty second- and third-parity pregnant sows (Duroc × Yorkshire × Landrace) were randomly distributed to three groups: basal diet (CON; n 10), CON + 100 mg/kg garcinol (Low Gar; n 10) and CON + 500 mg/kg garcinol (High Gar; n 10) from day 90 of gestation to the end of farrowing. The garcinol was obtained from Biomol/Enzo Life Sciences International, Inc. and extracted from dried fruit rind of Garcinia indica with a purity of 95 %, as measured by HPLC. During pregnancy, sows were placed in separate gestation crates (2·1 × 0·58 m). All the crates were equipped with a concrete trough where pregnancy diet was supplied (08.00 hours) by an automatic feeding system and a stainless-steel nipple for water. Throughout the experiment, water was freely available for piglets and sows. All experimental diets were prepared in accordance with National Research Council (2012) requirements (online Supplementary Table S1). Feeding and fasting blood samples (2 h after feeding; 12 h fasting) were collected via jugular venipuncture for glucose measurement at days 90, 95, 100, 105 and 110 of gestation. Sows were euthanised through chemical sedation using Telazol administered at 0·1 ml/kg intramuscular injection followed by exsanguination. The livers of sows were collected for examination at the end of farrowing. The body weight (BW), average daily feed intake and backfat thickness were measured at the end of the experiment.
Plasma and liver tissue analyses
The plasma concentrations of TAG and total cholesterol were quantified using commercial ELISA kits (R&D Systems, Inc.) according to the manufacturer’s instructions. Plasma insulin and glucagon levels were measured using an ultrasensitive insulin ELISA kit (Mercodia, Inc.) and a glucagon ELISA kit (Crystal Chem), respectively. Sows liver tissue was weighed, minced and homogenised in phosphate buffer at pH 7·0. The homogenates were centrifuged (9500 rpm for 10 min at 4°C), and the supernatant was used to determine glucose and glycogen concentration using a glucose assay kit (Nanjing Jiancheng Bioengineering Institute) and a glycogen assay kit (Nanjing Jiancheng Bioengineering Institute), respectively.
Hepatocyte culture and treatment
Isolation of hepatocytes was performed by collagenase perfusion and mechanical destruction from the livers of pregnant sows (after farrowing)(Reference Gradilone, Carreras and Lehmann20). Hepatocytes were cultured in accordance with a previous publication(Reference Wang, Yao and Li21). Cells from pregnant sows were treated with garcinol (0, 5, 10 and 15 μm) for 48 h. Besides, hepatocytes were transfected by Lipofectamine 2000 (Invitrogen) with a mixture of four small interfering RNA (siRNA) or control siRNA (scrambled siRNA; Qiagen) directed isolation of primary hepatocytes and cell culture towards PCAF (Qiagen) for 4 h. To confirm PCAF knockdown, Western blot was used to analyse PCAF expression.
Glucose production
Primary sows hepatocytes were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 5 mm glucose supplemented with 1 % penicillin/streptomycin and 10 % fetal bovine serum (FBS). To measure glucose production, the cells were treated with garcinol (0, 5, 10 and 15 μm) or vehicle in FBS-free, low-glucose DMEM for 5 h, washed and then incubated in glucose production media (glucose- and phenol-free DMEM containing 20 mm sodium lactate and 2 mm sodium pyruvate) in the continued presence or absence of either the vehicle or garcinol for 3 h. Glucose released in the media was measured using a glucose assay kit (Nanjing Jiancheng Bioengineering Institute) and normalised to protein content of the same samples.
Real-time quantitative-PCR
Extract total RNA from the liver and detect the relative RNA abundance according to previous publications(Reference Huang, Rena and Jiang22,Reference Zhou, Zhao and Li23) . The reference gene glyceraldehyde-3-phosphate dehydrogenase and the primers (online Supplementary Table S2) of tested genes were mentioned in our previous study(Reference Sun, Zhang and Zhu24). Fold changes in mRNA expression levels were calculated using the 2–ΔΔCt method.
Western blots and immunoprecipitation
Western blot analyses were performed as described previously(Reference Yan, Pepper and Vatamaniuk25). For the analyses, the major antibodies, including PGC-1α, FOXO1, PEPCK, G6Pase and β-actin (ACTB), are submitted in online Supplementary Table S3. Detection of concentrations of protein in the liver was performed by the bicinchoninic acid assay. Western blotting was performed for acetylation detection. For blocking, 50 mm Tris (pH 7·5) with 1 % peptone (AMRESCO) and 10 % (v/v) Tween-20 were used. For the primary and secondary antibodies, 50 mm Tris (pH 7·5) with 0·1 % peptone was prepared(Reference Zhao, Xu and Jiang26).
Enzymatic activity assay
The supernatants of cell culture were gathered at specific time points and were stored at –80°C. Tissue and plasma were homogenised in DMEM and were stored at –80°C. The tissue was thawed and plasma samples were centrifuged at 5000 g and 4°C for 20 min before taking the measurements and collecting the supernatants. The enzyme activities of PCAF, PGC-1α and FOXO1 in hepatocytes and liver were identified by Activity Assay Kits (Sigma). The PCAF activity in hepatocytes and liver was detected as described by Herrera et al.(Reference Herrera, Bergel and Yang27).
Statistical analysis
SPSS 20.0 software (SPSS, Inc.) was used for statistical analysis. Data are submitted as mean values and standard deviations. The standard t test was performed to analyse data between two groups. Prior to ANOVA analysis, Levene’s variance uniformity test was used to assess unequal variances. Data were analysed by one-factor ANOVA with a significance level of P < 0·05, and the Tukey–Kramer method was used for multiple mean comparisons. Welch’s ANOVA was used in case of unequal variances, followed by Dunnett’s T3 test for post hoc comparisons.
Results
Garcinol had no effect on performance of late-pregnancy sows
Garcinol had no effect (P ≥ 0·05) on BW, average daily feed intake, backfat and BW gain (Fig. 1(A)–(D)) among all the groups. Feeding blood glucose levels in pregnant sows with or without garcinol treatment were not significantly (P ≥ 0·05) different as compared with non-pregnant sows (Fig. 1(E)). At days 105 and 110 of gestation, fasting blood glucose levels in the Low and High Gar groups were increased significantly (P < 0·05) by approximately 40 and 45 %, respectively, compared with those in the control group (Fig. 1(F)). Plasma glucose and litter size weight of newborn piglets in the Low Gar group and the High Gar group were significantly higher (P < 0·05) than in the pregnant group (Fig. 1(G) and (H)).
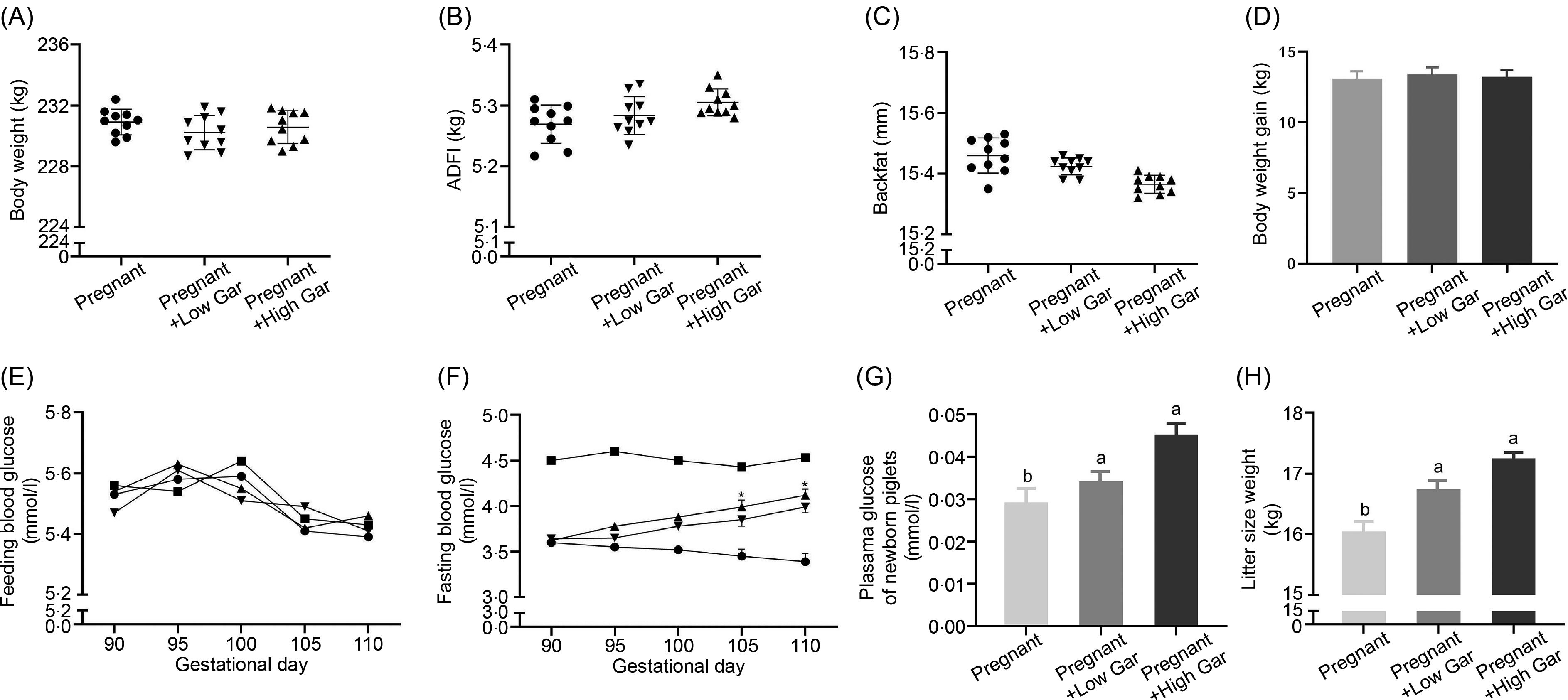
Fig. 1. Body weight, average daily feed intake (ADFI), backfat and body weight gain (A–D) in pregnant sows fed 100–500 mg/kg garcinol from day 90 of gestation to the end of farrowing. Effects of dietary garcinol on the plasma glucose levels (E, F, G) of pregnant sows and newborn piglets. Effects of dietary garcinol on litter size weight (H) of newborn piglets. Values are means and standard deviations; n 10, * Significantly different from control group (P < 0·05). a,b Means with unlike letters are significantly different (P < 0·05). (E, F) ![]() , non-pregnant;
, non-pregnant; ![]() , pregnant;
, pregnant; ![]() , pregnant + low garcinol (Low Gar);
, pregnant + low garcinol (Low Gar); ![]() , pregnant + high garcinol (High Gar).
, pregnant + high garcinol (High Gar).
Garcinol increased hepatic glucose levels and gluconeogenic enzyme expression in late-pregnancy sows
The concentrations of plasma TAG and total cholesterol were significantly decreased (P < 0·05) in the High Gar groups compared with those in the pregnant group (Fig. 2(A) and (B)). The plasma insulin and glucagon did not differ (P ≥ 0·05) among all the groups (Fig. 2(C) and (D)). All two Gar-supplemented groups had a higher hepatic glucose concentration compared with the pregnant group (P < 0·05, Fig. 2(E)). However, the hepatic glycogen concentrations did not change significantly in all groups (P ≥ 0·05, Fig. 2(F)). The protein levels of hepatic PEPCK and G6Pase were significantly higher (P < 0·05) in the High Gar group than the Low Gar and pregnant groups. However, the hepatic PGC-1α and FOXO1 expression did not differ (P ≥ 0·05, Fig. 2(G) and (H)).

Fig. 2. Effects of dietary garcinol on the plasma TAG (A), total cholesterol (TC, (B)), insulin (C), glucagon (D), hepatic glucose levels (E), hepatic glycogen levels (F) and hepatic gluconeogenic protein expression (G, H) in sows fed diets from day 90 of gestation to the end of farrowing. Values are means and standard deviations, n 10. a,b Means with unlike letters are significantly different (P < 0·05). (G) ![]() , pregnant;
, pregnant; ![]() , pregnant + low garcinol (Low Gar);
, pregnant + low garcinol (Low Gar); ![]() , pregnant + high garcinol (High Gar). PEPCK, phosphoenolpyruvate carboxykinase; G6Pase, glucose-6-phosphatase; PGC-1α, PPAR-γ co-activator 1α; FOXO1, Forkhead box O1.
, pregnant + high garcinol (High Gar). PEPCK, phosphoenolpyruvate carboxykinase; G6Pase, glucose-6-phosphatase; PGC-1α, PPAR-γ co-activator 1α; FOXO1, Forkhead box O1.
Garcinol reduced the acetylation of PPAR-γ co-activator 1α/Forkhead box O1 and increased its activity in the liver of late-pregnancy sows
The enzyme activity of hepatic PCAF was decreased in the Low Gar group (P = 0·07) and significantly decreased (P < 0·05) in the High Gar group compared with that of controls (Fig. 3(A)). The activities of PGC-1α and FOXO1 were increased (P < 0·05) in the High Gar group compared with those in other two groups (Fig. 3(B) and (C)). Furthermore, the PGC-1α and FOXO1 acetylation levels were significantly reduced (P < 0·05) in the High Gar group compared with the other two groups (Fig. 3(D) and (E)).

Fig. 3. Effects of dietary garcinol (Gar) on the activity of P300/CBP-associating factor (PCAF), PPAR-γ co-activator 1α (PGC-1α) and Forkhead box O1 (FOXO1) ((A)–(C)), acetylation level of PGC-1α and FOXO1 (D, E) in sows fed diets from day 90 of gestation to the end of farrowing. Values are means and standard deviations, n 10. a,b Means with unlike letters are significantly different (P < 0·05). WB, Western blot.
Garcinol up-regulated hepatic gluconeogenic enzyme expression through P300/CBP-associating factor in hepatocytes from late-pregnancy sows
There was no difference (P ≥ 0·05) in the expression of phosphofructokinase and pyruvate kinase among all groups (Fig. 4(A)). However, the expression of PEPCK was significantly increased (P < 0·05) in 10 and 15 μm dosages of the garcinol treatment group (Fig. 4(B)). The G6Pase expression was significantly increased (P < 0·05) in 15 μm dosages of the garcinol treatment group (Fig. 4(B)). The PGC-1α and FOXO1 expression did not differ (P ≥ 0·05) among all the garcinol treatment groups (Fig. 4(C)). In addition, garcinol treatment directly increased (P < 0·05) the glucose production in hepatocytes (Fig. 4(D)). After transfecting with siRNA of PCAF in pregnant sow hepatocytes, the protein expression of PCAF was decreased by about 78 % (P < 0·05, Fig. 4(E)). Knockdown of PCAF significantly increased (P < 0·05) the mRNA levels of PEPCK and G6Pase. The treatment of garcinol in the hepatocytes from late-pregnancy sows led to increased PEPCK and G6Pase mRNA levels. There were no differences (P ≥ 0·05) between these two groups (Fig. 4(F)). Results for PEPCK and G6Pase protein levels are consistent with those of mRNA (Fig. 4(G)). Consistently, both garcinol treatment and transfecting with siRNA of PCAF significantly increased (P < 0·05) the glucose production (Fig. 4(H)).
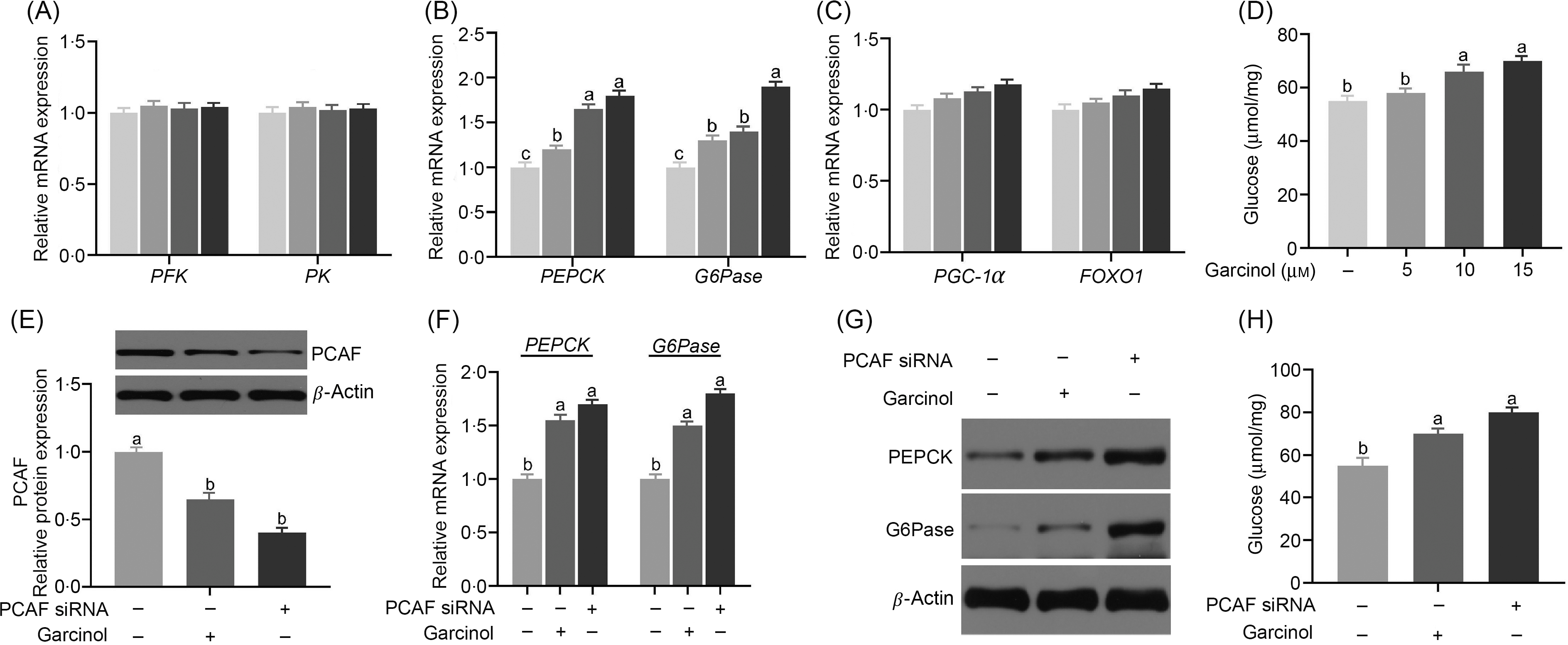
Fig. 4. Effects of dietary garcinol on the mRNA expression of phosphofructokinase (PFK) and pyruvate kinase (PK) (A), phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) (B, F), PPAR-γ co-activator 1α (PGC-1α) and Forkhead box O1 (FOXO1) (C), protein expression of PEPCK and G6Pase (G), P300/CBP-associating factor (PCAF) (E), glucose production (H) in hepatocytes from pregnant sows. Values are means and standard deviations, n 10. a,b,c Means with unlike letters are significantly different (P < 0·05). (A–C) ![]() , 0 µm garcinol;
, 0 µm garcinol; ![]() , 5 µm garcinol;
, 5 µm garcinol; ![]() , 10 µm garcinol;
, 10 µm garcinol; ![]() , 15 µm garcinol. siRNA, small interfering RNA.
, 15 µm garcinol. siRNA, small interfering RNA.
Garcinol inhibits the P300/CBP-associating factor-dependent acetylation of PPAR-γ co-activator 1α and Forkhead box O1 in hepatocytes of late-pregnancy sows
The PGC-1α and FOXO1 activities in pregnant sow hepatocytes were significantly increased (P < 0·05) after transfecting with siRNA of PCAF compared with the controls. Garcinol treatment also increased significantly (P < 0·05) the PGC-1α and FOXO1 activities (Fig. 5(A) and (B)). Interestingly, we detected a decrease (P < 0·05) in the acetylation levels of PGC-1α and FOXO1 in hepatocytes from late-pregnancy sows (Fig. 5(C) and (D)).
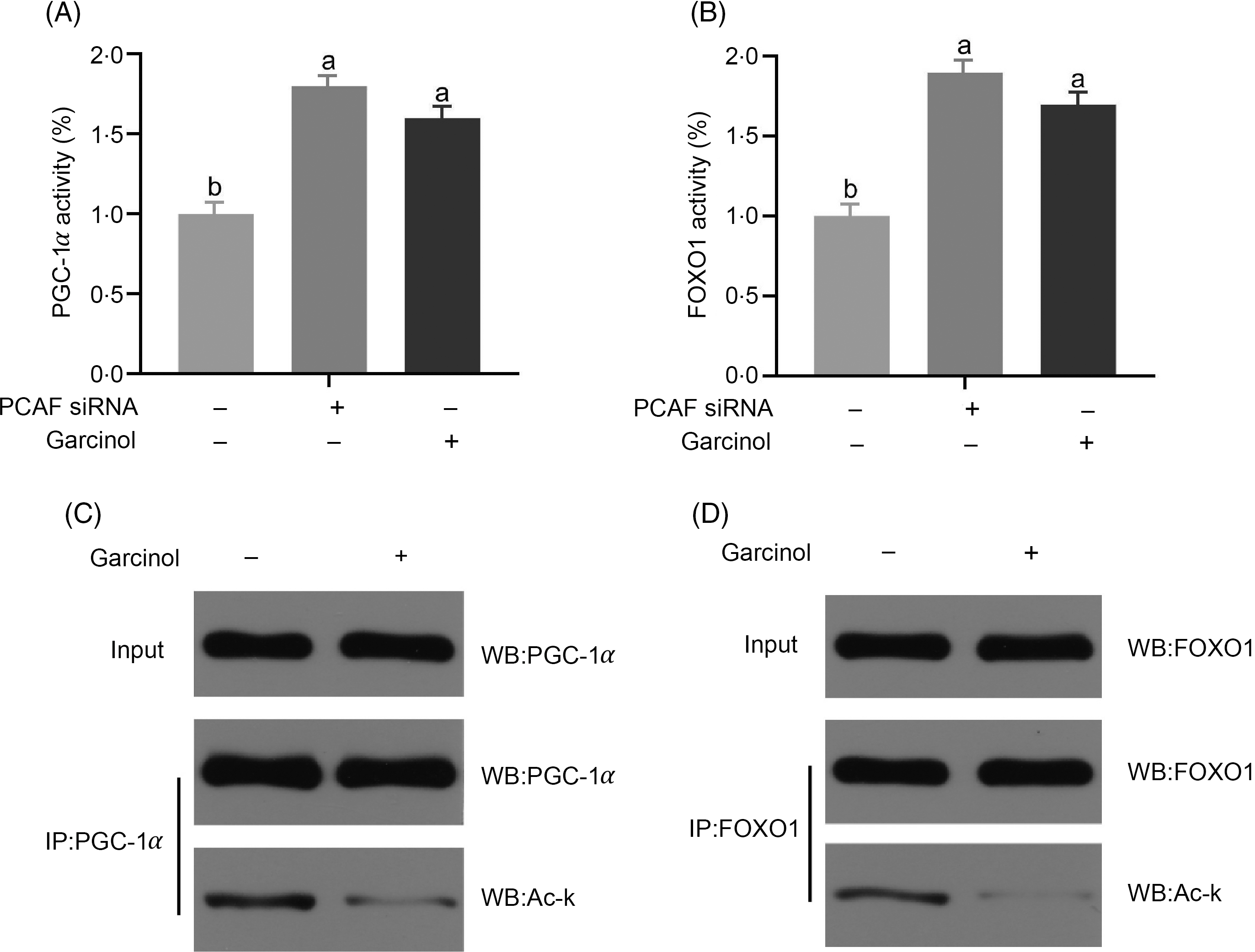
Fig. 5. Effects and mechanisms of dietary garcinol on the activity of PPAR-γ co-activator 1α (PGC-1α) and Forkhead box O1 (FOXO1) (A, B), acetylation level of PGC-1α and FOXO1 (C, D) in hepatocytes from pregnant sows. Values are means and standard deviations, n 10. a,b Means with unlike letters are significantly different (P < 0·05). PCAF, P300/CBP-associating factor; siRNA, small interfering RNA; WB, Western blot.
Discussion
In the present study, we demonstrated that the dietary garcinol supplementation for 4 weeks restored glucose homoeostasis, independent of BW change. Importantly, garcinol did not affect the metabolic phenotypes of pregnant sows, which indicated that the health benefits of garcinol rely upon the metabolic state and the potential side effect of nutritional supplementation of garcinol was minor. Meanwhile, garcinol may have antihyperlipidaemic effects, as the plasma concentrations of TAG and total cholesterol were significantly decreased in the High Gar group compared with those in the pregnant. To identify the potential therapeutic effects of garcinol on glucose metabolism, the blood of pregnant sows was collected as the gestation period increases. In the present study, we found that garcinol increased fasting blood glucose levels without altering BW gain, backfat, insulin and glucagon, suggesting that the improving glucose levels of garcinol is not a secondary effect, whereby it modulated these metabolic parameters. These results, together with our recent findings that dietary garcinol intake prevented pregnancy-induced glucose metabolism disorder in pregnant sows, suggesting that garcinol may be an inexpensive and safe natural compound(Reference Wang, Yao and Xia9).
The observed metabolic effect of garcinol in pregnant sows was linked to a promotion of production of hepatic glucose. Available data from in vitro and in vivo indicated that garcinol was an inhibitor of hepatic PCAF. On the one side, we found that pregnant sows under treatment of garcinol displayed higher hepatic PGC-1α and FOXO1 transcriptional activities, which could improve glucose homoeostasis by increasing the production of hepatic glucose. Activation of PEPCK and G6Pase, the predominant hexokinases, is proposed to be a potential target for hypoglycaemia treatment because of its decisive role in regulating glucose homoeostasis. Multiple studies proved that PGC-1α and FOXO1 played an important role in regulating PEPCK and G6Pase expression(Reference Park, Kim and Bae28–Reference Gu, Ding and Wang31). Function of PCAF is crucial for regulating the transcription of FOXO1 and PGC-1α through acetylation in gluconeogenesis(Reference Wang, Yao and Shao32,Reference Zhang, Yao and Xia33) . The transcriptional activity of FOXO1 and PGC-1α is decreased by insulin, which can decrease PGC-1α and FOXO1 binding to promoters of gluconeogenic genes(Reference Sajan, Lee and Foufelle34). In addition, when PGC-1α and FOXO1 were acetylated by PCAF, the interaction between PGC-1α and FOXO1 and DNA was susceptible to Akt-mediated phosphorylation, which lead to a decrease in activity of transcription(Reference Southgate, Bruce and Carey35,Reference Oliveri, Davio and Batlle36) . These reports demonstrated that the acetylation of PGC-1α and FOXO1 was considerable in regulation of genes. The interrelationship between these modifications is worth studying.
We have studied a molecular mechanism of hepatic gluconeogenesis under the control of PCAF with regard to PGC-1α and FOXO1 action. To this end, the hepatocytes were treated with garcinol and the role of PGC-1α and FOXO1 was observed in the transcriptional activation of G6Pase and PEPCK. Garcinol treatment increased pregnancy-suppressed G6Pase and PEPCK mRNA levels. Pregnancy is associated with deregulated activity and expression of enzymes in gluconeogenesis, which control the production of glucose such as G6Pase and PEPCK, leading to deficient gluconeogenesis and thus glucose output in the liver, which primarily contribute to fasting hypoglycaemia(Reference Masuyama, Mitsui and Maki37–Reference Sun, Yan and Peng39). Results in our study showed that PEPCK and G6Pase protein levels were significantly increased in the garcinol groups. Transfection of PCAF siRNA into hepatocytes increased the mRNA levels of G6Pase and PEPCK, suggesting that the up-regulation of gluconeogenic genes by garcinol might occur by PCAF. The levels of acetylated PGC-1α and FOXO1 in the garcinol-treated group decreased after PCAF siRNA transfection, suggesting that PGC-1α and FOXO1 acetylation by garcinol might happen in a PCAF-dependent mode. Based on these results, it is conjectured that the pregnancy-mediated inhibition of expression of hepatic gluconeogenic genes could be reversed by garcinol, which inhibits PGC-1α and FOXO1 acetylation in a PCAF-dependent manner.
Here, we show that administration of garcinol ameliorated hypoglycaemia in late-pregnancy sows. These effects might largely be due to the ability of garcinol to improve gluconeogenesis in the liver by decreasing the acetylation of PGC-1α and FOXO1 induced by PCAF. In short, these results suggest that garcinol could be an effective natural compound by increasing gluconeogenesis in the liver of late-pregnancy sows. Further studies are guaranteed to define whether garcinol can be proposed as an effective way to prevent and/or treat hypoglycaemia in the pregnant mammals.
Acknowledgements
The research is supported by the National Natural Science Foundation of China (grant no. 32072742), the Hubei Provincial Natural Science Foundation of China (grant no. 2018CFA071) and the National Key Research and Development Program (grant no. 2018YFD0500600).
The authors’ contributions were as follows: W. Y., J. X. and F. H. designed the experiment; W. Y., T. W. and L. H. conducted the experiment; W. Y. and J. L. analysed the data and W. Y. wrote the manuscript. All authors read and approved the final manuscript.
The authors declare that they have no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S000711452000375X








