Obesity has reached epidemic proportions, due in large part to the adoption of a lifestyle with increased energy intake and reduced physical activity( 1 , Reference Block, Rosenberger and Patterson 2 ). The incidence of obesity during pregnancy has increased from 70 to 100 % during the last decade, which is reflected by almost every maternal complication during pregnancy and increases the odds of short-term and long-term disturbances in fetal health, such as congenital abnormalities, inadequate size and weight, and risk of obesity and the metabolic syndrome( Reference Guelinckx, Devlieger and Beckers 3 – Reference Heerwagen, Miller and Barbour 8 ). This evidence points to the existence of an effect of maternal obesity across generations, suggesting that the maternal environment during the prenatal period appears to programme for the development of obesity and the metabolic syndrome in the offspring during childhood and adulthood( Reference Desai, Beall and Ross 9 – Reference Stocker, Arch and Cawthorne 14 ). Some studies have suggested that resistance to insulin and leptin observed in mothers may modulate fetal metabolic programming( Reference McMillen, Muhlhausler and Duffield 15 – Reference Catalano, Kirwan and Haugel-de Mouzon 19 ); however, the mechanisms whereby maternal obesity affects offspring health are still unclear.
Another question that remains is whether maternal obesity alone can influence the genesis of obesity in offspring until adulthood or whether persistence of obesity into adulthood depends on the offspring following their obese mothers' dietary patterns. Some studies have found evidence of intra-uterine programming of obesity regardless of the offspring diet( Reference Howie, Sloboda and Kamal 5 , Reference Bayol, Farrington and Stickland 20 – Reference White, Purpera and Morrison 22 ). However, others have suggested that excessive weight and its consequent complications, such as the metabolic syndrome, occur only when the offspring of obese mothers are exposed to excessive dietary energy intake( Reference Akyol, McMullen and Langley-Evans 23 , Reference Tamashiro, Terrillion and Hyun 24 ).
In addition to seeking evidence on the harm caused by maternal obesity to some parameters related to the metabolism of male offspring, the present study sought to investigate whether an interaction exists between obesity in mothers and obesity in their offspring. Hence, the aim of the present study was to test the hypotheses that induced maternal obesity has negative effects on offspring metabolism and that the offspring of obese mothers that are also subjected to the induction of obesity exhibit even greater negative effects on this outcome.
Experimental methods
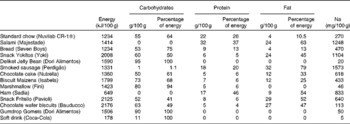
The present study was carried out on Wistar rats obtained from the Centre for Laboratory Animal Reproduction and Experimentation of the Federal University of Rio Grande do Sul (UFRGS), Porto Alegre, Brazil. All the experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the United States National Institutes of Health and were approved by the Animal Research Ethics Committee of UFRGS. The research project was approved with Committee judgement no. 21 224. Female rats (n 23) aged 21 d (average weight 45·79 (sem 0·9581) g) were kept in vivaria under controlled temperature (20–24°C) and luminosity (12 h light–12 h dark cycle) conditions. The rats were randomly divided into two treatment groups: CON, receiving only standard chow (Nuvilab CR-1®) and water ad libitum from their own weaning to the weaning of their offspring, and CAF, receiving standard chow, water and other items from a cafeteria diet (Table 1) ad libitum from their own weaning to the weaning of their offspring.
Table 1 Nutritional composition of food items in the cafeteria diet
At 120 d of age, checking of the oestrous cycle by vaginal smears began. Material was collected with the aid of a dropper containing 70 % saline. The vaginal epithelium of the female rats was rinsed with this solution, and the material thus collected was immediately analysed by optical microscopy to determine the current phase of the oestrous cycle. Female rats in the pro-oestrus phase that exhibited signs of sexual receptivity were mated with a control male rat. Female rats were considered possibly pregnant if spermatozoa were found in the vaginal canal the following morning. After birth and weaning, male offspring were divided into four groups depending on the maternal and offspring diets (Fig. 1): CON-CON (n 16), offspring of CON dams fed the control diet after weaning; CON-CAF (n 16), offspring of CON dams fed the cafeteria diet after weaning; CAF-CON (n 21), offspring of CAF dams fed the control diet after weaning; CAF-CAF (n 18), offspring of CAF dams fed the cafeteria diet after weaning. From day 16 of lactation to the day after weaning of their litter (day 22), CAF dams whose pups had been allocated to the CAF-CON group were fed the control diet so as to prevent the pups from consuming the cafeteria diet, as recommended by Tozuka et al. ( Reference Tozuka, Kumon and Wada 25 , Reference Tozuka, Wada and Wada 26 ). The dietary intake of the pups was recorded every 2 d from week 5 of treatment (age 49 d) to the end of treatment (age 120 d). Foods were provided in excess amounts, and intake was assessed by weighing the quantity provided and the quantity remaining of each item of the control and cafeteria diets individually. The daily data obtained for each food item were summed, generating total intake per d, and total daily intakes were summed to obtain total weekly intake, which was then divided by the number of days of the week and the number of animals. The complete procedure has been described in detail in a previously published work( Reference Goularte, Ferreira and Sanvitto 27 ). Energy intake was evaluated on the basis of the nutritional composition of each item, and the results are expressed as weekly intake/animal per d. Dams and male offspring alike were weighed once a week; results are expressed as the weight on the day of weaning, weight on the day of death and weekly evolution of weight.
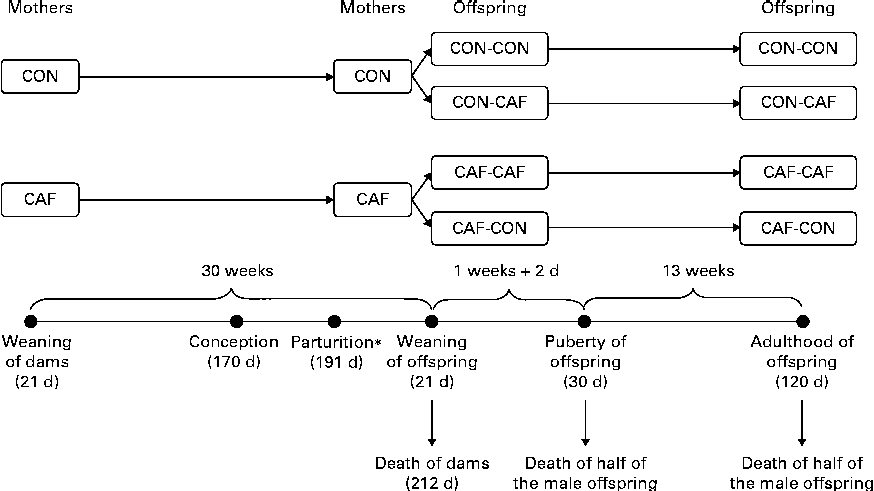
Fig. 1 Experimental design (see text for details). * From day 16 of lactation to the day after weaning (day 22), CAF dams whose pups had been allocated to the CAF-CON group were fed the control diet. CON-CON, control offspring born to control mothers (n 16); CON-CAF, cafeteria offspring born to control mothers (n 16); CAF-CAF, cafeteria offspring born to cafeteria mothers (n 18); CAF-CON, control offspring born to cafeteria mothers (n 21).
Dams were decapitated on the morning after weaning of their pups, at 210 d of age (30 weeks of treatment), and their male offspring were decapitated at one of two time points: at 30 d of age (9 d of treatment) or at 120 d (14 weeks of treatment) (Fig. 1). Decapitation was carried out using a rodent guillotine, after 12–14 h of fasting, between 08.30 and 12.00 hours. Blood was collected to obtain serum and plasma by centrifugation. The concentrations of total cholesterol, TAG and glucose in serum were quantified by a colorimetric method (Colesterol Liquiform, Triglicérides Liquiform, Glicose PAP Liquiform; Labtest®), and the concentrations of insulin and leptin in plasma were quantified by ELISA, using commercial reagents (Rat/Mouse Insulin and Rat Leptin ELISA Kits; Millipore). Visceral adipose tissue was dissected by separating the adipose tissue around the stomach, spleen, pancreas, small and large intestines and reproductive tract and the retroperitoneal adipose tissue corresponding to the fat around and below the kidneys. Shortly after dissection, the fatty tissue samples were weighed and discarded.
Statistical analyses
The Kolmogorov–Smirnov test was used to assess the normality of data, and F test was used to test the equality of variances. Student's t test was used for between-group comparisons, and two-way ANOVA with Bonferroni post hoc correction was used to evaluate the effects of dam and pup exposures and the interactions between them. Multivariate analysis of repeated measures was used to evaluate the effect of dam and offspring exposures on the evolution of body weight and energy intake, as well as the interaction between treatments. Data are expressed as means with their standard errors. In all the cases, differences were considered significant when P <0·05. Statistical analyses were carried out using GraphPad Prism® 5.00 for Windows (GraphPad Software) and the Statistical Package for the Social Sciences (SPSS)® 19.0 for Windows (SPSS, Inc., IBM).
Results
Maternal body weight, adipose tissue weight, and total cholesterol, TAG, glucose, insulin and leptin concentrations at age 210 d
At the end of the lactation period, CAF dams had significantly greater body weight and visceral and retroperitoneal adipose tissue weights than CON dams (Table 2). The total cholesterol concentrations of CAF dams were higher than those of CON dams at the same time point. The concentrations of TAG and glucose were similar between the groups. However, CAF dams had significantly higher concentrations of insulin and leptin than CON dams.
Table 2 Maternal metabolic characteristics at the end of treatment (210 d of age) (Number of rats and mean values with their standard errors)
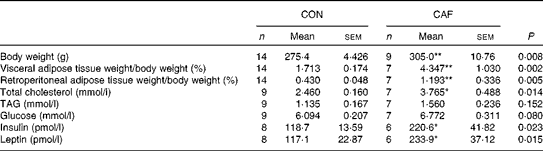
CON, control mothers; CAF, cafeteria mothers.
Mean value was significantly different from that of the control group: * P <0·05, ** P <0·01.
Effect of maternal and offspring diets on the body weight, adipose tissue weight, and total cholesterol, TAG and glucose concentrations of male offspring at age 30 d
On the day of weaning, at 21 d of age, corresponding to the first day of exposure to the different diets, the male offspring of CON and CAF dams had similar body weights (P =0·857) (data not shown). At 30 d of age, body weights were still similar among the offspring groups (Table 3), with no effect of maternal exposure and no interaction between the maternal and offspring diets, although there was a trend towards an effect of the offspring diet. At the same time point, CAF offspring had greater retroperitoneal and visceral adipose tissue weights than CON offspring, regardless of the maternal diet, and there was no interaction between the maternal and offspring diets. At 30 d of age, the concentrations of total cholesterol, TAG and glucose were also similar across all the offspring groups (Table 4), with no effect of offspring or maternal diet and no interaction between the offspring and maternal exposures. The evolution of body weight over time was influenced by the maternal diet, and there was a trend towards an interaction between the maternal and offspring diets, but there was no effect of the offspring diet (Fig. 2). The evolution of the energy intake of offspring over time was influenced by the offspring diet; there was no effect of maternal diet and no interaction between the maternal and offspring diets (Fig. 3).
Table 3 Body and adipose tissue weights of male offspring during puberty (30 d) and adulthood (120 d) (Number of rats and mean values with their standard errors)

CON-CON, control offspring born to control mothers; CAF-CON, control offspring born to cafeteria mothers; CON-CAF, cafeteria offspring born to control mothers; CAF-CAF, cafeteria offspring born to cafeteria mothers.
* Mean value was significantly lower than that of the CON-CAF offspring (P< 0·01; Bonferroni post hoc test).
Table 4 Metabolic profile of male offspring during puberty (30 d) and adulthood (120 d) (Number of rats and mean values with their standard errors)

CON-CON, control offspring born to control mothers; CAF-CON, control offspring born to cafeteria mothers; CON-CAF, cafeteria offspring born to control mothers; CAF-CAF, cafeteria offspring born to cafeteria mothers.
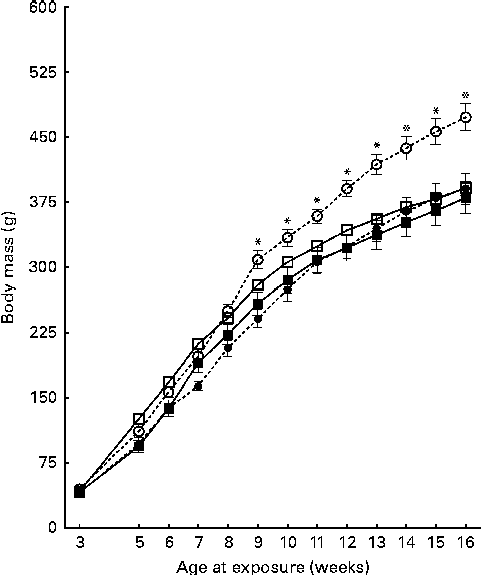
Fig. 2 Evolution of the body weight of pups over the 14-week experimental period. Values are means, with their standard errors represented by vertical bars. Treatment was initiated at 21 d of age in male offspring. However, intake data were recorded only from week 5 of treatment (age 7 weeks) to the end of treatment at week 14 (age 16 weeks). CON-CON (![]() ), control offspring born to control mothers (n 16); CON-CAF (
), control offspring born to control mothers (n 16); CON-CAF (![]() ), cafeteria offspring born to control mothers (n 16); CAF-CON (
), cafeteria offspring born to control mothers (n 16); CAF-CON (![]() ), control offspring born to cafeteria mothers (n 21); CAF-CAF (
), control offspring born to cafeteria mothers (n 21); CAF-CAF (![]() ), cafeteria offspring born to cafeteria mothers (n 18). The evolution of body weight over time was influenced by the maternal diet, and there was a trend towards an interaction between the maternal and offspring diets (for further details, see text). * Mean value was significantly different for the CON-CAF group from that of the other groups from week 10 (P< 0·05; Bonferroni post hoc test).
), cafeteria offspring born to cafeteria mothers (n 18). The evolution of body weight over time was influenced by the maternal diet, and there was a trend towards an interaction between the maternal and offspring diets (for further details, see text). * Mean value was significantly different for the CON-CAF group from that of the other groups from week 10 (P< 0·05; Bonferroni post hoc test).
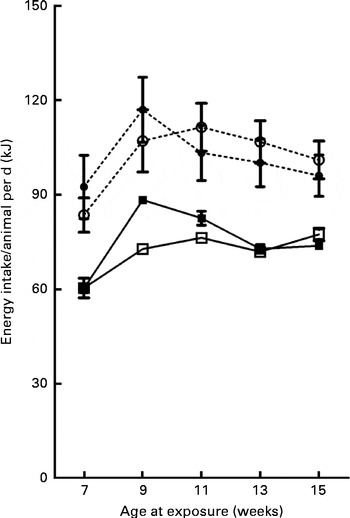
Fig. 3 Evolution of energy intake/animal per d over the 14-week experimental period. Values are means, with their standard errors represented by vertical bars. Treatment was initiated at 21 d of age in male offspring. However, intake data were recorded only from week 5 of treatment (age 7 weeks) to the end of treatment at week 14 (age 16 weeks). CON-CON (![]() ), control offspring born to control mothers (n 16); CON-CAF (
), control offspring born to control mothers (n 16); CON-CAF (![]() ), cafeteria offspring born to control mothers (n 16); CAF-CON (
), cafeteria offspring born to control mothers (n 16); CAF-CON (![]() ), control offspring born to cafeteria mothers (n 21); CAF-CAF (
), control offspring born to cafeteria mothers (n 21); CAF-CAF (![]() ), cafeteria offspring born to cafeteria mothers (n 18). According to multivariate repeated-measures ANOVA, energy intake was influenced by the offspring diet (for further details, see text).
), cafeteria offspring born to cafeteria mothers (n 18). According to multivariate repeated-measures ANOVA, energy intake was influenced by the offspring diet (for further details, see text).
Effect of maternal and offspring diets on the body weight, adipose tissue weight, and total cholesterol, TAG and glucose concentrations of male offspring at age 120 d
At 120 d of age, the body weight of offspring was affected by the offspring diet (Table 3), by the maternal diet, and by the interaction between the offspring and maternal exposures. At the same time point, visceral and retroperitoneal adipose tissue weights were influenced by the offspring diet alone, with no effect of maternal diet and no interaction between the offspring and maternal exposures. At 120 d of age, the concentrations of total cholesterol and TAG were also similar across all the offspring groups (Table 4), with no effect of offspring or maternal diet and no interaction between the offspring and maternal exposures. The concentrations of glucose were also influenced by the offspring diet, with no effect of maternal diet and no interaction between the offspring and maternal exposures. Similarly, at 120 d of age, the concentrations of insulin and leptin of offspring were affected by the offspring diet, with no effect of maternal diet and no interaction between the offspring and maternal exposures.
Discussion
The findings of the present study suggest that maternal diet does not affect the metabolism of male offspring that are exposed to a control diet during their lifetime. Furthermore, when both mother and offspring ingested the cafeteria diet, they exhibited metabolic effects the same as those exhibited by the offspring of control dams that were fed the cafeteria diet after weaning. However, when both mother and offspring were ingested the cafeteria diet, the offspring exhibited a different pattern of change in body weight compared with the offspring that were fed the cafeteria diet after weaning and were born to control dams, suggesting that the maternal environment during gestation and lactation affects the body composition of offspring in adulthood.
Dams that ingested the cafeteria diet developed a body habitus and a metabolic profile characteristic of obesity, as indicated by significant increases in body weight, visceral and retroperitoneal adipose tissue weights, and concentrations of total cholesterol, insulin and leptin. However, circulating concentrations of glucose and TAG remained the same in the two treatment groups. Even if insulin and leptin were dosed in the post-gestational period, exactly on the day after weaning of offspring, it is believed that the concentrations of these hormones would already have been high during pregnancy, as gestation is a condition in which physiological metabolic changes that promote resistance to these hormones occur( Reference Trujillo, Spuch and Carro 28 – Reference Grattan, Ladyman and Augustine 30 ). Furthermore, studies in obese females fed hyperenergetic diets found hyperinsulinaemia and hyperleptinaemia during pregnancy( Reference Nivoit, Morens and Assche 31 , Reference Samuelsson, Matthews and Argenton 32 ). This evidence suggests that CAF dams were possibly (at the very least) hyperinsulinaemic and hyperleptinaemic while pregnant and, therefore, that their offspring were probably exposed to a metabolically altered environment during the fetal period.
The total energy intake of offspring that were fed the control diet and were descendants of dams that were fed the cafeteria diet was similar to that of pups that were fed the control diet and descendants of control dams. Many studies have attempted to investigate the influence of hyperenergetic maternal feeding on feeding behaviour of offspring, with contradictory results. Some have found no differences in energy intake( Reference Bayol, Farrington and Stickland 20 , Reference Akyol, McMullen and Langley-Evans 23 , Reference Wright, Fone and Langley-Evans 33 , Reference Shankar, Harrell and Liu 34 ), as has been observed in the present study, while others have reported higher energy intake among offspring fed a control diet but born to mothers exposed to hyperenergetic diets( Reference Nivoit, Morens and Assche 31 , Reference Samuelsson, Matthews and Argenton 32 ). The above-cited studies have major methodological differences, thus precluding any strict comparisons between them. As expected, the energy intake of pups that were fed the cafeteria diet and born to control dams was higher than that of control pups born to control dams, a difference attributable to the fact that the cafeteria diet consists of hyperenergetic items and that intake of chow is progressively replaced by these items( Reference Bayol, Farrington and Stickland 20 , Reference Goularte, Ferreira and Sanvitto 27 , Reference Shafat, Murray and Rumsey 35 , Reference Prats, Monfar and Castell 36 ). Pups that were fed the cafeteria diet from the fetal period to adulthood exhibited an energy intake greater than that of control pups, but similar to that of pups that were fed the cafeteria diet only after weaning. This is consistent with the findings of previous studies( Reference Akyol, McMullen and Langley-Evans 23 , Reference Shankar, Harrell and Liu 34 , Reference Ong and Muhlhausler 37 ).
Treatment exposures were initiated in all the pups at 21 d of age with similar body weights, which shows that ingestion of the cafeteria diet by dams before pregnancy and during gestation and lactation did not affect total body weight; this is consistent with the findings of previous research( Reference Bayol, Simbi and Stickland 21 , Reference Akyol, McMullen and Langley-Evans 23 , Reference Samuelsson, Matthews and Argenton 32 , Reference Wright, Fone and Langley-Evans 33 ). The body weights and visceral and retroperitoneal adipose tissue weights of pups fed the cafeteria diet during the prenatal and lactation periods were similar to those of control pups born to control dams at puberty, which is consistent with the findings of some prior studies( Reference Akyol, McMullen and Langley-Evans 23 , Reference Wright, Fone and Langley-Evans 33 ). While pubertal body weight of pups fed the cafeteria diet after weaning was similar to that of control pups born to control dams, retroperitoneal and visceral adipose tissue weights of these pups were already significantly higher, suggesting that, at least during puberty, body composition is different despite similar total body weights( Reference Bayol, Simbi and Stickland 21 , Reference Benkalfat, Merzouk and Bouanane 38 ). Furthermore, this demonstrates that periods as short as 9 d of exposure to a cafeteria diet (from weaning to puberty) can affect fat accretion. In addition, despite the fact that pups fed the cafeteria diet from the prenatal period to adulthood had pubertal body weight comparable to that of the other groups, these pups had visceral and retroperitoneal adipose tissue weights greater than those of pups fed the control diet, regardless of the maternal diet. Again, this indicates that the body composition of pups fed the cafeteria diet was different from that of pups fed the control diet, even though all the four groups had similar total body weights.
The pubertal concentrations of total cholesterol, TAG and glucose of pups fed the cafeteria diet only during the fetal and lactation periods were similar to those of control pups born to control dams. To date, similar results have been reported only by a few studies( Reference Akyol, McMullen and Langley-Evans 23 , Reference Nivoit, Morens and Assche 31 , Reference Samuelsson, Matthews and Argenton 32 ). However, these findings indicate that even in pups born to obese mothers, ingestion of the control diet prevents the expression of a metabolic phenotype characteristic of obesity. Similar results were observed during puberty in pups that began eating the cafeteria diet immediately after weaning, as there were no changes in circulating cholesterol, TAG or glucose concentrations; similarly, pups born to obese dams and fed the cafeteria diet had circulating glucose, TAG and total cholesterol concentrations similar to those of the other groups.
Pups that were born to control dams and fed the cafeteria diet after weaning (CON-CAF) had higher total body weight and visceral and retroperitoneal adipose tissue weights in adulthood when compared with pups born to control dams and fed the control diet (CON-CON), showing that the cafeteria diet had an obesogenic effect in these pups( Reference Shafat, Murray and Rumsey 35 , Reference Rothwell and Stock 39 ). On the other hand, pups born to obese dams and fed the cafeteria diet (CAF-CAF) had a total body weight similar to that of control pups born to control dams and lower than that of pups fed the cafeteria diet from weaning.
These data can be explained in several ways. For instance, an increase in maternal leptin levels (especially in milk) would prevent increases in energy intake (and consequently body weight) in offspring continuously exposed to the cafeteria diet( Reference Picó, Oliver and Sanchéz 40 , Reference Sanchéz, Priego and Paulou 41 ) when compared with pups born to control dams and first exposed to the cafeteria diet after weaning. These body weight findings run counter to the results of many studies that have found that maternal diet energy increases offspring susceptibility to obesity( Reference Bayol, Farrington and Stickland 20 , Reference Samuelsson, Matthews and Argenton 32 , Reference Shankar, Harrell and Liu 34 ), but are consistent with the findings of recent studies( Reference Howie, Sloboda and Kamal 5 , Reference Ong and Muhlhausler 37 ) describing that offspring born to mothers exposed to a high-energy diet are protected from obesity induction. Exposure to a hyperenergetic diet in utero may have led to adaptations conferring a greater ability to deal with an equally energy-rich environment after birth, thus serving as a form of predictive adaptive response to this exposure to high levels of lipids and carbohydrates during the fetal and postnatal periods( Reference Gluckman and Hanson 42 , Reference Gluckman and Hanson 43 ), possibly through epigenetic modification in the central control of intake( Reference Garratt, Vickers and Gluckman 44 , Reference Plagemann, Harder and Brunn 45 ).
Despite this, in adulthood, the retroperitoneal and visceral adipose tissue weights of CAF-CAF pups were greater than those of CON-CON pups and similar to those of CON-CAF pups. Considering that the total body weight of CAF-CAF pups was lower than that of CON-CAF pups, but that these two groups had similar visceral and retroperitoneal adipose tissue weights, one could propose that maternal obesity exerts an effect on body structure, possibly by increasing fat mass relative to lean mass and by further reducing lean mass( Reference Bayol, Simbi and Stickland 21 , Reference Ong and Muhlhausler 37 ). This theory is consistent with studies that showed that obese mothers have higher chances of having children with low birth weight( Reference McDonald, Han and Mulla 46 ), but that these offspring have greater adiposity, altered muscle development and increased risk for the metabolic syndrome( Reference Drake and Reynolds 47 ) with the passing of years, possibly also through epigenetic modulation( Reference Yan, Zhu and Xu 48 , Reference Tong, Yan and Zhu 49 ). However, further research into the musculoskeletal composition of all the treatment groups is required to confirm this hypothesis.
The concentrations of TAG and cholesterol remained the same across all the four groups in adulthood. However, in adulthood, blood glucose, insulin and leptin concentrations of pups that were fed the cafeteria diet were elevated compared with those of pups that were fed the control diet, regardless of the maternal diet, thus confirming the finding of leptin and insulin resistance and glucose intolerance in animals exposed to a cafeteria diet( Reference Sampey, Vanhoose and Winfield 50 , Reference Castell-Auví, Cedó and Pallarès 51 ). These findings refute the hypothesis of an influence of maternal obesity and its interaction with offspring diet in the metabolic state, but instead provide evidence of a greater influence of the food choices of each generation on the biochemical parameters of the individuals of that generation.
Given the conditions under which the present study was carried out, it can be concluded that the cafeteria diet induced obesity in dams and that this condition did not affect the metabolic parameters of their male offspring at 30 and 120 d of age, but it did modify body weight in adulthood among offspring also exposed to the cafeteria diet. Nevertheless, further studies on body composition are needed to elucidate these results. The present study emphasises the importance of food preferences throughout life and suggests that choices can reverse the effects of a metabolically altered maternal environment and its programming. Furthermore, it suggests that both maternal and offspring dietary intakes must be appropriate to ensure optimal body composition in the next generations.
Acknowledgements
The authors appreciate the collaboration of Grace Violeta Espinoza Pardo during the experimental procedures.
The present study was supported by the National Council for Technological and Scientific Development (CNPq), the Coordination for Improvement of Higher Education Personnel (Capes) and the Graduate Studies Division at UFRGS (PROPG). The financial backers played no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: A. B. M. was involved in all stages of the study design, experimental procedures, data analysis and manuscript writing; J. F. G., C. N. and C. S. B. contributed to the experimental procedures, data analysis and manuscript writing; A. C. A. C. and R. C. C. were involved in all stages of the experimental procedures; P. P. S. and G. L. S. contributed to the study design, data analysis and manuscript writing.
The authors have no conflicts of interest to declare.









