Hepatitis B virus (HBV)- and hepatitis C virus (HCV)-related chronic infections represent a worldwide public health problem, with more than 300 and 170 million people being infected, respectively(Reference Kane1–4). HBV, a DNA virus, and HCV, an RNA virus, are both hepatotropic, and both lead to chronic hepatitis in many patients with potentially fatal complications including decompensated cirrhosis and hepatocellular carcinoma.
The current treatment of chronic HBV infection includes immunomodulating agents (pegylated interferon-α (PEG-IFN-α)) and several antiviral molecules specifically targeting the virus polymerase(4, Reference Delaney and Borroto-Esoda5). Interferon-based therapies have a fixed duration and are able to induce a sustained suppression of HBV viraemia but carry a high probability of relapse after the end of the treatment. Moreover, they can give rise to adverse events, so that their use in advanced liver disease is not recommended. Antiviral drugs, such as nucleos(t)ide analogues, need to be continued for a very long time or even indefinitely, and their efficacy is seriously hampered by the onset of drug-resistant strains.
A combination therapy with PEG-IFNα and ribavirin is currently the treatment of choice for chronic HCV infection. However, this dosage regimen is effective in approximately 50–60 % of patients and can induce severe side effects(Reference Manns, McHutchison and Gordon6–Reference Hadziyannis, Sette and Morgan8). So far, several HCV protease and polymerase inhibitors have been developed, but they are still under investigation(Reference Soriano, Peters and Zeuzem9, Reference Vento, Cainelli and Temesgen10).
Despite remarkable virological differences, HBV and HCV seem to share common behaviours, such as the induction of long-term chronic liver disease, and similar pathogenetic mechanisms of liver damage(Reference Guidotti and Chisari11). In both infections, inflammatory liver injury is largely mediated by the host's cellular immune response to infected hepatocytes(Reference Guidotti and Chisari11–Reference Bertoletti and Gehring13). Conversely, a defective or weak specific immune response has been postulated as the main factor leading to chronic evolution of the infection and an unfavourable disease outcome(Reference Bertoletti and Gehring13, Reference Rehermann14).
Several data suggest that HBV and HCV favour the generation of reactive oxygen species within the infected cells. This contributes to liver damage and carcinogenesis via oxidative stress, as extensively shown by several studies(Reference Medina and Moreno-Otero15–Reference Farinati, Cardin and Bortolami25). In addition, reactive oxygen species are produced by both neutrophils and macrophages during the immune response against pathogens(Reference Fialkow, Wang and Downey26) and during the accumulation of viral components in infected hepatocytes(Reference Okuda, Li and Beard27–Reference Majano, Lara-Pezzi and López-Cabrera30).
On this basis, compounds exerting immune-stimulatory and antioxidant properties could be effective in reducing liver damage(Reference Sprengers and Janssen31, Reference Rocchi, Casalgrandi and Ronzoni32). It is well known that several vitamins have an essential role in neutralising reactive oxygen species activity(Reference Fialkow, Wang and Downey26–Reference Majano, Lara-Pezzi and López-Cabrera30) and in regulating both innate and adaptive immune response(Reference Fialkow, Wang and Downey26, Reference Maggini, Wintergerst and Beveridge33), so that their deficiency is associated with an increased susceptibly to infections. The immunomodulation of vitamins is driven through multiple pathways, such as promoting the differentiation into Th1 and Th2 subsets and enhancing lymphocyte proliferation and cytokine production.
This background represents the rationale to evaluate the effectiveness of vitamins in attenuating liver damage in chronic viral hepatitis. Interestingly, although not limited to hepatic disease of viral aetiology, significant reductions in serum levels of vitamins such as β-carotene, vitamin C, vitamin D and vitamin E in patients with chronic viral hepatitis have been reported(Reference Lin and Yin34–Reference Grønbaek, Sonne and Ring-Larsen40), but the underlying mechanisms have not been elucidated.
The aim of the present study was to review clinical studies investigating the efficacy of vitamins, alone or in addition to standard therapy, in the treatment of HBV- and HCV-related chronic hepatitis.
Materials and methods
In order to identify clinical studies involving vitamins in the treatment of chronic hepatitis B and C, a literature search was performed in the following electronic databases: PubMed, MEDLINE, the Cochrane Library and EMBASE. No a priori selection regarding a specific vitamin was performed so as to extract all the available experience in this context. The search terms used were chronic hepatitis, chronic hepatitis B, chronic hepatitis C, vitamins, vitamin A, retinol, vitamin B1, thiamine, vitamin B2, riboflavin, vitamin B3, niacin, vitamin B5, pantothenic acid, vitamin B6, pyridoxine, vitamin B7, biotin, vitamin B9, folic acid, folate, vitamin B12, cobalamin, vitamin C, ascorbic acid, vitamin D, cholecalciferol, vitamin E, alpha tocopherol, vitamin K and antiviral treatment. The search was carried out in February 2009 without a lower limit on the search and was restricted to peer-reviewed, full-text English language publications.
Only studies evaluating the effects of vitamins alone or in combination with standard antiviral treatment on viral replication and/or serum transaminases or liver histology parameters were included. Both controlled and uncontrolled clinical trials, pilot studies and case series in adult and paediatric patients with different clinical and serovirological features were evaluated. In vitro studies, or studies with other major aims such as the role of vitamins in counteracting the standard antiviral treatment side effects, were excluded.
Results
Hepatitis B virus infection
Vitamin B1 (thiamine) supplementation
An association between high serum and/or hepatic iron level and either an increased risk of disease progression or a reduced response to IFN therapy has been reported in patients with chronic hepatitis B(Reference Van Thiel, Friedlander and Fagiuoli41). Thiamine is required for the formation of dihydrolipoate, a complex able to remove ferritin-bound Fe(Reference Bonomi and Pagani42). Thus, it was hypothesised that thiamine might slow or reverse liver damage in chronic HBV infection (Table 1) via the reduction of Fe overload.
Table 1 Summary of clinical studies of vitamin use in the treatment of chronic hepatitis B
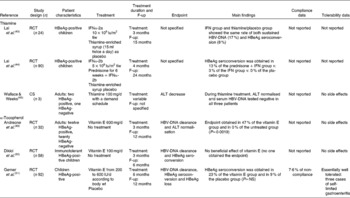
F-up, post-treatment follow-up; RCT, randomised clinical trial; HBeAg, hepatitis B e antigen; IFN, interferon; tiw, three times a week; HBV, hepatitis B virus; CS, case series; ALT, alanine aminotransferase.
On this topic, the search strategy generated three studies eligible for inclusion. The first study randomised twenty-four Chinese children with hepatitis B e antigen (HBeAg)-positive chronic hepatitis to receive either recombinant IFNα-2a or thiamine-enriched syrup as placebo for 12 weeks(Reference Lai, Lok and Lin43). The two groups underwent the same rate of sustained HBV-DNA and HBeAg loss (17 and 8 %, respectively). The second study randomised ninety HBeAg-positive Chinese children to be treated with recombinant IFNα-2a, either with or without prednisone, or vitamin B complex, as placebo. In contrast with the results of the previous trial(Reference Lai, Lin and Lau44), none of the children who had received vitamin B underwent HBeAg seroconversion, which occurred in 3 and 13 % of cases treated with IFN alone or IFN plus steroid, respectively. Considering the controversial results of these two early studies, no benefit of thiamine can be demonstrated in children with chronic hepatitis B.
In a more recent study, three adult patients with HBV-related chronic hepatitis who had failed to respond to or did not tolerate IFN were given thiamine at 100 mg/d, in different time schedules(Reference Wallace and Weeks45). Thiamine administration was stopped whenever transaminases normalised and was resumed when they increased again. During thiamine administration, alanine aminotransferase (ALT) became normal, and HBV-DNA fell to undetectable levels in each patient. In addition, the two HBeAg-positive patients seroconverted to hepatitis B e antibody (HBeAb). However, the extremely small cohort, the descriptive design of the study and the possibility that transaminase fluctuations and spontaneous HBe seroconversion can occur during the natural history of chronic hepatitis B make this result largely inconclusive.
Vitamin E (α-tocopherol) supplementation
Antioxidant activity is one of the most important biological properties of vitamin E (α-tocopherol), a liposoluble vitamin widely available in nature(Reference Meydani46). Vitamin E acts as a free radical ‘scavenger’ and effectively contributes to neutralise free radicals and reactive oxygen species. Therefore, it plays an important role in stabilising and protecting cellular structures from oxidative damage(Reference Sies and Murphy47, Reference Halliwell and Gutteridge48). Vitamin E also exerts immunomodulant properties, as it can enhance cell-mediated immunity(Reference Meydani46). Three studies evaluated the effect of vitamin E on chronic hepatitis B.
A small clinical trial randomised thirty-two patients with chronic hepatitis B to receive either vitamin E or no treatment(Reference Andreone, Fiorino and Cursaro49). The patient population included both HBeAg- and HBeAb-positive adult subjects, as well as naive patients and non-responders to previous IFN treatment. At the end of the study period, seven patients (47 %) in the vitamin E group and none of the controls (P = 0·0019) achieved a complete response, defined as ALT normalisation and undetectable serum HBV-DNA.
In a more recent study, fifty-eight HBeAg-positive children with normal ALT levels and a high viral load received either vitamin E or no treatment(Reference Dikici, Dagli and Ucmak50). Vitamin E failed to either reduce the viral load or promote HBeAg seroconversion. Divergent results seem to emerge from another study involving ninety-two children randomised in a 3:1 ratio to receive either vitamin E according to body weight or placebo for 6 months. The intention-to-treat analysis showed that HBeAg seroconversion was obtained in sixteen of sixty-nine (23 %) vitamin E-treated children and in two of twenty-three (9 %) placebo-treated children. Although such a difference was not statistically significant, this study suggests that vitamin E could favour HBeAg seroconversion(Reference Gerner, Posselt and Krahl51).
These studies in children and adults have major limitations represented not only by the small size but also by the serological and clinical heterogeneity of patients enrolled, making the results inconclusive. However, the use of vitamin E in the treatment of chronic hepatitis B provides some interesting cues. Only one study reported a complete clinical failure, but it was conducted on immunotolerant children treated with a lower vitamin E dosage than that administered in the other paediatric study(Reference Dikici, Dagli and Ucmak50, Reference Gerner, Posselt and Krahl51). This may suggest that ‘successful’ stimulation of the immune system by vitamin E could be obtained mainly in the immunoclearance phase, since the immune system is too silent and weak in immunotolerance to be reinforced.
Hepatitis C virus infection
Vitamin A (retinoic acid) supplementation
An HCV replicon model has recently shown that HCV inhibits the activity of gastrointestinal glutathione peroxidase(Reference Morbitzer and Herget52), a potent antioxidant enzyme expressed by epithelial cells of the gastrointestinal tract and liver, which contains retinoic acid response elements(Reference Kelner, Bagnell and Montoya53) (Table 2). The same study reported an effect of all-trans retinoic acid (ATRA) in inducing a down-regulation of the HCV replicon(Reference Morbitzer and Herget52). The addition of ATRA to HCV-transfected liver tumour cell lines also up-regulated type-I IFN receptors, which in turn enhanced the antiviral effects of IFNα in vitro (Reference Hamamoto, Fukuda and Ishimura54). These experiments provided the rationale for evaluating the effectiveness of ATRA in patients with chronic hepatitis C.
Table 2 Summary of clinical studies of vitamin use in the treatment of chronic hepatitis C

RCT, randomised clinical trial; F-up, post-treatment follow-up; ATRA, all-trans retinoic acid; qd, once daily; PEG-IFNα, pegylated interferon-α; HCV, hepatitis C virus; tiw, three times a week; CS, case series; ALT, alanine aminotransferase; NAC, N-acetylcysteine; SPV complex, one pill contains 15 mg vitamin E and 47 mg silybin.
So far, the only available study(Reference Böcher, Wallasch and Höhler55) randomly assigned twenty so-called difficult-to-treat patients defined by HCV genotype 1 infection and/or non-response to previous antiviral treatments to receive either ATRA plus sodium selenite or a combination of ATRA, sodium selenite and PEG-IFNα-2a. An end-of-treatment virological response, defined as a drop ≥ 2 log of serum HCV-RNA, was obtained in one (10 %) patient in the ATRA group and in four (40 %) patients in the ATRA plus PEG-IFNα group. During the subsequent follow-up period, this response was only maintained in the patient from the ATRA group. Adverse events were reported in detail (Table 2). In particular, no evidence for liver toxicity was observed, but typical retinoid-associated toxicity (skin dryness, pruritus, headache, asthenia and hypertriacylglycerolaemia) occurred in the majority of patients requiring three ATRA dose reductions and four pre-term withdrawals. Based on these results, the authors argued that ATRA has an in vivo anti-HCV activity and suggested a potential additive or synergistic effect of ATRA and PEG-IFNα, warranting larger controlled clinical trials. This conclusion is questionable because the endpoint is not correct as it does not refer to sustained virological response defined as HCV-RNA negativisation, and the authors emphasised an event that occurred in just a single patient. Furthermore, a major concern in the use of vitamin A is its tolerability profile and well-known hepatotoxicity, which can limit the potential benefits.
Vitamin E supplementation
The safety and potential efficacy of vitamin E in patients with chronic hepatitis C have been evaluated in several trials.
A double-blind randomised placebo-controlled trial with a cross-over design administered vitamin E to twenty-three HCV patients not responding to previous IFN treatment(Reference von Herbay, Stahl and Niederau56). At the end of the study, even though eleven patients (48 %) were classified as responders because ALT was reduced by >35 %, none normalised ALT or obtained HCV-RNA negativisation.
Another study blindly randomised twenty-four naive chronic hepatitis C patients to receive IFNα-2a or IFNα plus N-acetylcysteine (NAC) and sodium selenite or IFNα plus NAC, sodium selenite and vitamin E(Reference Look, Gerard and Rao57). At the end of the treatment, a complete response (normalisation of ALT and negativisation of HCV-RNA) was obtained in three of eight (37·5 %) patients treated with IFN monotherapy, two of eight (25 %) patients treated with IFN plus NAC and sodium selenite and six of eight (75 %) patients treated with IFN plus NAC, sodium selenite and vitamin E. However, IFN plus antioxidant therapy was not effective, since a virological and/or biochemical relapse occurred in seven of the eleven (63 %) responders within 6 months after the end of the treatment(Reference Look, Gerard and Rao57).
In an attempt to demonstrate the efficacy of the association between IFNα and antioxidant drugs in chronic hepatitis C, 120 patients not responding to a previous treatment with IFN were randomised to receive natural IFNα or IFNα plus NAC and vitamin E for 6 months. This study also disclosed no difference in the biochemical or virological responses observed in the two groups(Reference Idéo, Bellobuono and Tempini58).
Houglum et al. (Reference Houglum, Venkataramani and Lyche59), in a series of six patients with chronic hepatitis C refractory to IFN therapy, showed that treatment with vitamin E inhibited important steps in hepatic fibrogenesis such as stellate cell activation and collagen-α1(I) gene expression, suggesting a potential role of this vitamin in preventing hepatic fibrosis, which is a critical step in the progression of HCV-related liver disease. However, no change in either serum ALT and HCV-RNA levels or histological degree of inflammation and fibrosis was observed throughout the study period.
In addition, a subsequent small double-blind, placebo-controlled trial of supplementation with antioxidant compounds such as vitamin C, vitamin E and Se did not produce any clinical effect in well-compensated patients with chronic hepatitis C and did not affect the erythrocyte activities of antioxidative enzymes or the plasma levels of oxidative markers(Reference Groenbaek, Friis and Hansen60).
In a more recent study, naive patients with chronic hepatitis C were divided into two groups: thirty received silybin-phospholipids and vitamin E complex for 3 months and ten did not receive any treatment(Reference Falasca, Ucciferri and Mancino61). The aim of this observational study was to evaluate the liver anti-inflammatory effect of this antioxidant compound. Treated patients only obtained a significant reduction in ALT levels, while the viral load did not significantly change. However, in the silybin-phospholipids and vitamin E complex group, a significant increase in serum IL-2 and a significant reduction in IL-6 were observed. This finding, suggesting a preferential shift towards the Th1 profile, led the authors to conclude that the silybin-phospholipids and vitamin E complex exert liver anti-inflammatory effects, even though data on liver histology were not reported.
In summary, there is no evidence for a direct anti-HCV effect by vitamin E or C. However, two studies(Reference von Herbay, Stahl and Niederau56, Reference Falasca, Ucciferri and Mancino61) described a transient improvement in liver necroinflammatory activity. This finding deserves further investigation to establish the potential of these vitamins as a supportive treatment in patients who cannot undergo standard therapy for HCV.
Discussion
The available clinical experiences on the use of vitamins to treat chronic viral hepatitis, either alone or in combination with IFN-based therapies, are inconsistent and hampered by major methodological drawbacks, such as small sample size, the lack of double-blinding or placebo controls, the heterogeneous features of patients and the use of surrogate endpoints. The different dosage regimens, treatment durations and endpoints also make it difficult to formulate a patient-relevant clinical message. Furthermore, a number of studies have reported the administration of multi-complex formulations, including more vitamins or vitamins in addition to other antioxidant and mineral compounds(Reference Böcher, Wallasch and Höhler55, Reference Look, Gerard and Rao57, Reference Idéo, Bellobuono and Tempini58, Reference Groenbaek, Friis and Hansen60, Reference Falasca, Ucciferri and Mancino61). This does not allow the role of a single vitamin to be established even when an appropriate randomisation has been performed.
Disappointingly, almost all the reviewed studies lack some critical information. With very few exceptions (Tables 1 and 2), no data on compliance with vitamin therapy were found, making it difficult to extrapolate the real effectiveness of experimental treatment. Similarly, data on side effects are not available or fairly elusive, providing a misleading message on a universal vitamin good safety profile. Indeed, vitamins have been demonstrated to induce a wide range of toxic effects albeit at higher dosages than those commonly used(Reference Harrison's62, Reference Goodman and Gilman's63).
Although the antiviral activity of vitamins in chronic hepatitis B or C is not demonstrated, some data seem to show that vitamin E alone or in combination with a silybin–phosphatidylcholine complex is able to reduce inflammation-induced oxidative stress and to restore the immune response. Thus, vitamin E could have a role in improving the necroinflammatory state once the principal aim of the treatment, that is viral eradication and/or viral suppression, cannot be achieved. On the other hand, viral eradication often does not prevent the progression of liver disease, mainly when obtained after a long course of inflammation leading to fibrosis. So, achieving a suppression of necroinflammatory activity could play a role in the prevention of the long-term sequelae of chronic viral infection.
Based on the studies reviewed here, no vitamin can be recommended for the treatment of chronic viral hepatitis.
Clinical trials must compare the therapeutic efficacy of vitamins, if any, to standard state-of-the-art treatment measured using hard endpoints as established by international guidelines(4, Reference Ghany, Strader and Thomas64). Future research directions should include well-designed randomised clinical studies evaluating whether vitamins in combination with standard antiviral treatment increase the current response rate, or whether they serve as a monotherapy for patients with contraindications or intolerance to standard therapy to decrease the incidence of cirrhosis and its complications.
Acknowledgements
There is no conflict of interest. There are no funding sources for the present study. The contribution of authors was as follows: S. F. conceived the study and coordinated the search activity of co-workers; F. C. screened the literature and contributed to writing the first draft of the manuscript; A. G. supervised the literature search analysis and wrote the final draft of the manuscript; E. L. contributed to writing the manuscript; C. C. contributed to writing the manuscript; R. D. D. contributed to part of the search analysis (HCV) by consulting the above-reported database; L. M. contributed to part of the search analysis (HBV) by consulting the above-reported database; S. G. contributed to the search analysis by consulting the above-reported database; A. C. contributed to the design of the review and commented on drafts of the manuscript; M. B. supervised and critically reviewed the manuscript; P. A. was responsible for the final approval of the manuscript.




