It is well established that nutrition plays an important role in the management and prevention of type 2 diabetes (T2D) through its effect on weight and metabolic control. Therefore, the American Diabetes Association has recommended that nutrition therapy should be regarded as an effective component of the overall treatment plan for all patients with either type 1 diabetes or T2D( 1 ). One of the major obstacles in the implementation of nutrition therapy is poor compliance, which may be improved using a meal replacement (MR) programme. In patients with T2D, the MR has been demonstrated to exert beneficial effects on weight management( Reference Chaiyasoot, Sarasak and Pheungruang 2 – Reference Xu, Sun and Chen 6 ), lipids( Reference Gulati, Misra and Tiwari 3 , Reference Kempf, Schloot and Gartner 4 , Reference Xu, Sun and Chen 6 ), blood pressure (BP)( Reference Gulati, Misra and Tiwari 3 ), liver function( Reference Gulati, Misra and Tiwari 3 ), insulin sensitivity( Reference Gulati, Misra and Tiwari 3 , Reference Kempf, Schloot and Gartner 4 , Reference Gonzalez-Ortiz, Martinez-Abundis and Hernandez-Salazar 7 , Reference Wang, Zhang and Zhou 8 ) and glycaemic control( Reference Chaiyasoot, Sarasak and Pheungruang 2 – Reference Stenvers, Schouten and Jurgens 9 ).
The sustained chronic hyperglycaemia, hypoglycaemic episodes and glycaemic variability (GV) have been depicted as the three main components of dysglycaemia in diabetes( Reference Monnier, Colette and Owens 10 ). Of them, GV refers to short-term (intra-day) or long-term (inter-day) fluctuations in glucose around the mean value( Reference Suh and Kim 11 ). Although chronic hyperglycaemia is the main risk factor for diabetic complications( Reference Nathan and Genuth 12 – Reference Orchard and Nathan 14 ), there is emerging evidence that GV contributes independently to the development of diabetic complications in both type 1 diabetes( Reference Picconi, Parravano and Ylli 15 , Reference Soupal, Skrha and Fajmon 16 ) and T2D( Reference Matsutani, Sakamoto and Iuchi 17 , Reference Su, Mi and Tao 18 ). In vivo ( Reference Horvath, Benko and Kiss 19 ), in vitro ( Reference Quagliaro, Piconi and Assaloni 20 ) and clinical( Reference Monnier, Mas and Ginet 21 , Reference Wentholt, Kulik and Michels 22 ) studies demonstrated that glucose fluctuations induce endothelial dysfunction and oxidative stress, a key player in the pathogenesis of diabetic complications( Reference Brownlee 23 ). Previous studies on MR in patients with T2D mainly focused on indices of hyperglycaemia including fasting and postprandial glucose and, most importantly, glycated Hb (HbA1c)( Reference Chaiyasoot, Sarasak and Pheungruang 2 , Reference Kempf, Schloot and Gartner 4 – Reference Xu, Sun and Chen 6 , Reference Stenvers, Schouten and Jurgens 9 ), which reflects the average glucose level during the past 2–3 months. However, none has examined whether MR exerts beneficial effects on GV. Therefore, we conducted this randomised clinical trial (NCT02248714) to evaluate the effects of lifestyle intervention (LI) with or without breakfast replacement with a liquid formula on the indices of GV, as assessed by continuous glucose monitoring (CGM) and markers of metabolic control.
Methods
Study populations
In this study, subjects referred to the outpatient clinic at the Department of Endocrinology of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital were enroled from March 2011 to March 2018. The inclusion criteria were as follows: (1) newly diagnosed and untreated T2D, (2) BMI ≥18·5 kg/m2 and (3) HbA1c ≥6·5 %. The exclusion criteria were as follows: (1) severe liver disease, (2) severe kidney disease, (3) history of unstable angina or myocardial infarction or New York Heart Association class III or IV congestive heart failure, (4) acute or chronic disorders interfering with digestion or absorption, (4) history of malignancy or mental disorders and (5) pregnancy or lactation. T2D was defined in accordance with the 1999 WHO criteria.
Following study enrolment (visit 1), each subject underwent a 75-g oral glucose tolerance test (OGTT). Participants who met the eligibility criteria were then monitored continuously for 72 h (visit 2 to visit 3) with a retrospective CGM system (Medtronics). After 3 d of CGM (visit 3), the subjects were randomised (1:1) to receive either LI or LI+MR for 4 weeks. At the end of the 4-week intervention, subjects underwent another CGM from visit 4 (day 29) to visit 5 (day 32), at which a final OGTT was conducted in the fasting state. The schematic outline of study conduction is illustrated in Fig. 1.
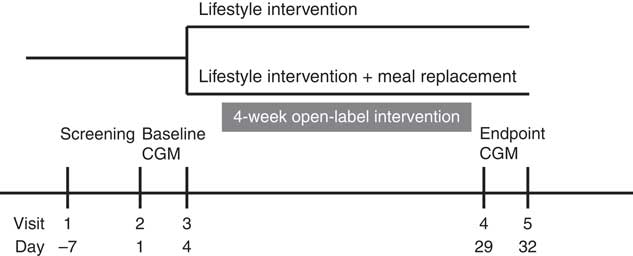
Fig. 1 Schematic outline of study conduction. CGM, continuous glucose monitoring.
The study protocol was in accordance with the Helsinki Declaration and approved by the Ethics Committees of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Written informed consent was obtained from all participants.
This trial is registered at www.ClinicalTrials.gov with clinical trial registration number NCT02248714.
Intervention
Before the intervention (visit 3), both groups received lifestyle education delivered by an experienced nutritionist who assessed each patient’s medical history and clinical characteristics and prescribed an individualised meal plan comprising the quantity and type of food products, ensuring a total daily energy intake of 105–126kJ/kg ideal body weight/d. Participants were instructed to have three main meals (i.e. breakfast, lunch and dinner) per d and avoid snacks. The prescribed diet consisted of 45–60 % of energy from carbohydrate, 25–35 % from fat and 15–20 % from protein, according to the Chinese guidelines to the medical nutrition therapy for diabetes. Breakfast, lunch and dinner comprised approximately 20, 40 and 40 % of total daily energy intake, respectively. Participants were also advised to replace refined carbohydrates with low-glycaemic index foods such as whole grains and vegetables, avoid sugar-sweetened beverages, minimise the consumption of foods with added sugar, limit the intake of fried and fatty foods and cut down on salt and cooking oil. Besides, they were encouraged to engage in 150 min or more of moderate-intensity physical activity per week and reduce the amount of time spent being sedentary. At the end of week 2, all study subjects were asked to visit the nutritionist again and the instructions on diet and exercise were reinforced.
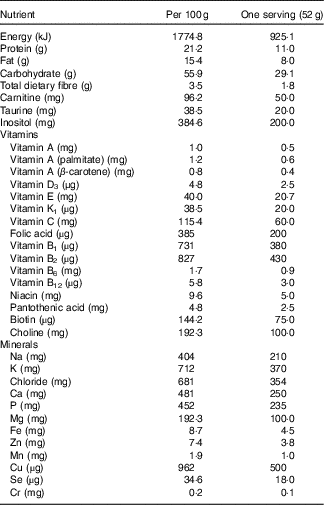
In addition to the lifestyle education, participants in the LI+MR group were instructed to replace breakfast with Glucerna SR (Abbot Nutrition) powder 52 g dissolved in 200 ml of warm water, providing 923·0 kJ, 29·1 g of carbohydrate, 11·0 g of protein and 8·0 g fat. The composition of Glucerna SR is listed in Table 1. The average energy content of the breakfast in the LI group was 1450·8 kJ, containing 42·6 g of carbohydrate, 11·8 g of fat and 18·2 g of protein. The macronutrients composition and energy content in lunch and dinner did not differ between the two groups.
Table 1 The composition of Glucerna SR powder
Continuous glucose monitoring parameters
Time in range (TIR) was defined as the percentage of time in the target glucose range of 3·9–10·0 mmol/l during a 24-h period. The incremental AUC of postprandial blood glucose (AUCpp) during CGM was calculated as the area between the glucose concentration–time curve and the pre-prandial baseline glucose value measured at 4 h after each meal. Intra-day GV parameters include the standard deviation of blood glucose (SDBG) values, glucose CV and the mean amplitude of glycaemic excursions (MAGE). CV was calculated by dividing the sd by the mean of the corresponding glucose readings. MAGE was calculated as the arithmetic mean of the differences between consecutive peaks and nadirs. Only excursions of more than 1sd of the mean glycaemic values were considered for the calculation of MAGE.
Anthropometric and biochemical measurements
Anthropometric and biochemical parameters were measured at visit 1 (baseline) and visit 4 (post-intervention). BMI was calculated as the weight (kg) divided by squared height (m). BP was measured twice using a standard mercury sphygmomanometer and the average was documented. Venous blood samples were collected after an overnight fast. Biochemical parameters including fasting plasma glucose (FPG), 2-h plasma glucose (2h-PG) during OGTT, HbA1c, glycated albumin (GA), insulin, TAG, total cholesterol, HDL-cholesterol and LDL-cholesterol were assayed as previously reported( Reference Lu, Ma and Zhou 24 ).
Insulin sensitivity was evaluated by homoeostasis model assessments of insulin resistance (HOMA-IR) and Matsuda index using the following formulae: HOMA-IR=fasting insulin (in mU/l)×FPG (in mmol/l)/22·5 and Matsuda index=10 000/square root of (FPG (in mmol/l)×fasting insulin (in mU/l))×(mean glucose×mean insulin during OGTT)( Reference Matsuda and DeFronzo 25 ). Early phase (0–30 min) and total (0–120 min) incremental insulin response to the increase in plasma glucose concentration (ΔI/ΔG) were calculated as indices of insulin secretion.
Determination of sample size and statistical analyses
Because the effect of MR on GV has not been reported previously, sample size calculation was not performed and a convenience sample size was adopted. Based on the current sample size, our study had more than 80 % power (α=0·05) in each arm to detect the effect sizes of 150·0 (sd 368·0) min × mmol/l, 0·3 (sd 0·7) mmol/l, 3·0 (sd 8·0) % and 0·8 (sd 2·1) mmol/l for AUCpp, SDBG, CV and MAGE, respectively.
The primary outcomes were the improvements in metrics of GV. The secondary outcomes were the improvements in cardiometabolic risk factors including body weight, BMI, BP, lipid profiles and indices of glucose control, insulin secretion and insulin sensitivity.
Continuous variables at baseline were compared between the two groups by Student’s t test. Comparison of categorical variables between groups was performed by χ 2 tests. Differences in the parameters before and after treatment were analysed by paired t test. The changes (Δ) in variables were compared with Student’s t test or ANCOVA where indicated. Multivariate linear regression analysis was conducted to examine the risk factors of improvements in metrics of GV after adjusting for covariates. Statistical analyses were performed using SPSS software version 17.0 (SPSS Inc.). A P value of <0·05 (two tailed) was considered statistically significant.
Results
In total, 131 patients with T2D met the enrolment criteria and were randomised into the two groups (LI+MR: n 66 and LI: n 65). A total of four patients were excluded from the LI+MR group because they were lost to follow up (n 2) or exhibited a major protocol deviation (n 2). Another four patients were excluded from the LI group because they were lost to follow up. Finally, 62 (93·9 %) and 61 (93·8 %) participants completed the study in the LI+MR group and LI group, respectively.
The characteristics of the participants who completed the study before and after the intervention are depicted in Table 2. At baseline, there were no significant differences in all the clinical parameters except ΔI30/ΔG30 (LI+MR: 4·6 (sd 2·7) mU/mmol, LI: 6·5 (sd 6·4) mU/mmol, P=0·031). After the 4-week intervention, body weight, BMI, TAG, FPG, 2h-PG, HbA1c, GA, TIR, ΔI120/ΔG120, HOMA-IR and Matsuda index were significantly improved in both the LI+MR and LI groups. Compared with baseline, systolic BP (P<0·001) and diastolic BP (P=0·002) at the end of the study were significantly reduced, and ΔI30/ΔG30 (P=0·013) was significantly increased in the LI+MR group but not in the LI group. The changes in clinical parameters following interventions did not differ significantly between the two groups, except that the improvement in systolic BP (P=0·046) and TIR (P=0·033) was significantly higher in the LI+MR group compared with the LI group.
Table 2 Participant characteristics before and after intervention (Mean values and standard deviations)
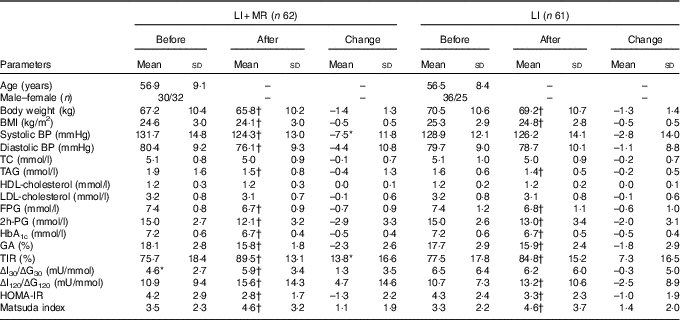
LI, lifestyle intervention; MR, meal replacement; BP, blood pressure; TC, total cholesterol; FPG, fasting plasma glucose; 2h-PG, 2-h plasma glucose during oral glucose tolerance test; HbA1c, glycated Hb A1c; GA, glycated albumin; TIR, time in range; HOMA-IR, homoeostasis model assessment for insulin resistance.
* P<0·05 compared with the LI group.
† P<0·05 compared with baseline.
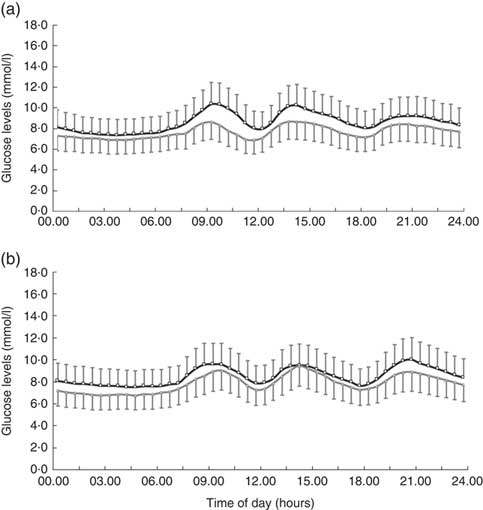
In all, 4-week treatment of LI+MR exerted beneficial effects on all the GV-related measures of CGM including SDBG (before: 1·9 (sd 0·7) mmol/l, after: 1·3 (sd 0·5) mmol/l, P<0·001), CV (before: 21·5 (sd 7·3) %, after: 16·8 (sd 6·0) %, P<0·001), MAGE (before: 5·0 (sd 2·0) mmol/l, after: 3·5 (sd 1·5) mmol/l, P<0·001) and AUCpp (before: 522·5 (sd 335·9) min × mmol/l, after: 234·6 (sd 186·4) min × mmol/l, P<0·001). In contrast, only MAGE (before: 4·7 (sd 1·7) mmol/l, after: 4·1 (sd 1·6) mmol/l, P=0·032) was significantly reduced in the LI group. LI+MR caused significantly greater improvements in SDBG (LI+MR: Δ=–0·6 (sd 0·8) mmol/l, LI: Δ=–0·2 (sd 0·7) mmol/l, P=0·005), CV (LI+MR: Δ=–4·7 (sd 8·1) %, LI: Δ=–0·2 (sd 7·6) %, P=0·002), MAGE (LI+MR: Δ=–1·6 (sd 2·3) mmol/l, LI: Δ=–0·6 (sd 2·1) mmol/l mmHg, P=0·016) and AUCpp (LI+MR: Δ=–287·8 (sd 368·7) min × mmol/l, LI: Δ=–31·2 (sd 301·6) min × mmol/l, P<0·001) compared with LI (Figs 2 and 3).
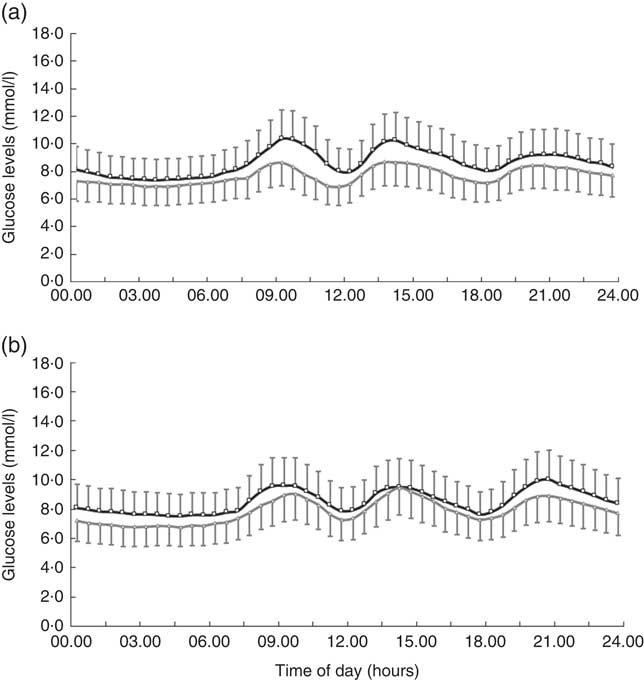
Fig. 2 Comparison of average continuous glucose monitoring tracings between the (a) lifestyle intervention (LI)+meal replacement (MR) group and the (b) LI group. The black curve represents the baseline values, and the grey curve represents the endpoint values. Data are reported as means and standard deviations.
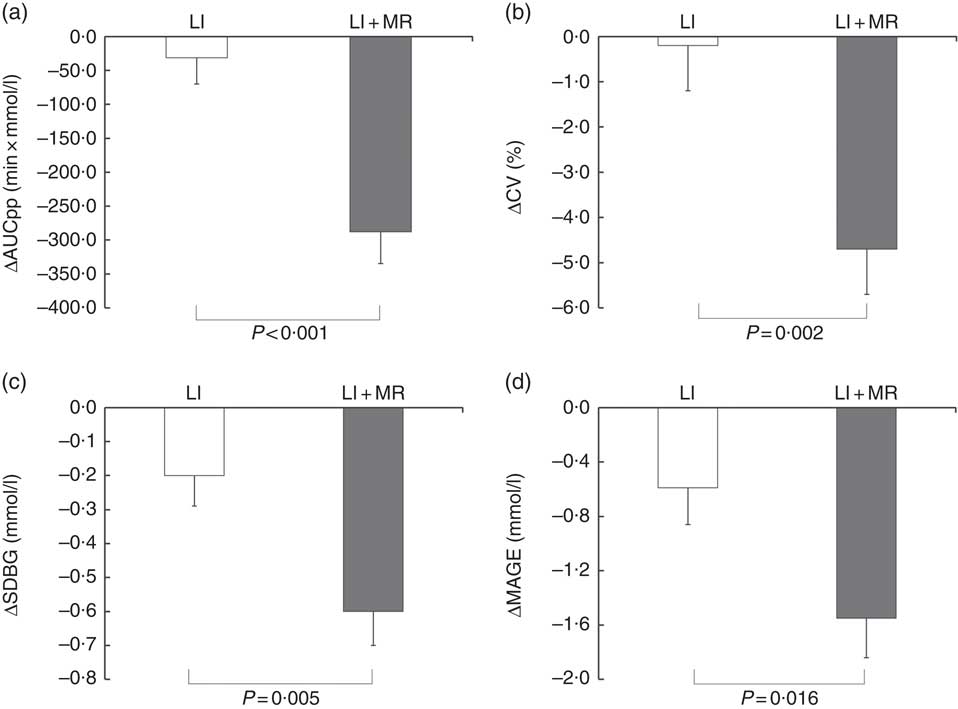
Fig. 3 Comparison of mean changes in (a) postprandial blood glycaemic excursions (incremental AUC of postprandial blood glucose (AUCpp)), (b) CV of glucose, (c) standard deviation of blood glucose (SDBG) and (d) mean amplitude of glycaemic excursions (MAGE) in the lifestyle intervention (LI) and LI+ meal replacement (MR) groups. Data are reported as means with their standard errors.
After combining the two groups of participants, multivariate linear regression analysis revealed that LI+MR and baseline ΔI30/ΔG30 were consistently and independently associated with the improvements in SDBG, CV, MAGE and AUCpp after adjusting for baseline clinical variables including age, sex, BMI, FPG, 2h-PG, HbA1c, ΔI120/ΔG120, Matsuda index and corresponding baseline CGM metrics (i.e. baseline SDBG, CV, MAGE or AUCpp) (Table 3).
Table 3 Multivariate regression analysis investigating the determinants of improvements in metrics of glycaemic variability (β-Coefficients and 95 % confidence intervals)

AUCpp, AUC of postprandial blood glucose; MAGE, mean amplitude of glycaemic excursions; LI, lifestyle intervention; MR, meal replacement.
* P was adjusted for baseline clinical variables (age, sex, BMI, fasting plasma glucose, 2-h plasma glucose, HbA1c, ΔI120/ΔG120 and Matsuda index) and baseline AUCpp.
† P was adjusted for baseline clinical variables as mentioned above and baseline sd.
‡ P was adjusted for baseline clinical variables as mentioned above and baseline CV.
§ P was adjusted for baseline clinical variables as mentioned above and baseline MAGE.
Discussion
Previous studies investigating the effects of full or partial MR on blood glucose have produced conflicting results regarding FPG. However, all these studies reported that MR significantly reduced postprandial glucose levels( Reference Gulati, Misra and Tiwari 3 , Reference Xu, Sun and Chen 6 , Reference Gonzalez-Ortiz, Martinez-Abundis and Hernandez-Salazar 7 , Reference Stenvers, Schouten and Jurgens 9 ). Postprandial glucose is considered as a contributor to glucose excursions, and there is mounting evidence that postprandial glucose is more strongly associated with CVD than with FPG( Reference Cavalot, Pagliarino and Valle 26 , Reference Coutinho, Gerstein and Wang 27 ). Nevertheless, no study has examined the effect of nutrition therapy on parameters reflecting intra-day GV assessed by CGM. In this study, we found that 4-week replacement of breakfast with Glucerna SR significantly reduced AUCpp, which is in accordance with the findings of previous reports. More importantly, there were significant improvements in GV metrics assessed by CGM including SDBG, CV and MAGE following intervention with LI+MR but not with LI alone. SDBG was reported to be significantly associated with diabetic retinopathy in patients with type 1 diabetes and T2D( Reference Sartore, Chilelli and Burlina 28 ), and Soupal et al. ( Reference Soupal, Skrha and Fajmon 16 ) found a positive relationship between SDBG and peripheral neuropathy in type 1 diabetes. Matsutani et al. ( Reference Matsutani, Sakamoto and Iuchi 17 ) previously observed in type 1 diabetes that CV was significantly related to baroreflex sensitivity, an early indicator of cardiovascular autonomic neuropathy. Regarding MAGE, it has been shown to be associated with the presence and severity of coronary artery disease in T2D patients with chest pain( Reference Su, Mi and Tao 18 ) and was observed to be predictive of 1-year major adverse cardiac events in T2D patients with acute myocardial infarction( Reference Wang, Zhao and Dorje 29 ).
Regarding the mechanism underlying the impact of MR on intra-day measures of GV (i.e. SDBG, CV and MAGE), since postprandial glucose is an important component of glucose excursions, it is possible that the effect of MR on postprandial glucose contributes to improvements in SDBG, CV and MAGE. However, it is noteworthy that MR was only applied at breakfast, and a previous study reported that breakfast MR did not affect postprandial glucose levels after lunch( Reference Stenvers, Schouten and Jurgens 9 ). Therefore, other mechanisms may be involved in the promotion of GV by MR. There is emerging evidence that in addition to its physicochemical properties, dietary fibre also affects the composition of the gut microbiota, and the fermentation of dietary fibre in the intestine leads to increased production of SCFA, which stimulate the secretion of incretin hormones including glucagon-like peptide 1 (GLP-1)( Reference Zhou, Martin and Tulley 30 , Reference Zhao, Zhang and Ding 31 ). GLP-1 inhibits glucagon secretion and gastrointestinal motility, and acts as a physiological regulator of appetite. Moreover, it stimulates insulin secretion in a glucose-dependent manner( Reference Holst 32 ). Therefore, GLP-1 is regarded as a crucial player in the regulation of glucose homoeostasis. Interestingly, increases in GLP-1 during OGTT were reported to be significantly correlated with early phase insulin response (ΔI30/ΔG30)( Reference Shen, Chen and Chen 33 ), which was consistently and independently associated with improvements in GV parameters in our study, suggesting that GLP-1 may, in part, account for the beneficial effect of MR on GV.
Glucerna SR is characterised by a higher MUFA:carbohydrate ratio, the presence of fructose and high amounts of fibre, which all lead to lower postprandial glucose levels. Of note, an indirect effect of MR on postprandial glucose in the real world may be of concern, although this could not be confirmed in the current study as the participants were instructed not to skip breakfast. In this regard, a clinical trial conducted by Jakubowicz et al. ( Reference Jakubowicz, Wainstein and Ahren 34 ) showed that skipping breakfast resulted in a significantly higher glycaemic response after subsequent isoenergetic lunch and dinner accompanied by impaired responses of insulin and GLP-1. Moreover, they found that skipping breakfast acutely disrupts the circadian clock and the expression of clock-controlled genes, which are crucial for glucose metabolism( Reference Jakubowicz, Wainstein and Landau 35 ). Considering that MR decreases the need for intensive dietary counselling, and is convenient and easy to prepare, especially for individuals staying away from home, it may possibly reduce the chance of breakfast skipping and therefore prevent postprandial glucose excursions.
As for other cardiometabolic risk factors, LI+MR demonstrated more pronounced improvements in systolic BP and TIR than LI. Two previous studies reported the beneficial effect of MR on BP as well. Chaiyasoot et al. ( Reference Chaiyasoot, Sarasak and Pheungruang 2 ) speculated that lower total energy intake caused by MR may partially account for this observation. However, the decrease in body weight did not differ significantly between the two intervention arms in our study, suggesting that MR may exert a weight-independent effect on BP. Although the two interventions led to similar improvements in HbA1c and GA, significantly greater improvement in TIR was achieved with LI+MR than with LI. HbA1c and GA reflect the average glucose level for a period of time and are widely used in clinical practice. However, it is recognised that they are far from perfect in the evaluation of glucose control. On the other hand, TIR refers to the time spent in the target glucose range and provides information about whether hypoglycaemia or hyperglycaemia improves over time, which is not reflected by HbA1c or GA. Our findings further support the notion that TIR is a valuable metric for glucose control( Reference Danne, Nimri and Battelino 36 ) in addition to HbA1c and GA.
Several limitations of the study design should be noted. First, the intervention period was relatively short (i.e. 4 weeks). Therefore, the long-term effects of MR on the cardiometabolic profile and GV could not be determined. Second, the open-label design of the current study may introduce some extent of bias. However, since Glucerna SR looks and tastes different compared with a Chinese diet, a blinded trial was not feasible. Third, the energy content of the breakfast in the LI+MR groups were lower than those in the LI group, which may be a confounding factor for the relationship between MR and GV. However, since the weight loss was similar in the two groups and was only about 2 %, it is unlikely that the lower energy intake related with MR accounts for the improvements in GV. Finally, only untreated T2D patients were admitted in the study. Our findings may not be generalised to those patients receiving hypoglycaemic agents, and the possibility of evaluating the interaction between MR and different hypoglycaemic agents was precluded.
In conclusion, breakfast replacement with a liquid formula for 4 weeks significantly improved GV in T2D patients. Our results suggest that MR could be an effective treatment to alleviate glucose fluctuations, which is associated with the development of diabetic complications. Future studies with longer intervention times are warranted to confirm the beneficial effects of MR on GV.
Acknowledgements
The authors would like to thank all of the involved clinicians, nurses and technicians for dedicating their time and skill to the completion of this study.
This work was funded by the Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20161430).
X. M. and J. Z. contributed to study design, data analysis and interpretation of data. J. P. and J. L. contributed to data analysis and writing the paper. L. Y., W. L. and W. Z. conducted the study and contributed to data collection. Y. B. interpreted the data and revised the manuscript. X. M. and J. Z. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors declare that there are no conflicts of interest.