There were 1·4 million colorectal cancer cases and 693 900 deaths caused by this disease worldwide in 2012( 1 ). In China, the incidence of colorectal cancer risk presents an increasing trend and has become the fourth common cancer, with an estimated 376 300 cases occurring in 2015( Reference Chen, Zheng and Baade 2 ). The epidemiological evidence supported that dietary and lifestyle factors may play a pivotal role in the aetiology of colorectal cancer risk( Reference Huxley, Ansary-Moghaddam and Clifton 3 ). However, on the basis of the current available evidence, only alcohol consumption and red and processed meat are the firm causes of colorectal cancer risk( Reference Wiseman 4 ). Thus, the association between other dietary factors and colorectal cancer risk needs more epidemiological studies to confirm.
It was reported that diabetes was related to an increased risk of colorectal cancer( Reference Deng, Gui and Zhao 5 , Reference De Bruijn, Arends and Hansen 6 ). The most important mechanism underlying the carcinogenesis was hyperglycaemia, hyperinsulinaemia or inflammation( Reference Giovannucci, Harlan and Archer 7 ). Insulin could enhance the concentration of insulin-like growth factor (IGF) by decreasing the level of IGF-binding proteins( Reference Suikkari, Koivisto and Rutanen 8 ), and IGF play a vital role in cell growth and differentiation( Reference Kaaks and Lukanova 9 ). Carbohydrate is the main dietary component affecting blood glucose and insulin concentrations( Reference Greenwood, Threapleton and Evans 10 ). Carbohydrate-rich diet results in hyperglycaemia, and then hyperinsulinaemia; thus, it may further induce the carcinogenesis of colorectal cancer. However, the effect of carbohydrate on blood glucose may depend on the type and amount of carbohydrate consumed. For example, fibre cannot be absorbed in the intestine, whereas the non-fibre carbohydrate, including starch, can be absorbed in the small intestine and lead to an increase of blood glucose( Reference Aune, Chan and Lau 11 ). Glycaemic index (GI) of foods is an index for ranking their carbohydrate content based on their postprandial blood glucose effects, which is defined as the area under the 2-h blood glucose response curve, divided by the corresponding area of the same amount of carbohydrate from a standard reference (glucose or white bread)( Reference Jenkins, Wolever and Taylor 12 ). Glycaemic load (GL) is an index further considering the quantity of carbohydrate consumed, which is calculated as the product of the GI value and the amount of available carbohydrate, and dividing the total by 100( Reference Du, van der and van Bakel 13 ). Therefore, GL is a comprehensive measure of both the blood glucose effects (quality) of the food and the total quantity of carbohydrate consumed( Reference Sieri, Krogh and Agnoli 14 ).
Some observational studies examined the association between dietary carbohydrate, GI and GL and colorectal cancer risk, but failed to draw a consistent conclusion. A cohort study found that high dietary GI and carbohydrate intake from high-GI foods, but not GL, were associated with an increased risk of colorectal cancer( Reference Sieri, Krogh and Agnoli 14 ). Similarly, a meta-analysis analysing eight cohort studies and five case–control studies found that dietary GI was related to an increased risk of colon cancer( Reference Galeone, Pelucchi and La Vecchia 15 ). Another meta-analysis considering only cohort studies also revealed that dietary GI had a borderline positive relationship with colorectal cancer risk( Reference Choi, Giovannucci and Lee 16 ). However, one meta-analysis including fourteen cohort studies concluded that there was no clear association between dietary carbohydrate, GI and GL and the risk of colorectal cancer( Reference Aune, Chan and Lau 11 ). Regarding the different types of carbohydrate, a meta-analysis including sixteen studies revealed that total dietary fibre intake was related to a reduced risk of colorectal cancer( Reference Aune, Chan and Lau 17 ). Some studies focused on dietary non-fibre carbohydrate intake. One cohort study found no clear association between non-fibre carbohydrate intake and colorectal cancer risk( Reference Sieri, Krogh and Agnoli 14 ). However, another cohort study( Reference Higginbotham, Zhang and Lee 18 ) and one case–control study( Reference Borugian, Sheps and Whittemore 19 ) observed that consumption of non-fibre carbohydrate was associated with an increased risk of colorectal cancer. So far, two case–control studies providing inconsistent conclusions have been conducted to examine the association between starch intake and colorectal cancer risk( Reference Franceschi, La Vecchia and Russo 20 , Reference Levi, Pasche and Lucchini 21 ). To date, only one relevant study has been conducted among Chinese population, yet it focused on only women( Reference Li, Yang and Shu 22 ).
This case–control study aimed to evaluate the association between dietary intake of total carbohydrate, non-fibre carbohydrate, total fibre, starch, GI and GL and colorectal cancer risk. We hypothesised that diets high in total carbohydrate, non-fibre carbohydrate, starch, dietary GI and GL were associated with increased colorectal cancer risk, whereas high intake of total fibre was inversely associated with colorectal cancer risk.
Methods
Study subjects
This is an ongoing case–control study that began in July 2010, and the study design has been described previously( Reference Zhong, Fang and Pan 23 ). In brief, potential case subjects were consecutively recruited from Sun Yat-sen University Cancer Center, Guangzhou, China, from July 2010 to April 2017. Eligible patients were aged 30–75 years and natives of Guangdong province or had lived in Guangdong for at least 5 years, with histologically confirmed colorectal cancer diagnosed no more than 3 months. Patients were excluded if they could not understand or speak Mandarin/Cantonese or had a prior history of any cancer. A total of 2230 eligible cases were identified and 1961 were successfully interviewed. In all, 269 patients failed to complete the investigation, mainly because of fatigue, communication barriers and refusal. Thus, the response rate was 87·93 %. A total of seventeen subjects with an extreme energy intake (<2510 or >14644 kJ/d (<600 or>3500 kcal/d) for women, <3347 or >17 573 kJ/d (<800 or>4200 kcal/d) for men) were excluded in the analysis. Finally, 1944 cases were included in the analysis.
Two control groups were used in this study, which were frequency matched by age (5-year internal) and sex to the case patients. The first control group was recruited from Departments of Vascular Surgery, Otorhinolaryngology and Plastic and Reconstructive Surgery in the First Affiliated Hospital of Sun Yat-sen University during the same time period as the cases. They mainly suffered from chronic otitis media, chronic sinusitis, sudden deafness, vocal cord polyp, trigeminal neuralgia, varicose veins, orthopaedics and facial paralysis. The second control group was recruited from residents in the same community via community advertisements, written invitations or subjects’ referrals. The same inclusion and exclusion criteria that were used to the cases were applied to the controls, except that they had no prior history of colorectal cancer. Totally, 1327 hospital-derived controls were identified and 1168 were successfully interviewed, with a participation rate of 88·02 %. In addition, 859 community-derived controls were interviewed. The total number of controls was 2027.
We assumed that there were 25 % people with higher carbohydrate intake, GI and GL among the general population, and the estimated OR between carbohydrate intake, GI and GL and colorectal cancer risk was 1·51, 1·35 and 1·43( Reference Sieri, Krogh and Agnoli 14 ), respectively, the type I error rate was <0·05 (α=0·05), the power of test was 90 % (β=0·10) and the response rate was 80 %. On the basis of these assumptions, we required a sample size of 755 cases for carbohydrate, 1454 cases for GI and 1013 cases for GL.
This study was conducted according to the Declaration of Helsinki. The Ethical Committee of School of Public Health, Sun Yat-sen University, approved all procedures. Written informed consent was obtained from all participants before the interview.
Data collection
All study subjects were interviewed face-to-face by trained interviewers using a structured questionnaire, in order to collect information on socio-demographic characteristics, lifestyle factors (e.g. active smoking, passive smoking, alcohol drinking and physical activity), family history of cancer and body measurements (weight and height). The body weight was measured and the height was self-reported. BMI was calculated as the ratio of weight (kg) to squared height (m2). The diagnosis and histological findings were collected from the medical records. The study subjects were also asked to answer the question ‘Have you had physician-diagnosed diabetes mellitus?’. For women, menstrual conditions and reproductive experiences were also obtained. In this study, active smokers were defined as people who smoked at least 1 cigarette/d for more than 6 months consecutively or accumulatively in their lifetime( 24 ). Passive smokers were defined as non-smokers who reported being exposed to the smoke exhaled by smokers for at least 15 min/d over a week. Regular drinking was defined as drinking alcohol at least once per week over the previous year. The intensity of physical activity was assessed based on self-reported occupational, household and leisure activity in the past year. Information on frequency (d/week) and duration (h/d) for household and leisure physical activities were obtained. According to labour intensity, occupational activity was classified as follows: (a) not working; (b) sitting for a long time; (c) low intensity; (d) moderate intensity; or (e) vigorous intensity, with examples provided. Household and entertainment activities were combined in this study. They were categorised into light physical activity (e.g. walking), moderate physical activity (e.g. jogging, mountaineering, playing table tennis) and vigorous physical activity (e.g. running, playing football/basketball). The mean metabolic equivalent task-hours (MET) value of each physical activity was acquired by estimating the average of all comparable activities in the Compendium of Physical Activities( Reference Ainsworth, Haskell and Whitt 25 , Reference Ainsworth, Haskell and Herrmann 26 ). MET-h/week over the past one year was calculated as follows: number of days/week×number of hours/day×MET of a specific type of activity=MET-h/week.
The subjects completed an interviewer-administered FFQ that involved eighty-one food items and relevant dietary habits. The FFQ has been validated and tested for reproducibility( Reference Zhang and Ho 27 ), and has been used in previous studies( Reference Zhong, Fang and Pan 28 ). The food groups mainly included cereal products, soya and soya products, vegetables, fruit, red and processed meat, poultry, fish and other seafood, egg, dairy products and nuts. Dietary information was obtained from the cases during the preceding 12 months before diagnosis and from controls during the year before interview. The subjects were asked to report how often they consumed each food and how much they ate each time. Food photographs were used to help participants to quantify their dietary intakes.
The average daily intakes of total carbohydrate, fibre and starch were measured by summing the product of the frequency of consumption, portion consumed each time and the nutrient contents of each food item. The 2002 Chinese Food Composition Table was used to calculate energy, carbohydrate, fibre and other nutrient intakes( Reference Yang, Wang and Pan 29 ), and starch value was obtained from the US Department of Agriculture Food Composition Database( 30 ). The intake of non-fibre carbohydrate was calculated by excluding the total fibre intake from the total carbohydrate. The GI values of major carbohydrate-contributing foods (93·03 % of total carbohydrate) were obtained from the Chinese Food Composition Table( Reference Yang, Wang and Pan 29 ) and supplemented by the online GI database from University of Sydney (www.glycemicindex.com). The values of GI from Chinese Food Composition Table used glucose as the reference food. GL represents the glycaemic effect of food, and this effect is inherently a function of the carbohydrate available for digestion and absorption( Reference George, Mayne and Leitzmann 31 ). To calculate the GL, the available carbohydrate content was defined as the non-fibre carbohydrate, which excluded fibre from the total carbohydrate. Thus, the GL of a food was calculated by multiplying the non-fibre carbohydrate content of the food by its GI value, and then dividing the total by 100. Dietary GL for a participant was the sum of the GL of all food items( Reference Du, van der and van Bakel 13 ). Overall dietary GI for each participant was derived from the sum of the GI of each food consumed, multiplied by the average daily amount consumed and non-fibre carbohydrate content, all divided by the daily intake of the non-fibre carbohydrate( Reference Du, van der and van Bakel 13 ).
Statistical analysis
The t test or Wilcoxon signed-ranks test was used for the continuous variables, and the χ 2-test was used for the categorical variables to test the difference between the cases and controls. Dietary nutrient intake, GI and GL was adjusted based on total energy intake of men and women, using the regression residual method( Reference Willett, Howe and Kushi 32 ). Quartiles (Q1–Q4) of dietary intakes of total carbohydrate, non-fibre carbohydrate, total fibre and starch, dietary GI and GL were defined based on the distribution among the controls. Unconditional logistic regression model was used to estimate the OR and 95 % CI for the associations between dietary intakes of total carbohydrate, non-fibre carbohydrate, total fibre and starch, dietary GI and GL and colorectal cancer risk. The lowest-quartile group (Q1) served as the reference group. According to the characteristics comparison between cases and controls, or previous reported confounders, the following variables were selected in the multivariable-adjusted models: age, sex, marital status, residence, education, occupation, income level, BMI, active smoking, passive smoking, alcohol drinking, family history of cancer, occupational physical activity, household and leisure-time activities. All confounding factors were included as categorical variables except for age, BMI, household and leisure activities, which were regarded as continuous variables. Tests for trend were performed by entering the categorical variables (Q1–Q4) as continuous variables in the regression models; the quartiles (Q1–Q4) were coded as 1, 2, 3 and 4, respectively. Stratified analysis by sex, subgroup analysis by cancer site (colon or rectal cancer) and by sources of controls (community-derived controls and hospital-derived controls) were conducted. Sensitivity analysis was also performed by excluding study subjects who had physician-diagnosed diabetes mellitus. All of the statistical analyses were performed using SPSS 20.0 and the significant level was set at 0·05 (two-sided).
Results
There were 1944 cases in total, and 1079 of them were men and 865 were women. Among the cases, 1172 colon cancers and 772 rectal cancers were diagnosed. Table 1 shows the distribution of participants’ socio-demographic and relevant characteristics. Compared with control subjects, more cases were married and lived in rural area, were less educated, had a higher proportion of farmers and lower income, tended to have more active smoking and passive smoking, had a higher frequency of alcohol drinking, reported having a first-degree relative with cancer, engaged in more occupational activities and engaged in fewer household and leisure activities. No statistically significant differences were found in terms of age, sex, BMI, women’s postmenopausal status and age at menarche between cases and controls.
Table 1 Demographic and selected risk factors of colorectal cancer cases and controls in Chinese populationFootnote * (Numbers and percentages; mean values and standard deviations; medians and 25th, 75th percentiles)

MET, metabolic equivalent task.
* Continuous variables were evaluated using t tests or Wilcoxon rank-sum tests. Categorical variables were evaluated using χ 2 tests.
† Among the subgroup of women.
Compared with the controls, the cases had a higher dietary GI and GL, a higher intake of starch and a lower intake of total energy and total fibre. Total carbohydrate intake was 265·0 (sd 50·2) g/d for cases and 267·8 (sd 50·5) g/d for controls; the intake of non-fibre carbohydrate was 255·8 (sd 49·7) g/d for cases and 257·3 (sd 49·9) g/d for controls. Nevertheless, neither total carbohydrate nor non-fibre carbohydrate was observed to be statistically significantly different between two groups (Table 2).
Table 2 Intakes of energy, total carbohydrate, non-fibre carbohydrate, total fibre, starch, glycaemic index and glycaemic load among case and control subjects in Guangdong, ChinaFootnote * (Mean values and standard deviations; medians and 25th, 75th percentiles)
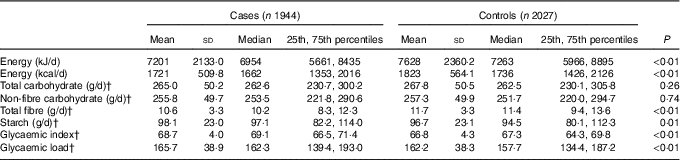
* Wilcoxon rank-sum test comparing the median consumption levels between cases and controls.
† Adjusted for the consumption for total energy intake by the regression residual method.
Among control subjects, two main food sources of total carbohydrate, non-fibre carbohydrate, fibre, starch and GL were cereal grains, vegetable and fruit. Cereal grains contributed 75·34 % of total carbohydrate, 77·67 % of non-fibre carbohydrate, 91·29 % of starch and 87·95 % of GL. Vegetables and fruit contributed 18·08 % of total carbohydrate, 16·07 % of non-fibre carbohydrate, 6·34 % of starch and 9·82 % of GL. However, vegetables and fruit contributed 61·31 % and cereal grains contributed 25·58 % of total fibre. As for daily GI, cereal grains, vegetables and fruit contributed 69·15 and 21·49 % to non-fibre carbohydrate for the first quartile of GI, and 84·73 and 11·23 % to non-fibre carbohydrate for the last quartile of GI.
The OR and 95 % CI of colorectal cancer for the intakes of total carbohydrate, non-fibre carbohydrate, total fibre, starch, dietary GI and GL are presented in Table 3. Total carbohydrate intake was not found to be associated with colorectal cancer risk, with an adjusted OR of 0·85 (95 % CI 0·70, 1·03) comparing the highest with the lowest quartile (P trend=0·08). For the intake of total fibre, the highest quartile intake showed a risk reduction of 53 % compared with the lowest quartile (OR 0·47; 95 % CI 0·39, 0·58, P trend<0·01). Dietary GI was positively associated with colorectal cancer risk, with an adjusted OR of 3·10 (95 % CI 2·51, 3·85, P trend<0·01) comparing the highest with the lowest quartile. However, no significant association was found between the intakes of non-fibre carbohydrate, starch, dietary GL and colorectal cancer risk.
Table 3 Colorectal cancer according to quartiles (Q) of the intakes of total carbohydrate, non-fibre carbohydrate, total fibre, starch, dietary glycaemic index and glycaemic load (Odds ratios and 95 % confidence intervals)
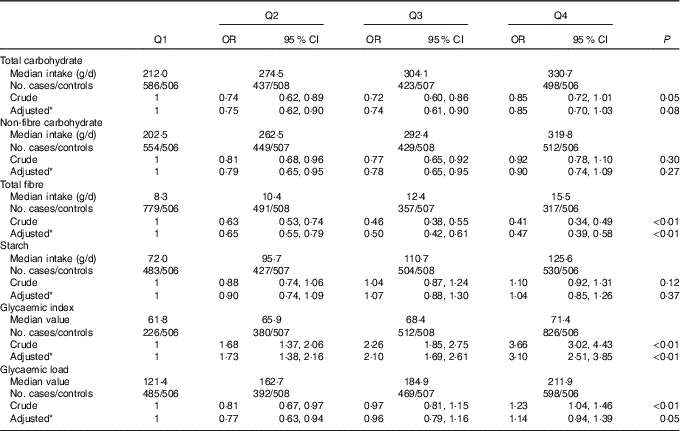
* OR was adjusted for age, sex, marital status, residence, education, occupation, income level, smoking status, passive smoking, alcohol drinking, family history of cancer, occupational physical activity, household and leisure-time activities.
Stratified analysis by sex showed that dietary GI was positively associated with colorectal cancer among both sexes. The adjusted OR for the highest quartile v. the lowest quartile was 3·49 (95 % CI 2·62, 4·64, P trend<0·01) for men and 2·44 (95 % CI 1·72, 3·46, P trend<0·01) for women. Intakes of total carbohydrate, non-fibre carbohydrate and total fibre were inversely associated with colorectal cancer risk among men but not among women. Compared with the lowest quartile, the adjusted OR of the highest quartile was 0·66 (95 % CI 0·51, 0·85, P trend<0·01) for total carbohydrate, 0·72 (95 % CI 0·55, 0·93, P trend=0·01) for non-fibre carbohydrate and 0·36 (95 % CI 0·27, 0·47, P trend<0·01) for total fibre among men. Among women, dietary GL was positively related to colorectal cancer risk. The adjusted OR of the highest quartile compared with the lowest-quartile intake was 1·42 (95 % CI 1·04, 1·95, P trend<0·01). No significant association was found between consumption of total carbohydrate, non-fibre carbohydrate or total fibre and colorectal cancer risk among women. In addition, there was no notable association between the intake of starch and colorectal cancer risk in either sex (Table 4).
Table 4 Colorectal cancer according to quartiles (Q) of the intakes of total carbohydrate, non-fibre carbohydrate, total fibre, starch, dietary glycaemic index and glycaemic load by sex (Odds ratios and 95 % confidence intervals)
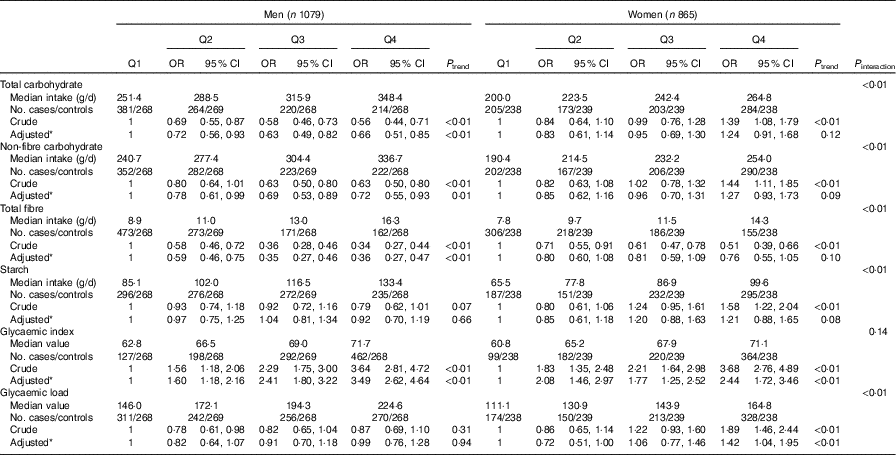
* OR was adjusted for age, marital status, residence, education, occupation, income level, smoking status, passive smoking, alcohol drinking, family history of cancer, occupational physical activity, household and leisure-time activities.
As presented in Table 5, subgroup analysis by cancer site was similar to the analysis among all colorectal cancer cases. Subgroup analysis by community-derived controls and hospital-derived controls also showed that no significant difference between the intakes of non-fibre carbohydrate, fibre, starch, dietary GI and GL and colorectal cancer risk was found when using either group, except that total carbohydrate intake was inversely associated with colorectal cancer risk when using the community-derived controls (Table 6).
Table 5 Colorectal cancer according to quartiles (Q) of the intakes of total carbohydrate, non-fibre carbohydrate, total fibre, starch, dietary glycaemic index and glycaemic load by cancer site (Odds ratios and 95 % confidence intervals)
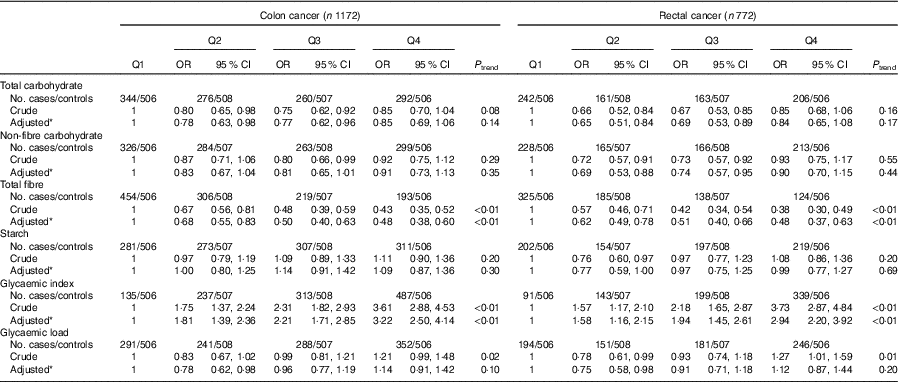
* OR was adjusted for age, sex, marital status, residence, education, occupation, income level, smoking status, passive smoking, alcohol drinking, family history of cancer, occupational physical activity, household and leisure-time activities.
Table 6 Colorectal cancer by quartiles (Q) of total carbohydrate, non-fibre carbohydrate, total fibre, starch, dietary glycaemic index and glycaemic load in two control groups (Odds ratios and 95 % confidence intervals)
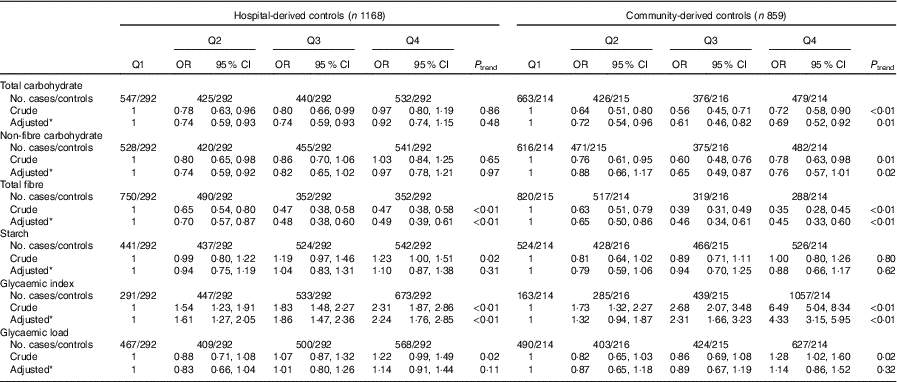
* OR was adjusted for age, sex, marital status, residence, education, occupation, income level, smoking status, passive smoking, alcohol drinking, family history of cancer, occupational physical activity, household and leisure-time activities.
In the present study, there were 159 colorectal cancer cases (8·18 %) and 113 controls (5·57 %) with physician-diagnosed diabetes mellitus. The prevalence of physician-diagnosed diabetes mellitus was significantly higher in colorectal cancer cases than in controls. Sensitivity analysis excluding study subjects with diabetes mellitus did not materially change the results (data not shown).
Discussion
This case–control study found that dietary GI was positively associated with colorectal cancer risk, whereas total fibre intake was negatively associated with colorectal cancer risk. However, there was no clear association between total carbohydrate, non-fibre carbohydrate, starch or dietary GL and colorectal cancer risk.
In 2012, three meta-analyses( Reference Aune, Chan and Lau 11 , Reference Galeone, Pelucchi and La Vecchia 15 , Reference Choi, Giovannucci and Lee 16 ) were conducted to detect the association between dietary carbohydrate, GI and GL and colorectal cancer risk. Our findings were partially consistent with them. Regarding dietary carbohydrate, a meta-analysis including fourteen cohort studies concluded that there was no clear association between dietary total carbohydrate and colorectal cancer; the summary RR for high v. low intake was 1·00 (95 % CI 0·87, 1·14)( Reference Aune, Chan and Lau 11 ). For dietary GI, two meta-analyses including the same twelve cohort studies observed a borderline positive association between dietary GI and colorectal cancer risk; the summary RR for high v. low intake was 1·07 (95 % CI 0·99, 1·16)( Reference Aune, Chan and Lau 11 ) and 1·08 (95 % CI 1·00, 1·17)( Reference Choi, Giovannucci and Lee 16 ), respectively. Similarly, a meta-analysis analysing eight cohort studies and five case–control studies also found that dietary GI was related to an increased risk of colon cancer; the RR for the highest intake was 1·17 (95 % CI 1·00, 1·36) compared with the lowest intake( Reference Galeone, Pelucchi and La Vecchia 15 ). Furthermore, a cohort study conducted in Italy in 2015 with 47 749 participants including 421 colorectal cancer cases( Reference Sieri, Krogh and Agnoli 14 ) found that high dietary GI and high carbohydrate intake from high-GI foods were associated with increased risk of colorectal cancer. Compared with the lowest quintile, the hazard ratios (HR) of the highest quintile were 1·35 (95 % CI 1·03, 1·78) and 1·45 (95 % CI 1·04, 2·03), respectively, whereas low-GI carbohydrate was in relation to a decreased risk of colorectal cancer (HR 0·73; 95 % CI 0·54, 0·98, the highest quintile v. the lowest quintile). As regards dietary GL, the same cohort study mentioned above found that high dietary GL was significantly associated with increased risk of colon cancer( Reference Sieri, Agnoli and Pala 33 ). However, a meta-analysis reported no significant association between dietary GL and colon cancer risk( Reference Galeone, Pelucchi and La Vecchia 15 ), and another two meta-analyses also revealed a null association between dietary GL and colorectal cancer risk( Reference Aune, Chan and Lau 11 , Reference Choi, Giovannucci and Lee 16 ), which were consistent with our finding.
Fibre was the indigestible portion of total carbohydrate. Previous studies revealed that dietary fibre was probably a protective factor against colorectal cancer( Reference Aune, Chan and Lau 17 ). Therefore, the association between total carbohydrate and colorectal cancer risk might be biased by the protective effect of fibre. Some studies evaluated the association between dietary intake of non-fibre carbohydrate and colorectal cancer risk. The cohort study mentioned above found that carbohydrate intake excluding dietary fibre had no significant association with colorectal cancer risk( Reference Sieri, Krogh and Agnoli 14 ), which was similar to our finding. However, a cohort study conducted in the USA with 38 451 women including 174 colorectal cancer cases reported a positive association between non-fibre carbohydrate intake and colorectal cancer risk( Reference Higginbotham, Zhang and Lee 18 ). Another case–control study also drew the same conclusion( Reference Borugian, Sheps and Whittemore 19 ). One possible explanation for the inconsistent results might be that different studies adjusted for different confounders. In these two studies with positive results( Reference Higginbotham, Zhang and Lee 18 , Reference Borugian, Sheps and Whittemore 19 ), the important confounding factors such as physical activity, smoking status and alcohol use were not included in the multivariable regression models, which would bias the risk estimates and lead to a different conclusion from our study. In addition, the cohort study mentioned above( Reference Higginbotham, Zhang and Lee 18 ) only focused on women, which might potentially affect the generalisability of the conclusion.
Contrary to our initial assumption, the present study found no significant association between total carbohydrate intake and colorectal cancer risk. Lack of an association between total carbohydrate and colorectal cancer risk might partly attribute to dietary fibre and resistant starch. Fibre and resistant starch can be fermented by intestine flora, and then produced SCFA, such as butyrate( Reference Leonel and Alvarez-Leite 34 ). Butyrate was important for the intestinal homoeostasis, such as modulating PH and epithelial thickness, decreasing inflammation( Reference Higgins and Brown 35 ) and reducing expression of microRNA that are related to increase the proliferation colon cancer cell( Reference Peres 36 ). The protective effect of dietary fibre observed in the present study and the underlying resistant starch may have offset the adverse effect of other carbohydrate components, as it was presented that after excluding fibre from total carbohydrate, the OR of non-fibre carbohydrate was attenuated from 0·85 to 0·90 in our study.
In agreement with the findings from two previous meta-analyses( Reference Aune, Chan and Lau 11 , Reference Choi, Giovannucci and Lee 16 ), the present study found no significant association between dietary GL and colorectal cancer risk. Cereal grains, vegetables and fruit contributed 87·95 and 9·82 % to the daily dietary GL among control subjects. Although calculating GL excluded dietary fibre content, it was difficult to dissociate the effects of GL from other beneficial contents such as the phytochemicals in vegetables and fruit( Reference Xu, Chen and Huang 37 ). The protective effect of bioactive substances may dilute the adverse effect of GL. Therefore, it is possible that the relationship between dietary GL and colorectal cancer risk have been underestimated. More studies, especially prospective cohort studies, are required to clarify this issue.
To date, only two case–control studies examined the association between starch intake and colorectal cancer risk. One study from Italy including 1953 colorectal cancer cases and 4154 controls found a positive association between starch intake and colorectal cancer risk( Reference Franceschi, La Vecchia and Russo 20 ). However, another study from Swiss, which recruited 286 colorectal cancer cases and 550 controls, did not observe a significant association( Reference Levi, Pasche and Lucchini 21 ), which was consistent with our results. According to different digestive rate, starch was grouped into rapidly digestible starch, slowly digestible starch and resistant starch( Reference Englyst, Kingman and Cummings 38 ). It was reported that resistant starch could escape digestion in the small intestine and was beneficial for bowel health, by increasing faecal PH, faecal bulk, butyrate level and epithelial apoptosis and inhibiting cancer cell proliferation( Reference Birt, Boylston and Hendrich 39 ). The absence of an association between starch intake and colorectal cancer risk found in the present study might partially be due to the existence of resistant starch, which is not digested in the human small intestine. Owing to the limited evidence on the relationship between starch intake and colorectal cancer risk, further studies are needed to verify this issue.
The present study showed that GI, but not total carbohydrate, non-fibre carbohydrate, starch or GL, was positively associated with colorectal cancer risk. This indicated that increased colorectal cancer risk may depend more on the quality of carbohydrate consumed rather than a carbohydrate-rich diet. Consistent with our findings, a cohort study( Reference Sieri, Krogh and Agnoli 14 ) reported that higher intake of carbohydrates from high-GI foods was positively associated with colorectal cancer risk (highest v. lowest quartile: adjusted HR 1·45; 95 % CI 1·04, 2·03, P trend=0·03). However, higher intake of carbohydrates from low-GI foods was negatively associated with colorectal cancer risk (highest v. lowest quartile: adjusted HR 0·73; 95 % CI 0·54, 0·98, P trend=0·03). Another study also showed that a carbohydrate food with higher GI values, such as bread, tended to be more strongly associated with colorectal cancer risk than a carbohydrate food with lower GI values such as pasta( Reference Augustin, Malerba and Lugo 40 ). In the present study, cereal grains and vegetables and fruit were two main food sources of non-fibre carbohydrate. However, the proportion of food sources was different among different GI quartile groups. Compared with the first quartile of GI group, non-fibre carbohydrate of the last quartile of GI had a higher proportion of food sources from cereal grains, but a lower proportion from vegetables and fruit. It was reported that cereal grains consumed among Chinese were predominantly from white rice, which contained a high amount of refined carbohydrates and could provoke a rapid postprandial increase in blood glucose( Reference Yu, Shu and Li 41 ). The modulation of insulin and IGF might be the main mechanism underlying the positive association between dietary GI and colorectal cancer risk. Long-term intake of a high-GI diet results in hyperglycaemia, and consequently hyperinsulinaemia( Reference Sacks, Carey and Anderson 42 ). Insulin can regulate gene expression and mitogenicity( Reference Burgering, Medema and Maassen 43 ), and enhance the concentration of IGF by inhibiting the level of IGF-binding proteins( Reference Suikkari, Koivisto and Rutanen 8 ) or by activating insulin/IGF receptors( Reference Rosenzweig and Atreya 44 ). IGF plays an important role in the development of colorectal cancer by inhibiting apoptosis and stimulating cell proliferation( Reference Kaaks and Lukanova 9 ).
Stratified analysis by sex showed that the inverse association between intakes of total carbohydrate, non-fibre carbohydrate and total fibre and the risk of colorectal cancer was observed among men but not among women. Similarly, the Prostate, Lung, Colorectal and Ovarian screening study conducted in the USA found that carbohydrate intake was inversely associated with colorectal advanced adenomas only in men but not in women. The adjusted OR was 0·74 (95 % CI 0·57, 0·95) and 0·87 (95 % CI 0·62, 1·22), respectively( Reference Flood, Peters and Jenkins 45 ). The advanced adenomas included cancer in situ with high-grade dysplasia, which was a precancerous lesion of colorectal cancer. As presented in our study, the amount of carbohydrate and fibre intake among men was higher than that among women, which might partly explain the inverse association only observed in men. Moreover, the postprandial insulin response may be regulated by sex hormones. It was reported that girls demonstrate a greater blood insulin response than the boys after breakfast in a randomised controlled trail( Reference Cooper, Dring and Morris 46 ). The sex influenced the metabolism of carbohydrate, such as women had less reliance on carbohydrate oxidation to ensure fuel requirements during exercises, which was mediated by oestrogen( Reference Devries 47 ). However, the evidence about the sex difference is still limited and it may be a chance finding in our study. Thus, whether the association between carbohydrate and fibre intake and colorectal cancer risk is modified by sex requires further confirmation.
Subgroup analysis by sources of controls showed that the associations between the intakes of non-fibre carbohydrate, fibre, starch, dietary GI and GL and colorectal cancer risk were similar when only one set of controls was used. However, the intake of total carbohydrate was inversely associated with colorectal cancer risk when only using community-derived controls. One possible explanation might be that community-derived controls consumed more total carbohydrate than hospital-derived controls. In the present study, the intake of total carbohydrate was 277·6 (sd 48·8) g/d among community-derived controls and 260·6 (sd 50·5) g/d among hospital-derived controls. Another reason might be that community-derived controls were engaged in more household and leisure-time activities than hospital-derived controls (43·3 MET-h/week for community-derived controls and 37·7 MET-h/week for hospital-derived controls). As people who are more active in physical activity might consume more carbohydrate( Reference Greenwood, Threapleton and Evans 10 ), it is possible that diet with high carbohydrate indicates a healthy lifestyle( Reference Strayer, Jacobs and Schairer 48 ).
Our study has the following strengths. First of all, a validated FFQ was used to assess the frequency and quantity of intakes of total carbohydrate, non-fibre carbohydrate, total fibre and starch. Second, comprehensive indicators were used to assess dietary carbohydrate intake and colorectal cancer risk in the present study. GI ranks the foods’ carbohydrate quality based on their postprandial blood glucose effects, and GL further considers the quantity of carbohydrate consumed, which is a comprehensive measure of both the blood glucose effects (quality) of the food and the total quantity of carbohydrate consumed. Third, the GI values of most foods were from Chinese Food Composition Table, which could accurately reflect glucose response of local foods consumed by Chinese and offer an accurate and comprehensive estimate of dietary GI. Fourth, the potential confounders including dietary and non-dietary factors were adjusted for in the analysis. Moreover, the sample size was larger than that of most previous relevant case–control studies, so that we have enough power to detect small associations with colorectal cancer risk. Finally, this is the first study to detect association between dietary intake of non-fibre carbohydrate, starch and colorectal cancer risk among Chinese people.
Some potential limitations existed in the present study. First, selection bias may exist in hospital-based case–control studies. Even though the colorectal cancer patients were recruited from only one hospital, Sun Yat-sen University Cancer Center, it is the biggest cancer centre in Southern China. The colorectal cancer patients from this hospital shared similar clinical characteristics with those from other big hospitals in Guangdong or in mainland China( Reference Dai, Zheng and Zou 49 ). Furthermore, the high participation rate (87·93 % for cases and 88·02 % for hospital-derived controls) also helped to reduce selection bias in our study. Second, it is known that recall bias is difficult to rule out in case–control designed studies. To minimise this bias, we included only newly diagnosed cases and interviewed them as soon as possible after diagnosis. The average time interval between the diagnosis of colorectal cancer and study interview was 5·0 d for the case subjects. In addition, food photographs with usual portion sizes were also provided to the participants to help them better quantify their dietary intake. Third, random measurement error of diet is also of concern in the estimation of usual intake. As this misclassification bias is likely to be non-differential and lead the OR to the null, our results may tend to be conservative. Fourth, although we have tried our best to adjust a wide range of kinds of confounders in our analysis, some potential residual confounding factors may still remain. For example, information on drug usage was not collected which may influence the colorectal cancer risk. Fifth, the FFQ was not specifically developed to estimate dietary GI and GL, which may not accurately reflect the blood glucose response to mixed dishes compared with individual food items.
In conclusion, this study showed that dietary GI was positively associated with colorectal cancer risk. However, total carbohydrate, non-fibre carbohydrate intake, starch and dietary GL were not found to be related to an increased risk of colorectal cancer in a Chinese population.
Acknowledgements
The authors gratefully acknowledge the contribution of the study participants.
This study was supported by Guangdong Natural Science Foundation (nos: 2016A030313225, 2014A030313188). The funders had no role in the design, analysis or writing of this article.
The authors’ responsibilities were as follows: J. H. conducted the data collection and data analysis, and writing of this paper. M. X., H. L., N.-Q. Z. and W.-Q. H. participated in data collection and data entry. Y.-J. F. and Z.-Z. P. contributed to the field work. Y.-M. C. analysed and interpreted the data. C.-X. Z. was responsible for designing and writing grants, supervision of the research and writing of this paper.
The authors declare that there are no conflicts of interest.








