Gastric ulceration is a multifaceted disease with a complex, pluricausal aetiology, known to develop due to loss of balance between aggressive and protective factors(Reference Glavin and Szabo1). Aggressive factors include Helicobacter pylori, stress, smoking, alcohol consumption and non-steroidal anti-inflammatory drugs (NSAID). These factors increase the risk of gastric ulcer formation(Reference Glavin and Szabo1, Reference Singh, Kundu and Ganguly2). Naproxen, a representative of the NSAID family, causes gastric ulcers through various processes, including generation of reactive oxygen species (ROS), inhibition of PG synthesis, infiltration of polymorphonuclear leucocytes, induction of apoptosis and infiltration of lipid peroxidation(Reference Yoshikawa, Naito and Kishi3–Reference Fujii, Matsura and Kai5). Recent studies have indicated that gastric ulceration is associated with cleaving and remodelling of the extracellular matrix (ECM) by matrix metalloproteinases (MMP). MMP activate proteolytic cleavage of their prodomain comprised of a family of structurally related Zn-dependent endopeptidases(Reference Parks and Mecham6, Reference Lempinen, Inkinen and Wolff7). Several MMP are known as pathogenic factors for NSAID- and H. pylori-mediated gastric injury. The regulation of gastric inflammation by MMP is mediated through interplay with ECM proteins, growth factor receptors, cell adhesion molecules and cytokines(Reference Kundu, Mukhopadhyay and Patra8, Reference Crawford, Krishna and Israel9). MMP are divided into different subgroups, including collagenases, gelatinases, stromelysins, membrane-type MMP and others(Reference Parks and Mecham6). MMP are responsible for the physiological turnover of basement membrane proteins such as collagen IV, collagen I, gelatin, elastin and fibronectin(Reference Parks and Mecham6). The gelatinase family consists of matrix metalloproteinase-2 (MMP-2; 72 kDa) and matrix metalloproteinase-9 (MMP-9; 92 kDa). MMP-2 may participate in the physiological turnover of gastric ECM, whereas MMP-9 may be important in the early phase of gastric ulcer formation(Reference Lempinen, Inkinen and Wolff7).
The anthocyanin group of polyphenols is very important due to their demonstrated pharmacological activities. During the last decade, it has been shown that anthocyanins may have beneficial anti-inflammatory, antioxidant and chemoprotective properties that reduce the risks of CVD and cancers(Reference Middleton, Kandaswami and Theoharides10, Reference Jang, Cai and Udeani11).
In general, antioxidants act as radical scavengers to inhibit lipid peroxidation and other free radical-mediated processes which can be protective against gastric ulcers. The various antioxidants protect cells and tissues from the deleterious effects of ROS. This gastroprotective activity has been attributed to scavenging of various ROS(Reference Lempinen, Inkinen and Wolff7, Reference Swarnakar, Ganguly and Kundu12, Reference Ganguly, Maity and Reiter13).
The present study was undertaken to investigate the potential anti-ulcer effects of anthocyanins from black rice in an in vivo study by determining their effect on activation of MMP-2, via down-regulation of ROS. We found that anthocyanins regulated MMP-2 expression by blocking ROS generation, suggesting that anthocyanins from black rice may provide protection against naproxen-induced gastric ulceration through this mechanism.
Materials and methods
Extraction and isolation of anthocyanins from black rice bran
Milled black rice bran (5 g; Heugjinjubyeo) kindly provided by Dr Tae Young Kim (The Rural Development Administration of Korea, Suwon, Korea) was soaked in 500 ml ethanol containing 0·1 % trifluoroacetic acid for 24 h. The ethanol extract was pre-filtered through Whatman grade no. 4 filter paper and evaporated using a vacuum rotary evaporator set at 30°C. For further purification, the ethanol extract was dissolved in 100 ml purified water and applied to a column packed with Amberlite XAD-7 resin(Reference Ryu, Park and Kang14–Reference Park, Kim and Chang16).
The isolated anthocyanin extract was used for subsequent studies. The anthocyanin extract was analysed using HPLC apparatus (Waters, Milford, MA, USA) connected to a Millennium 32 data-processing computer. The column (250 mm × 4·6 mm) was a Luna 5μ C18(2) 100A (Phenomenex Inc., Torrance, CA, USA). The mobile phase flow rate was fixed at 1·0 ml/min. The detection wavelength was 520 nm. We used two elution solvents: A (water containing 0·5 % trifluoroacetic acid) and B (acetonitrile (MeCN) containing 0·5 % trifluoroacetic acid)(Reference Sander, Chang and Hamm15, Reference Park, Kim and Chang16). Our previous data identified the compounds as cyanidin 3-O-glucose (95 %) and peonidin 3-glucoside (5 %). These were also confirmed by cyanidin 3-O-glucoside (DaeHan R&D, Seoul, Korea)(Reference Park, Kim and Chang16).
Cell culture and assessment of cellular oxidative stress
AGS cells were maintained in Roswell Park Memorial Institute (RPMI)-1640 (Welgene, Seoul, Korea) supplemented with 10 % fetal bovine serum. Cellular oxidative stress was assessed using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Sigma-Aldrich, St Louis, MO, USA) cell-permeable fluorescent probes with fluorescent-activated cell sorting (FACS) analysis. For H2DCFDA, cells were treated with anthocyanins (25, 50 μg/ml) for the indicated time, the medium was aspirated, and the cells were then incubated in PBS containing a 6 μm probe at 37°C for 30 min. The probe was then removed, and warm medium was added back to the cells for 10 min. The cells were collected and re-suspended in cold PBS. All samples were analysed on a FACScan flow cytometer (Beckton, Dickinson and Co., Franklin Lakes, NJ, USA) with a laser excitation wavelength of 488 nm.
Experimental animals
Female 7-week-old Sprague–Dawley rats (180–220 g) were purchased from Orient Bio (Gyeonggi-do, Korea). Rats were bred in-house with free access to a standard laboratory diet and water for all experiments. All rats were subjected to alternating 12 h light–12 h dark cycles and housed at 25°C. Procedures involving animals were in accordance with the Guide for Experimental Animal Research from Institutional Animal Care and Use Committee at Korea University. Animals of all groups were fasted overnight with free access to water before each experiment.
Evaluation of effects of anthocyanins on gastric ulceration
To evaluate the potential protective effects of the anthocyanin extracts, the rats were divided into five groups (five rats per group). The untreated normal rats received only vehicle for 3 d, in comparable volume by the oral route. The control rats received only 80 mg naproxen/kg twice daily (at 09.00 and 21.00 hours) for 3 d. Each of the remaining three groups was treated with 80 mg naproxen/kg twice daily (at 09.00 and 21.00 hours) for 3 d after pretreatment with 5, 25 or 50 mg anthocyanins/kg body weight twice daily (at 09.00 hours and 21.00 hours) for 3 d(Reference Kim, Kim and Song17). All the rats were killed 12 h after the last oral administration of naproxen. The rat stomachs were promptly excised, weighed and washed with PBS. The mucosa was homogenised in 50 mm-potassium phosphate buffer at pH 7·5. Mitochondria and cytosolic fractions were prepared according to a previously described method(Reference Kim, Kim and Song17, Reference Hogeboom, Colowick and Kaplan18). The quantitative analysis of protein was measured by a Pro-Measure™ kit (iNtRON Biotechnology, Gyeonggi-do, Korea).
Measurement of lipid peroxidation
Lipid peroxidation was assayed by measuring malondialdehyde. Rat stomach tissue was homogenised and combined with 0·2 ml of 8·1 % SDS, 1·5 ml of 20 % acetic acid and 1·5 ml of 0·8 % thiobarbituric acid. The reaction mixture was incubated at 95°C for 1 h and added to 5 ml of a mixture of n-butanol and pyridine. The absorbance of the resulting supernatant fraction was measured at 532 nm. Lipid peroxidation was calculated from a standard malondialdehyde tetrabutylammonium salt curve and expressed as nmol malondialdehyde per g tissue(Reference Ohkawa, Ohishi and Yaki19).
Measurement of superoxide dismutase activity
The activity of superoxide dismutase (SOD) in the gastric mucosa of the rats was determined according to a previously described method(Reference McCord and Fridovich20). Standard assays were performed in 3 ml of 50 mm-potassium phosphate buffer (pH 7·8) containing 0·1 mm-EDTA in a cuvette at 25°C. The reaction mixture contained 0·1 mm-ferricytochrome c, 0·1 mm-xanthine and sufficient xanthine oxidase to produce a ferricytochrome c reduction rate of 0·025 absorbance units per min at 550 nm. Tissue homogenates were mixed with the reaction mixture and kinetic spectrophotometric analysis was initiated by adding xanthine oxidase at 550 nm. Under these conditions, the amount of SOD required to inhibit the reduction rate of cytochrome c by 50 % was defined as one unit of activity.
Measurement of catalase activity
The activity of catalase in the gastric mucosa of the rats was determined as previously described(Reference Aebi and Bergmeyer21). Standard assays were performed in 3 ml of 50 mm-potassium phosphate buffer at pH 7·0 (1·9 ml) containing 10 mm-H2O2 (1 ml) and tissue homogenate (100 μl). Under these conditions, the amount of catalase required to decompose 1·0 μmol H2O2 in 1 min at pH 7·0 and 25°C was defined as one unit of activity.
Measurement of glutathione peroxidase activity
The activity of glutathione peroxidase in the gastric mucosa of the rats was determined using a previously described method(Reference Lawrence and Burk22) with some modifications. The reaction mixture consisted of glutathione peroxidase assay buffer and NADPH assay reagent. A sample of the supernatant fraction fluid mixed with the homogenate solution and 50 mm-potassium phosphate buffer (pH 7·5) was prepared by centrifuging at 1000 g for 10 min at 4°C. Glutathione peroxidase assay buffer (900 μl), 50 μl of NADPH assay reagent, and 50 μl of sample were added to the cuvette and mixed by inversion. The reaction was initiated by adding 10 μl of 30 mm-tert-butyl hydroperoxide or 80 % cumene hydroperoxide. Absorbance was measured at 340 nm. The enzymic activity was the sum of the activity measured using tert-butyl hydroperoxide and cumene hydroperoxide. One unit of glutathione peroxidase will cause the formation of 1 μmol NADP+ from NADPH per min at pH 7·5 at 25°C.
Histopathology
Stomach tissues were fixed in 10 % neutral formalin and embedded in paraffin, and 5 μm thick sections were prepared and stained with haematoxylin and eosin using standard procedures(Reference Kim, Kim and Song17).
Tissue extraction and gelatin zymography
The stomach of each rat was washed with PBS, minced and incubated for 10 min at 4°C. After centrifugation at 12 000 g for 15 min, the supernatant fraction was saved. The pellet was extracted in lysis buffer (10 mm-2-amino-2-hydroxymethyl-propane-1,3-diol (Tris)-HCl, pH 8·0, 150 mm-NaCl, 1 % Triton X-100, and protease inhibitors) and centrifuged at 12 000 g for 15 min to obtain extracts. Tissue extracts were preserved at − 80°C until analysed. For assay of MMP-2 and MMP-9 activities, extracts were electrophoresed using 8 % SDS-PAGE containing 1 mg gelatin/ml under non-reducing conditions. The gels were washed in 2·5 % Triton X-100 and incubated in TNC buffer (50 mm-Tris-HCl, pH 7·4, 0·2 m-NaCl, 10 mm-CaCl2, 0·05 % Brij35, 0·02 % NaN3, and 2 % dimethyl sulfoxide) for 24 h at 37°C and stained with 0·1 % Coomassie blue followed by destaining(Reference Ganguly, Maity and Reiter13, Reference Kleiner and Stetler-Stevenson23). The activity of MMP-2 and MMP-9 were determined by scanning bands using a scanner (HP Officejet Pro L7380; Hewlett-Packard, Palo Alto, CA, USA) connected to a computer running ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Western blotting
The stomach of each rat was washed with PBS, and the tissue was minced and extracted in radio-immunoprecipitation assay (RIPA) buffer (50 mm-Tris-HCl, pH 7·5, 150 mm-NaCl, 1 % NP-40, 0·5 % deoxycholic acid, 0·1 % SDS and protease inhibitors) and then centrifuged at 12 000 g for 20 min. Protein concentration was measured by using a Pro-Measure™ kit (iNtRON Biotechnology). Proteins were resolved by 8 % SDS-PAGE and transferred to a nitrocellulose membrane(Reference Swarnakar, Ganguly and Kundu12). The membrane was blocked in 5 % non-fat dry milk solution in 20 mm-Tris-HCl, pH 7·4, 150 mm-NaCl and 0·1% Tween 20 (TBST) for 1 h at room temperature, and then incubated at 4°C in a 1:1000 dilution of MMP-2 antibody in TBST overnight. Each membrane was then washed with TBST and incubated with horseradish peroxidase-conjugated secondary antibody. Bands were visualised using an enhanced chemiluminescence detection system (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The activity was determined by scanning bands using a scanner (HP Officejet Pro L7380; Hewlett-Packard) connected to a computer running ImageJ software (National Institutes of Health).
Reverse transcription-PCR
Total cellular RNA was isolated from ulcer stomach tissues by RNA Iso-plus reagent (TaKaRa Bio, Shiga, Japan) according to the manufacturer's protocol and quantified by measuring the absorbance at 260 nm. Complementary DNA was synthesised using Maloney murine leukaemia virus RT and Taq polymerase with 1 μg of total RNA and 10 pmol of oligo (dT)15 primer. PCR amplification of the cDNA was performed using a thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The sequences of PCR primers were as follows: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense (5′-TGG GGT GAT GCT GGT GCT GAG-3′) and antisense (5′-GTT TTC TCC AGG CGG CAT GTC-3′); MMP2 sense (5′-ACG GCT TCC TCT GGT GTT-3′) and antisense (5′-CGT AGT TGG TTG TGG TTG C-3′); TNF-alpha (5′-CGC CCA GCC TTC CTT ACG GAA C-3′) and antisense (5′-GGC GAT TAC AGT CAC GGC TCC C-3′); IL-1beta sense (5′-GCT ACC TAT GTC TTG AAG AGA ACC-3′) and antisense (5′-GAC CAT TGC TGT TTC CTA GG-3′). After amplification, the products were separated on 1·5 % (w/v) agarose gels and stained with ethidium bromide. The expression was determined by scanning bands using Gel-Doc (Bio-Rad) connected to a computer running Quantity One® software (Bio-Rad).
Statistical analysis
Each experiment was repeated at least three times. Data are plotted as mean values and standard deviations. The statistical significance of the assay was evaluated using SPSS (SPSS, Inc., Chicago, IL, USA). The value of P < 0·05 was considered significant.
Results
Anthocyanins reduce intracellular oxidative stress
H2DCFDA was used to detect ROS production in cells. The fluorescence of this cell-permeable agent significantly increases after oxidation. Within the cell, esterase cleaves H2DCFDA to release H2DCFDA (non-fluorescent moiety), which is converted to a fluorescent product (2′,7′-dichlorodihydrofluorescein) when exposed to ROS. So, a fluorometric-based fluorescent-activated cell sorting (FACS) assay was used to examine if anthocyanins might reduce cellular oxidative stress.
The ROS level was increased by treatment of naproxen at 500 μg/ml (Fig. 1(b)), while it was decreased by treatment with anthocyanins. In particular, pretreatment with anthocyanins at 25 and 50 μg/ml significantly inhibited the generation of ROS in AGS cells, that is, decreased intracellular radical formation bxy 1·7 and 3·75 times, respectively (Fig. 1(c), (d)).

Fig. 1 Effect of anthocyanins on intracellular reactive oxygen species (ROS). Representative fluorescent-activated cell sorting (FACS) data showing that anthocyanins shift the fluorescence intensity of a ROS indicator (2′,7′-dichlorofluorescein diacetate) in AGS cells. A rightward shift in the peak of the signal indicates increased ROS production. (a) Normal cells; (b) cells treated with naproxen at 500 μg/ml; (c) cells treated with anthocyanins at 25 μg/ml; (d) cells treated with anthocyanins at 50 μg/ml; (e) relative inhibition of ROS in the four groups. M1, mean fluorescence intensity; FL1-H, channel name on FACS. Values are means (n 3), with standard deviations represented by vertical bars. Mean value was significantly different from that of the normal group: * P < 0·05, ** P < 0·01 (Student's t test).
Effect of anthocyanins on naproxen-induced gastric antral ulceration in rats
Superficial or deep erosions, bleeding and antral ulcers were observed in rats that received 80 mg/kg doses of naproxen for 3 d. Also, infiltration of inflammatory cells into the sub-mucosa was also detected in gastric tissues (Fig. 2(b)) as compared with untreated normal rats (Fig. 2(a)). However, pretreatment with 50 mg anthocyanins/kg for 3 d reduced the depth and severity of naproxen-induced gastric antral ulcers. No significant infiltration of inflammatory cells in the epithelial layer along with the continuous mucosal and sub-mucosal layer was observed in the pretreatment group (Fig. 2(c)).

Fig. 2 Protective effect of anthocyanins on naproxen-induced gastric ulcers in rats. Histological sections were stained with haematoxylin and eosin and photographs were taken at 40 × , respectively. (a) Normal gastric antrum from vehicle-treated normal rats. (b) Gastric antral ulcer in the naproxen-treated rats. (c) Gastric antrum in the anthocyanin-pretreated rats. Pretreatment with 50 mg anthocyanins/kg for 3 d reduced inflammation and gastric ulceration. Gastric mucosal epithelium (![]() ), muscularis mucosae ( ↑ ), infiltration of inflammatory cytokine (
), muscularis mucosae ( ↑ ), infiltration of inflammatory cytokine (![]() ) and disruption of gastric mucosa (*) are indicated.
) and disruption of gastric mucosa (*) are indicated.
Evaluation of anthocyanins on oxidative damage in naproxen-induced gastric ulcers
A major cause of gastric injury is ROS damage and NSAID-mediated gastric ulceration that generates oxidative damage by increasing lipid peroxidation and glutathione depletion. To determine whether oxidative damage was induced by naproxen, lipid peroxidation was evaluated by enzymic methods. The concentration of malondialdehyde in control rats (naproxen, 80 mg/kg) was elevated to 282·11 nmol/g tissue whereas the concentration of malondialdehyde in the untreated normal rats remained at 148·77 nmol/g tissue. This increase in the concentration of malondialdehyde was reduced in a dose-dependent manner in all anthocyanin-pretreated rats (Fig. 3). The activity of SOD in the control rats reduced to 4·36 U/mg protein, whereas activity of SOD in the untreated normal group was 10·04 U/mg protein. Pretreatment with anthocyanin extracts increased SOD activity in a dose-dependent manner (Fig. 4(a)). Likewise, the activity of catalase in the control rats was 5·29 U/mg protein, which was much lower than that in the untreated normal rats (36·08 U/mg protein). Pretreatment with anthocyanins increased the activity of catalase compared with that in the control rats in a dose-dependent manner (Fig. 4(b)). The activity of glutathione peroxidase in control rats was reduced to 5·18 U/mg protein, whereas the activity of glutathione peroxidase in the untreated normal rats remained at 12·15 U/mg protein. Pretreatment with the anthocyanin extracts increased glutathione peroxidase activity dose-dependently (Fig. 4(c)).
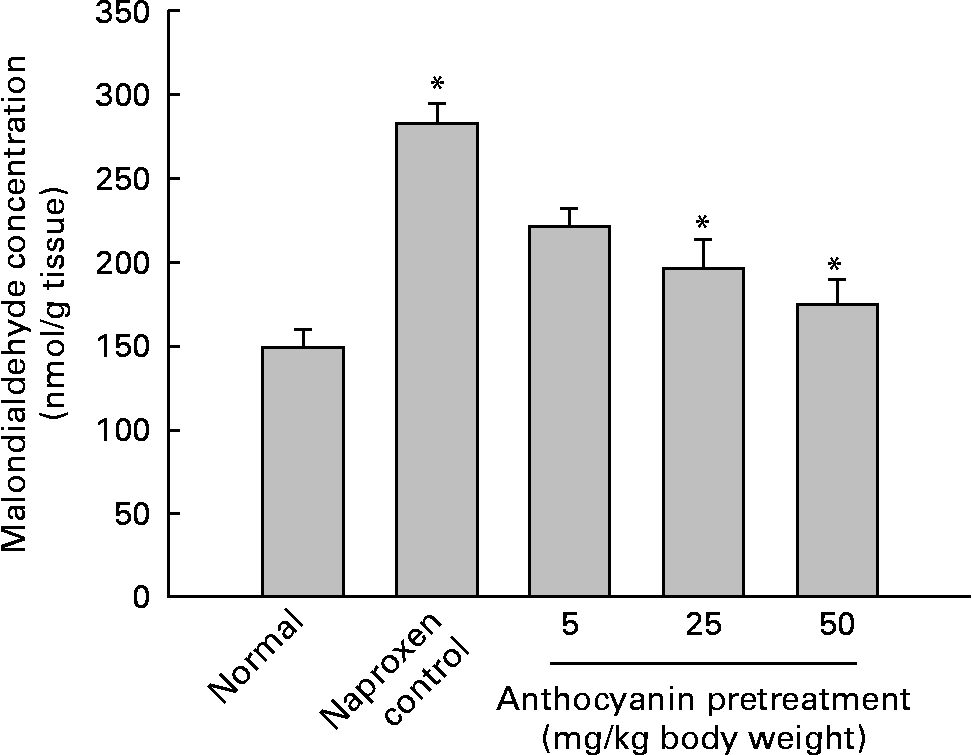
Fig. 3 Effect of anthocyanins on malondialdehyde concentration in naproxen-induced gastric ulceration. The normal group received only vehicle (no treatment). The control rats received only 80 mg naproxen/kg twice daily for 3 d. The other groups of rats were pretreated with anthocyanins (5, 25 or 50 mg/kg body weight) twice daily for 3 d and then treated with naproxen (80 mg/kg body weight) twice daily for 3 d. Values are means (n 5), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the normal group (P < 0·05; Student's t test).
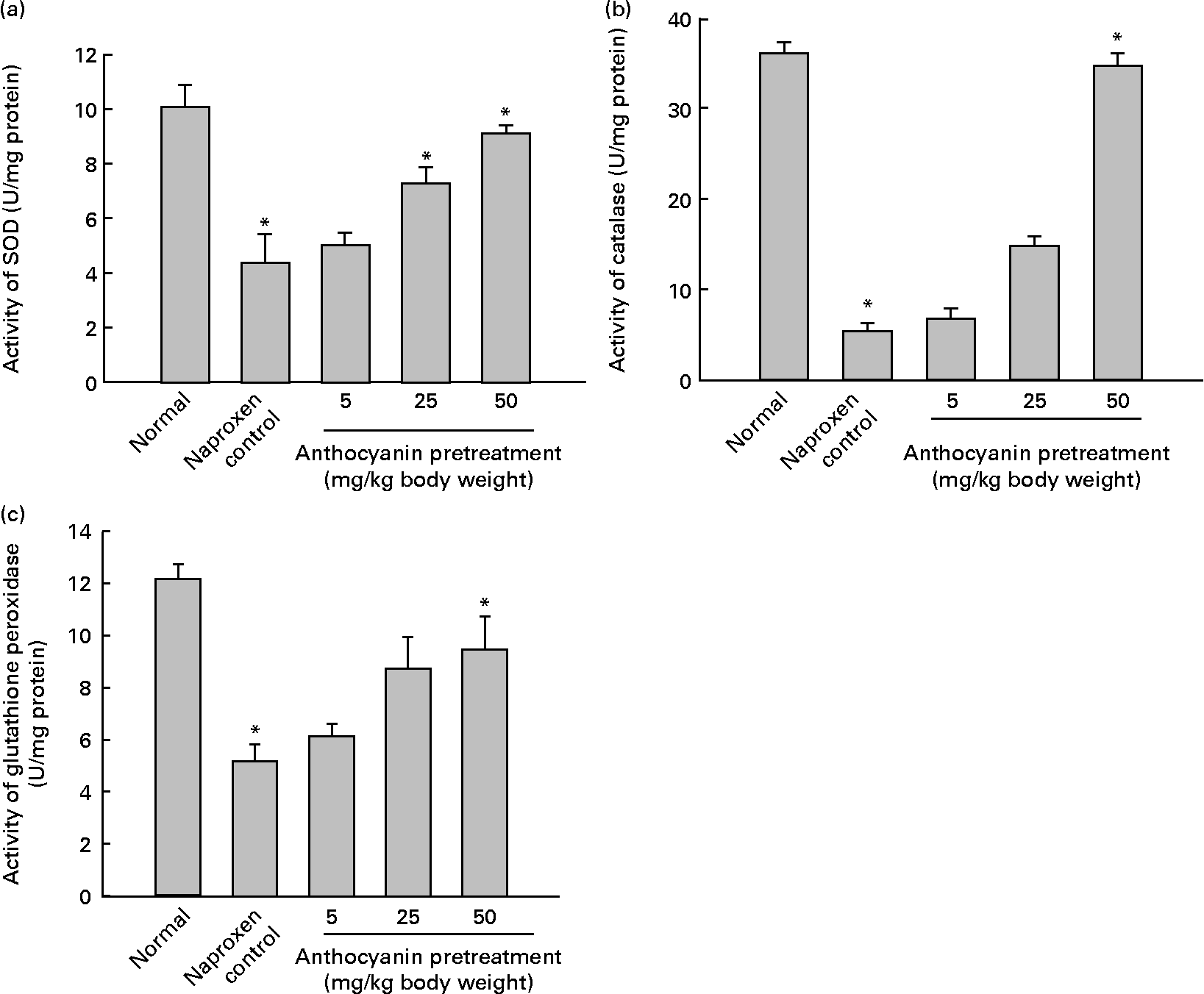
Fig. 4 Effect of anthocyanins on antioxidant enzyme activity on naproxen-induced gastric ulceration. The normal group received only vehicle (no treatment). The control rats received only 80 mg naproxen/kg twice daily for 3 d. The other groups of rats were pretreated with anthocyanins (5, 25 or 50 mg/kg body weight) twice daily for 3 d and then treated with naproxen (80 mg/kg body weight) twice daily for 3 d. Antioxidant enzymes are (a) superoxide dismutase (SOD), (b) catalase and (c) glutathione peroxidase. For definitions of units of enzyme activity, see the Materials and methods section. Values are means (n 5), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the normal group (P < 0·05; Student's t test).
These results suggest that pretreatment with anthocyanins reduced naproxen-induced gastric ulceration and removed the lipid peroxides induced by naproxen. Pretreatment with anthocyanins also resulted in a significant increase of the activities of SOD, catalase and glutathione peroxidase.
Evaluation of matrix metalloproteinase-2 and matrix metalloproteinase-9 activity in gastric ulcers
To obtain further insight into the mechanisms underlying the ROS-mediated induction of MMP-2 and MMP-9 activity, different doses of naproxen were administered in order to induce ulcer development. Ulcer index zymography indicated that the gelatinolytic activity of induced MMP-9 was gradually increased by naproxen in a dose-dependent manner. Notably, MMP-9 was detected at 80 mg/kg (Fig. 5). Meanwhile, in the case of MMP-2, a decrease in gelatinolytic activity was evident at 50 and 80 mg/kg (Fig. 5), and similarly, MMP-2 expression correlated with decreasing doses of naproxen as shown in Western blot analysis (Fig. 6(a)). The changes in the activity of MMP-9 and MMP-2 observed correlated with an increase in ulcers with naproxen in a dose-dependent manner.

Fig. 5 Effect of varying doses of naproxen on gastric tissues and associated matrix metalloproteinase-9 (MMP-9; ![]() ) and matrix metalloproteinase-2 (MMP-2;
) and matrix metalloproteinase-2 (MMP-2; ![]() ) activities. Different concentrations of naproxen were orally administration to each group of rats to induce gastric ulcers. Values are means (n 5), with standard deviations represented by vertical bars. Mean value was significantly different from that of the normal group: * P < 0·05, ** P < 0·01 (Student's t test).
) activities. Different concentrations of naproxen were orally administration to each group of rats to induce gastric ulcers. Values are means (n 5), with standard deviations represented by vertical bars. Mean value was significantly different from that of the normal group: * P < 0·05, ** P < 0·01 (Student's t test).

Fig. 6 Relationship of naproxen-induced ulceration and protective effect of anthocyanins with matrix metalloproteinase-2 (MMP-2) activity. (a) Gastric ulceration was induced in rats by oral administration of different concentration of naproxen. (b) The rats were pretreated with anthocyanins (5, 25 or 50 mg/kg body weight) twice daily for 3 d and then treated with naproxen (80 mg/kg body weight) twice daily for 3 d. Western blotting was performed using 30 μg protein extracted from gastric ulcer tissues of each group of rats and antibody with monoclonal anti-MMP-2. Values are means (n 5), with standard deviations represented by vertical bars. Mean value was significantly different from that of the normal group: * P < 0·05, ** P < 0·01 (Student's t test).
Anthocyanins associated with matrix metalloproteinase-2 activity in rats with naproxen-induced gastric ulcers
To investigate the effect of different concentrations of anthocyanins on MMP-2 activity, rats were pretreated with doses of 5, 25 or 50 mg/kg. Naproxen induced disruption of the gastric mucosal layer and down-regulation of MMP-2 activity. On the other hand, pretreatment with anthocyanins dose-dependently blocked naproxen-induced gastric ulcer formation through the induction of MMP-2 activity (Fig. 6). To substantiate the role of naproxen in the expression of MMP-2, protein extracts of ulcerated stomach tissue were evaluated using Western blotting. The up-regulation of MMP-2 was confirmed and determined to be due to attenuation of MMP-2 at the protein level as confirmed by Western blotting (Fig. 6). To evaluate the relationship between ROS damage and the expression of MMP-2 genes, cDNA from normal tissue, tissue treated with naproxen and tissue pretreated with anthocyanins was assessed with RT-PCR (Fig. 7). As expected, anthocyanin pretreatment blocked the suppression of MMP-2 gene expression with naproxen. These results clearly suggest that naproxen-induced gastric ulcers showed decreases in MMP-2 activity and anthocyanins reduced MMP-2 activity while protecting against gastric damage.

Fig. 7 Effect of anthocyanins on expression of matrix metalloproteinase-2 (MMP-2) and pro-inflammatory cytokines. The normal group received only vehicle (no treatment). The control rats received only 80 mg naproxen/kg twice daily for 3 d. The other groups of rats were treated with anthocyanins (5, 25 or 50 mg/kg of body weight) twice daily for 3 d and then treated with naproxen (80 mg/kg body weight) twice daily for 3 d. Total RNA was extracted from each group of rats and used for RT-PCR for expression of MMP-2, TNF-α, IL-1β and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. (![]() ), MMP-2:GAPDH; (
), MMP-2:GAPDH; (![]() ), TNF-α:GAPDH; (
), TNF-α:GAPDH; (![]() ), IL-1β:GAPDH. Values are means (n 5), with standard deviations represented by vertical bars. Mean value was significantly different from that of the normal group: * P < 0·05, ** P < 0·01 (Student's t test).
), IL-1β:GAPDH. Values are means (n 5), with standard deviations represented by vertical bars. Mean value was significantly different from that of the normal group: * P < 0·05, ** P < 0·01 (Student's t test).
Comparison of anthocyanins and other antioxidant substances on regulation of matrix metalloproteinase-2 activity in gastric ulcers
Naproxen-induced ulceration is related to cellular injury as well as inflammation and in turn suppression of MMP-2. With this in mind, we compared anthocyanins and ascorbic acid (50 mg/kg body weight; vitamin C) in gastric ulcers. Western blotting (Fig. 8) showed that the activity of MMP-2 was decreased by naproxen compared with normal. On the other hand, pretreatment with anthocyanins increased the activity of MMP-2 in gastric ulcers. It is well known that ascorbic acid has a strong antioxidant effect, so we hypothesised that ascorbic acid would increase the activity of MMP-2 in gastric ulcers. However, we found that ascorbic acid down-regulated the activity of MMP-2 compared with normal.

Fig. 8 Comparison of anthocyanins with another antioxidant (ascorbic acid; AA) on matrix metalloproteinase-2 (MMP-2) activity for the prevention of gastric ulcers. The normal group received only vehicle (no treatment). The control rats received only 80 mg naproxen/kg twice daily for 3 d. The other groups of rats were treated with anthocyanins (5, 25 or 50 mg/kg body weight) twice daily for 3 d and then treated with naproxen (80 mg/kg body weight) twice daily for 3 d. Western blotting was performed using 30 μg protein extracted from gastric ulcer tissues of each group of rats and antibody with monoclonal anti-MMP-2. Values are means (n 5), with standard deviations represented by vertical bars. Mean value was significantly different from that of the normal group: * P < 0·05 (Student's t test).
Inhibition of pro-inflammatory cytokine gene expression by anthocyanin pretreatment during blocking of naproxen-induced gastric ulcers
A recent study reported that NSAID induce erosions and ulcers through multifaceted processes, such as induced expression of pro-inflammatory cytokines(Reference Ganguly, Maity and Reiter13). The relative mRNA expressions were detected by RT-PCR (Fig. 7). It was shown that naproxen induced mRNA expression of TNF-α and IL-1β. On the other hand, pretreatment with anthocyanins decreased the expression of TNF-α and IL-1β in a dose-dependent manner in gastric ulcers. Attenuation of TNF-α and IL-1β expression by anthocyanins suggests that inhibition of secretion of pro-inflammatory cytokines to control level by anthocyanins mediates protection against gastric ulceration.
Discussion
This is the first report that we are aware of concerning the effect of anthocyanins from black rice on oxidative gastric damage caused by naproxen. Several researchers have reported that anthocyanins have antioxidant and free radical-scavenging activities. Moreover, we have previously shown that anthocyanins induced 2,2-diphenyl-1-picrylhydrazyl free radical scavenging and reduced superoxide anion radicals and H2O2(Reference Park, Kim and Chang16). We confirmed that anthocyanins have antioxidant effects via strong radical-scavenging activity and down-regulation of ROS (Fig. 1). ROS such as H2O2, and the superoxide and hydroxyl radicals, have been implicated in the regulation of many important cellular events. Excessive production of ROS gives rise to events that lead to the death of several types of cells(Reference Tanaka, Nakanishi and Ogawa24). Therefore these results suggest that the reduction of ROS by anthocyanins does not play a significant role in the ROS-mediated cellular damage response.
In a recent study, administration of NSAID such as aspirin and indomethacin was shown to cause damage in the stomach(Reference Bjarnason, Zanelli and Smith25). Also, NSAID non-selectively inhibit the cyclo-oxygenase inhibitor, through suppression of PGE synthesis and increased expression of pro-inflammatory cytokines such as IL-1 and TNF-α to cause gastric ulcers(Reference Swarnakar, Ganguly and Kundu12, Reference Konturek, Brzozowski and Stachura26, Reference Wallace27). Our previous studies documented that naproxen causes the up-regulation of lipid peroxidation and down-regulation of the radical-scavenging enzymes, such as SOD, catalase and glutathione peroxidase in conjunction with oxidative gastric mucosal damage(Reference Kim, Kim and Song17).
MMP, a family of Zn-dependent endopeptidases, have recently gained considerable attention partly because of their critical role in ECM remodelling during gastric mucosal damage(Reference Kundu, Mukhopadhyay and Patra8). The regulation of the activities of MMP at the gene and protein levels may be involved in some of the mechanisms of gastric damage and remodelling of the ECM during ulceration. An indirect role of NSAID via ROS-mediated MMP gene expression has been demonstrated(Reference Nelson and Melendez28). The present study in rats focused on the association of gastric ulcer damage by ROS and MMP activity. Naproxen at a dose of 80 mg/kg maximally induced gastric ulceration, as measured by histology (haematoxylin and eosin staining). Histological study of the stomach tissue indicated that naproxen induced disruption of the mucosal layer of the stomach. No damage to the mucosal layer was evident in rats pretreated with anthocyanins before naproxen exposure. Additionally, naproxen increased lipid peroxidation and decreased radical-scavenging enzymes. These results indicate that oxidative stress generated by naproxen is associated with the production of ROS and lipid peroxidation, which disrupts epithelial cells and the mucosal layer. Because increased lipid peroxidation is the major indication of ROS generation in gastric mucosa, the possibility that ROS generation plays a role in the regulation of MMP and the effect of anthocyanins is an interesting aspect for investigation(Reference Swarnakar, Ganguly and Kundu12). The potent anti-inflammatory and antioxidant properties of anthocyanins are thought to exert protective effects against ulceration. Our data show that anthocyanins have a protective effect against gastric ulcers by preventing oxidative damage caused by lipid peroxidation. In our opinion, these changes relate to an enormous shift in the level of MMP synthesis and secretion during ulceration. The results confirm that MMP-9 and MMP-2 expression are related to gastric ulceration. These data are consistent with other studies that have documented the involvement of MMP in gastric damage in rats(Reference Baragi, Qiu and Gunija-Smith29). In the case of induced acetic acid damage in the rat, gelatin zymography revealed that levels of gelatinase B (MMP-9) were greatly increased with gastric damage and declined during healing, whereas gelatinase A (MMP-2) levels remained constant(Reference Baragi, Qiu and Gunija-Smith29). Changes in MMP activities by treatment with naproxen appear to be important in ulceration via degradation of gastric ECM.
Another interesting finding is that during gastric ulcer development, an altered activation of MMP-2 in naproxen-treated rats was evident. This may account for the associated oxidative damage with the activation of MMP-2 upon naproxen-induced gastric ulceration in rats. Otherwise, protection from naproxen-induced ulcer formation was accelerated by anthocyanins through radical-scavenging enzymes and an MMP-2-dependent process. Our data show that anthocyanins not only decreased lipid peroxidation but also increased the activity of the radical-scavenging enzymes. Similarly, anthocyanins increased MMP-2 activity.
Also, we investigated whether anthocyanins prevent gastric ulcers induced by naproxen via their anti-inflammatory activity. The clinical outcome of naproxen-induced gastric ulcer is likely to be determined by ROS production, acid secretion, cytokine expression and cellular factors(Reference Pihan, Regillo and Szabo30). Ulceration led to the induction of gene expression levels of pro-inflammatory cytokines such as TNF-α and IL-1β, which were decreased by pretreatment with anthocyanins. There was also down-regulation of the inflammatory responses mediated by anthocyanins through attenuation of the expression and release of pro-inflammatory cytokines, thus exhibiting anti-ulcer effects.
In summary, the ulcerogenic response to naproxen in the stomach was markedly aggravated in ulcerated rats. Notably, gastric ulcers developed more rapidly after naproxen treatment than in untreated normal rats. However, anthocyanins inhibited the development of gastric ulcers in naproxen-treated rats. These findings may be accounted for by the protection from ROS damage in the stomach through blocking of the generation of ROS, inhibition of lipid peroxidation, and increased radical-scavenging enzymes. Likewise, anthocyanins inhibited the expression of pro-inflammatory cytokines and MMP-2 expression was elevated in rats. Thus, when using NSAID to treat patients with gastric ulcers, one should consider mucosal damage in the stomach.
Acknowledgements
The present study was supported by The Korea University Grant.
S.-J. K. was involved in study design, data interpretation and manuscript writing; Y. S. P. performed laboratory measurements of malondialdehyde, SOD, catalase and glutathione peroxidase; H.-D. P. was the coordinator of animal care; H. I. C. was involved in study design, data interpretation and manuscript editing.
There are no conflicts of interest.










