Introduction
The nature of cognitive impairment in psychotic patients has often been depicted as a progressive process (Mollon & Reichenberg, Reference Mollon and Reichenberg2018), involving pre-onset (Bang et al., Reference Bang, Kim, Song, Baek, Lee and An2015; Bolt et al., Reference Bolt, Amminger, Farhall, McGorry, Nelson, Markulev and Allott2019; Zhang et al., Reference Zhang, Cui, Tang, Xu, Li, Wei and Wang2016) and post-onset phases (Amoretti et al., Reference Amoretti, Rabelo-da-Ponte, Rosa, Mezquida, Sanchez-Torres, Fraguas and Group2021; Rodriguez-Sanchez et al., Reference Rodriguez-Sanchez, Setien-Suero, Suarez-Pinilla, Mayoral Van Son, Vazquez-Bourgon, Gil Lopez and group2022). While the concept of a prodromal stage, clinical high-risk (CHR) for psychosis, emerged in the early 1990s (Yung et al., Reference Yung, McGorry, McFarlane, Jackson, Patton and Rakkar1996), providing a crucial time window between the premorbid phase and the first episode of psychosis (FEP), it is essential to note that cognitive impairment is not consistently characterized as a linear decline but rather as an enduring presence, often observed since the risk phase. Although the link between psychosis progression and increased neurocognitive impairment is well-established among FEP and CHR populations (Bora & Murray, Reference Bora and Murray2014; Giuliano et al., Reference Giuliano, Li, Mesholam-Gately, Sorenson, Woodberry and Seidman2012), previous research on neurocognitive deficits and psychosis has typically focused on individual phases and specific populations. Many studies (Seidman et al., Reference Seidman, Shapiro, Stone, Woodberry, Ronzio, Cornblatt and Woods2016; Watson, Harrison, Preti, Wykes, & Cella, Reference Watson, Harrison, Preti, Wykes and Cella2022) concentrated on patients in a single phase, be it CHR or FEP, and often lacked the use of a standardized battery of tests, limiting their ability to compare and interpret data (Mam-Lam-Fook et al., Reference Mam-Lam-Fook, Danset-Alexandre, Pedron, Amado, Gaillard and Krebs2017).
Accumulating evidence underscores the crucial role of mild neurocognitive deficits (MCD) in predicting psychosis from the CHR phase (Cannon et al., Reference Cannon, Yu, Addington, Bearden, Cadenhead, Cornblatt and Kattan2016; Zhang et al., Reference Zhang, Xu, Li, Woodberry, Kline, Jiang and Wang2021, Reference Zhang, Cui, Wei, Tang, Xu, Hu and Wang2022) and differentiating FEP from healthy controls (HC) (Sawada et al., Reference Sawada, Kanehara, Sakakibara, Eguchi, Tada, Satomura and Kasai2017; Zhang et al., Reference Zhang, Li, Stone, Woodberry, Seidman, Tang and Wang2015a). However, previous studies did not differentiate between the two targeted populations, leaving unanswered questions about whether CHR individuals who later converted to psychosis exhibit a distinctive pattern of MCD compared to patients with FEP.
This study addresses the challenge of understanding the role of MCD in the development of psychosis by assessing and comparing various neurocognitive domains in three large groups: individuals at CHR, those with FEP, and matched HC. The specific objectives of our study are as follows: (1) Characterize MCD Patterns: Compare mild cognitive deficits between groups, specifically between FEP and HC, CHR and HC, and within the CHR group, comparing individuals who later converted to psychosis (CHR-C) with those who did not convert (CHR-NC). This aims to elucidate the distinctive MCD patterns using a standardized battery; (2) Develop Stage-Dependent Models: Distinguish various MCD models associated with different levels of psychosis risk across stages. This involves creating models specific to each stage of psychosis to capture nuanced cognitive impairments; and (3) Evaluate Model Discrimination: Examine the discriminatory power of specific MCD models in distinguishing FEP from HC, CHR-C from CHR-NC, and CHR from HC. This step assesses the effectiveness of the developed models in classifying individuals within different stages of psychosis. By delineating these specific objectives, our study aims to investigate MCD as a stage-dependent marker along the continuum of psychotic episodes from prodrome. We hypothesize that each stage condition will be associated with a unique MCD model, highlighting the heterogeneity of cognitive impairment across the psychosis continuum.
Methods
Projects and sample
The current research is part of the National Key R&D Program of the Ministry of Science and Technology of China (2016YFC1306800) conducted between 2016 and 2021. Five psychiatric tertiary hospitals in China participated in data collection and patient evaluation. This project aimed to collect cognitive and biological markers for the early stage identification of psychosis. The participants included 1000 individuals with FEP, 1000 individuals with CHR, and 2000 well-matched HC. A key element of the project is that, in contrast to many other samples, the participants had no treatment for any kind of psychiatric disorder, including psychotropic medications. In addition, they did not have any history of substance abuse or dependence according to specific exclusion criteria. In excluding substance-induced psychosis, we sought to concentrate our investigation on primary psychotic disorders, recognizing that individuals with psychosis resulting from substance use often demonstrate relatively preserved cognitive function. This deliberate focus enhances the specificity of our study in elucidating the comprehensive cognitive landscape during the initiation of primary psychosis.
Pooled data (baseline clinical and cognitive data) of 1059 patients with FEP and 774 HC from communities and schools were used for the current analysis. A total of 794 CHR individuals were recruited from a single site at the Shanghai Mental Health Center (SMHC) as an extended part of the Shanghai At Risk for Psychosis (SHARP) program (Li et al., Reference Li, Zhang, Xu, Tang, Cui, Wei and Wang2018; Zhang et al., Reference Zhang, Li, Woodberry, Seidman, Zheng, Li and Wang2014, Reference Zhang, Li, Tang, Niznikiewicz, Shenton, Keshavan and Wang2018), which was also used for early psychosis identification. Zhang et al. (Zhang et al., , Reference Zheng, Wang, Zhang, Li, Li and Jiang2012, Reference Zhang, Li, Woodberry, Seidman, Zheng, Li and Wang2014, Reference Zhang, Li, Woodberry, Seidman, Chow, Xiao and Wang2015b) provided further details regarding the SHARP methodology.
The Research Ethics Committees at the SMHC and local hospitals approved these studies. All participants provided written informed consent during the recruitment stage of the study. Participants younger than 18 years old had their consent forms signed by their parents, but they also expressed consent themselves. We recruited CHR individuals between January 2016 and November 2022 through a face-to-face interview using a structured interview for prodromal syndromes/scale of prodromal symptoms (SIPS/SOPS) (Miller et al., Reference Miller, McGlashan, Rosen, Cadenhead, Cannon, Ventura and Woods2003), and completed the baseline neurocognitive assessments using the Chinese version of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) (Kern et al., Reference Kern, Nuechterlein, Green, Baade, Fenton, Gold and Marder2008, Reference Kern, Gold, Dickinson, Green, Nuechterlein, Baade and Marder2011) (Shi, He, Cheung, Yu, & Chan, Reference Shi, He, Cheung, Yu and Chan2013). Among them, 561 completed the 2-year follow-up.
Measurements
The face-to-face interview of the SIPS (Miller et al., Reference Miller, McGlashan, Rosen, Cadenhead, Cannon, Ventura and Woods2003) was used to identify individuals with CHR, which has been widely used. In our previous studies, (Zhang et al., Reference Zhang, Li, Woodberry, Seidman, Zheng, Li and Wang2014, Reference Zhang, Li, Woodberry, Xu, Tang, Guo and Wang2017) the Chinese version of the SIPS, (Zheng et al., Reference Zheng, Wang, Zhang, Li, Li and Jiang2012) which was developed by the SHARP team, demonstrated good inter-rater reliability (interclass correlation coefficient: r = 0.96, p < 0.01; SIPS total score) and validity (26.4% of the subjects converted to psychosis in the following two years) in China. The first author received SIPS certification at a Yale University-sponsored SIPS training and had extensive experience with Chinese CHR research projects. Clinical psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS) (Kay, Fiszbein, & Opler, Reference Kay, Fiszbein and Opler1987) for patients with FEP. The PANSS consists of 30 items divided into three subscales: positive (PANSS-P; items P1–7), negative (PANSS-N; N1–7), and general psychopathology (PANSS-G; G1–16). Each item (symptom) was rated on a 7-point Likert scale (1 = absent to 7 = extreme). The PANSS interviews were conducted by 10 senior psychiatrists who completed the training required for this type of investigation.
The Chinese version of the MCCB (Shi et al., Reference Shi, He, Cheung, Yu and Chan2013) was used to assess neurocognition and was administered according to the standardized guidelines provided in the test manual. Consistent with the original version of the MCCB, (Kern et al., Reference Kern, Nuechterlein, Green, Baade, Fenton, Gold and Marder2008; Nuechterlein et al., Reference Nuechterlein, Green, Kern, Baade, Barch, Cohen and Marder2008) the Chinese version of the following eight subtests were included in the present study: (1) Part A of Trail Making Test (Trail Making A), (2) the Symbol Coding of the Brief Assessment of Cognition in Schizophrenia (BACS) (BACS symbol coding), (3) the Category Fluency Test (Category Fluency), (4) the Continuous Performance Test-Identical Pairs (CPT-IP), (5) the Spatial Span of the Wechsler Memory Scale-III (WMS-3 spatial span), (6) the Revised Hopkins Verbal Learning Test (HVLT-R), (7) the Revised Brief Visuospatial Memory Test (BVMT-R), and (8) the Neuropsychological Assessment Battery: Mazes (NAB mazes), which has been used to identify cognitive deficits in different populations, especially patients with psychosis, with a test-retest reliability of the subtests ranging from 0.73 to 0.94. (Shi et al., Reference Shi, He, Cheung, Yu and Chan2013)
CHR outcome and follow-up
Conversion to psychosis was the primary outcome used for the CHR group in this study, grouped as CHR-C and the remaining as CHR-NC, based on the criteria for the presence of Psychotic Symptoms, (McGlashan, Walsh, & Woods, Reference McGlashan, Walsh and Woods2010) which is part of the SIPS. Conversion was identified when the subject showed a level-6 positive symptom (severe and psychotic) that was either dangerous, disorganized, or occurred at an average of at least 1 h per day over 4 days in a week. Individuals with CHR were informed that the study involved a group of clinical and cognitive assessments at baseline with naturalistic follow-ups every year. The research procedure was independent of routine clinical treatment procedures at the SMHC. Both individuals with CHR and their caregivers were informed that they could contact the interviewer and study clinicians at any time to ask questions and request progress reports regarding patients' medical conditions.
Data analysis
The sociodemographic and clinical characteristics of the FEP, CHR, and HC groups were compared using the chi-square test (χ2) for nominal variables, and unidirectional analysis of variance (ANOVA) for continuous variables. The neurocognitive tests employed and their corresponding metrics were carefully selected to enhance discriminative power and were referenced from established literature (Nuechterlein et al., Reference Nuechterlein, Green, Kern, Baade, Barch, Cohen and Marder2008). The specific metrics included: Trail Making A (time to completion); BACS symbol coding (total number correct); Category Fluency (total number of animals named in 60 s); CPT-IP (mean d′ value across 2-, 3-, and 4-digit conditions); WMS-3 spatial span (sum of raw scores on forward and backward conditions); HVLT-R (total number of words recalled correctly over three learning trials), BVMT-R (total recall score over three learning trials), and NAB mazes (total raw score). To assess performance differences among the three groups, z-scores for the CHR and FEP groups based on the means and standard deviations (SDs) of HC, followed by ANOVAs. Cohen's d was employed for effect size calculations.
For discriminating FEP from HC, logistic models were constructed, both as an overall model and eight individual test models, adjusted for sex and age. Subsequently, we investigated the predictive capability of these discrimination models for CHR-C from CHR-NC. Receiver operating characteristic (ROC) methodology was employed to evaluate discriminative power in terms of the area under the ROC (AUC) for the conversion outcome.
To enhance discrimination effects, three models were constructed: MCD-H (high risk) to distinguish FEP from HC, MCD-M (medium risk) for CHR-C from CHR-NC, and MCD-L (low risk) for CHR from HC. Individual probabilities were generated for different stages and groups using related neurocognitive tests adjusted for sex and age. Specific cut-off values were determined to prioritize sensitivity or specificity based on clinical considerations. ROC analysis and proposed thresholds of PMCD−L, PMCD−M, and PMCD−H are presented.
Results
Demographics and clinical characteristics
Sex was not significantly different among the three groups. The CHR individuals were much younger than those in the other groups. The HC group had more years of education than the other groups (Table 1). During the 2-year follow-up, among 561 who completed the follow-up, 114 (20.3%) CHR individuals converted to psychosis. Demographic and clinical characteristic comparisons at baseline were conducted between the CHR-C and CHR-NC groups (Table 2). The differences between the CHR-C and CHR-NC groups were significant for Current GAF, GAF drop, and SIPS scores for positive symptoms, negative symptoms, disorganization symptoms, and total. There were more males than females in the CHR-C group than in the CHR-NC group.
Table 1. Demographic and clinical variables, comparison among CHR, FEP, and HC groups

GAF drop, Global Assessment of Functioning score baseline from highest in the past year; APSS, attenuated positive symptom syndrome; GRDS, genetic risk and deterioration syndrome; BIPS, brief intermittent psychotic syndrome; CHR, clinical high risk for psychosis; HC, healthy control; PANSS, Positive and Negative Syndrome Scale; SIPS, Structured Interview for Prodromal Symptoms. F/χ2: F for one-way analysis of variance test and χ2 for kappa test.
Table 2. Demographic and clinical variables, comparison between CHR-C and CHR-NC groups

GAF drop, GAF (Global Assessment of Functioning) score baseline from highest in the past year; low-risk family history, having any family members with mental disorders or a first-degree relative with non-psychotic disorders; high-risk family history, having at least one first-degree relative with psychosis; APSS, attenuated positive symptom syndrome; GRDS, genetic risk and deterioration syndrome; BIPS, brief intermittent psychotic syndrome; CHR, Clinical high risk for psychosis; CHR-converter, CHR individuals who were converted to fully psychosis; SIPS, the Structured Interview for Prodromal Symptoms; t/χ2: t for independent t test, χ2 for kappa test.
Neuropsychological profile comparison
Both the CHR and FEP groups demonstrated significantly poorer performances than the HC group on all neurocognitive tests, while the CHR-C group demonstrated significantly poorer performances than the CHR-NC group on BVMT-R (p < 0.001), NAB-mazes (p = 0.005), and BACS-symbol coding (p = 0.023) (Fig. 1). The original mean scores of the neurocognitive tests for group comparisons are shown in Table s1 and Table s2. The effect sizes of the comparisons across neurocognitive tests are presented in Fig. 1. Comparing the HC with CHR and FEP and CHR with FEP, the BACS-symbol coding test showed the highest effect size, while BVMT-R showed the highest effect size between the CHR-NC and CHR-C groups.
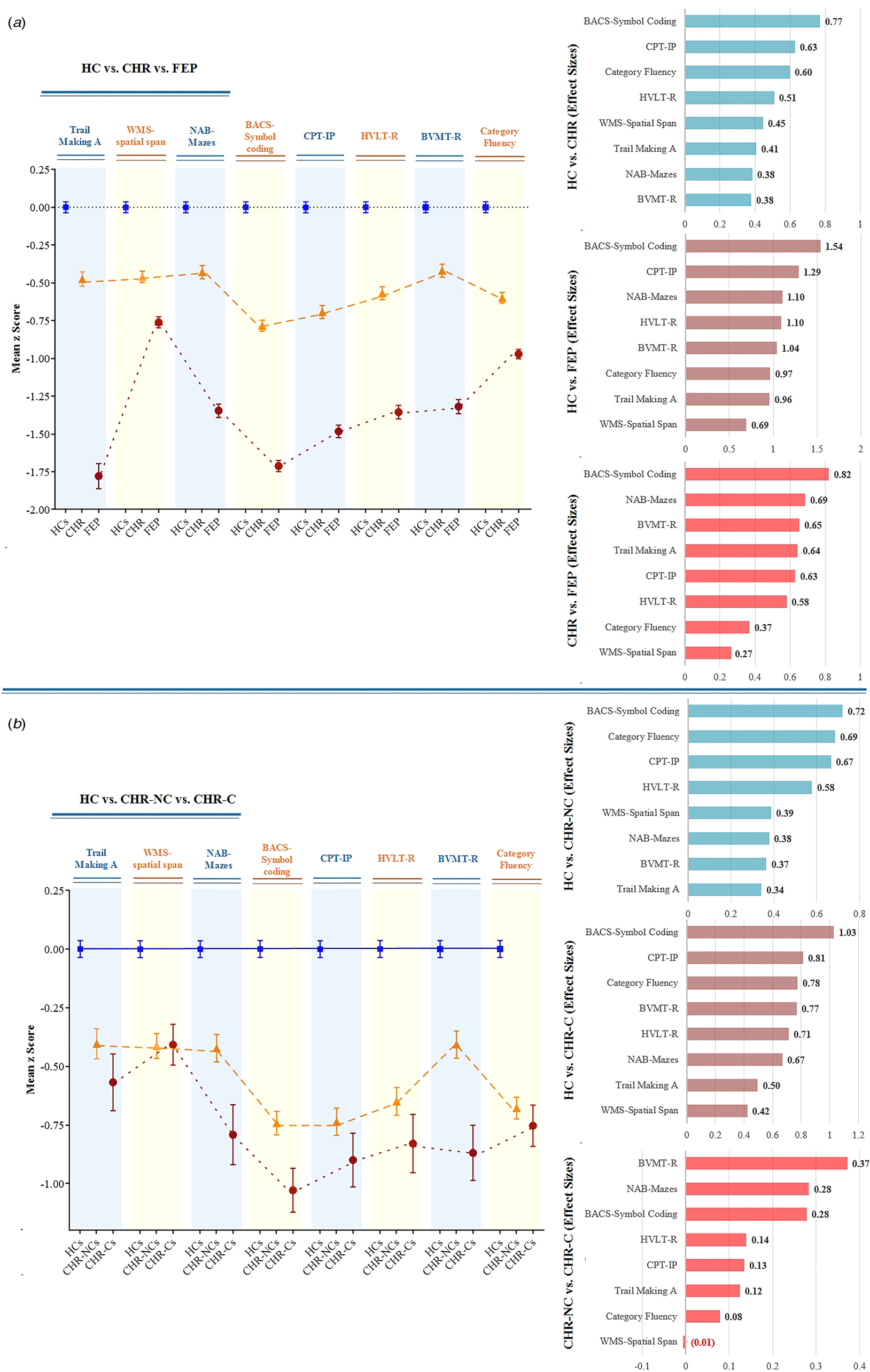
Figure 1. Neuropsychological profile, comparisons, and effect sizes (Cohen d) by groups of clinical high-risk (CHR), first-episode psychosis (FEP), CHR Converters to Psychosis (CHR-C), CHR Nonconverters (CHR-NC), and healthy controls (HC).
Note: Means from one-way ANOVA with Bonferroni correction analysis of variance were standardized with healthy controls' means (SDs) to convert to z scores. BACS, Brief Assessment of Cognition in Schizophrenia Symbol Coding; BVMT-R, Brief Visuospatial Memory Test–Revised; CPT-IP, Continuous Performance Test–Identical Pairs; HVLT-R, Hopkins Verbal Learning Test–Revised; NAB, Neuropsychological Assessment Battery mazes; WMS, Wechsler Memory Scale. The effect sizes are ranked from largest to smallest.
Discrimination performances
In general, Fig. 2 shows that the performance on the overall and individual cognitive tests was found to significantly differentiate FEP from HC. All adjusted probabilities showed significant values for distinguishing FEP from HC (Table s3). When eight test variables were included in the regression model, most cognitive tests (except WMS-3 spatial span and BVMT-R) still showed significance and were included in the overall model (Table s4). Detailed ROC performances for the individual and overall cognitive models for discriminating FEP from HC are presented in Table s5-s14. However, when these models were applied to differentiate CHR-C and CHR-NC, the discrimination performances were not satisfactory, with only significant probabilities in individual cognitive tests of BACS symbol coding (AUC = 0.577, p = 0.011), BVMT-R (AUC = 0.607, p < 0.001), NAB maze (AUC = 0.594, p = 0.002), and the overall model (AUC = 0.581, p = 0.008) (Table s15–17).

Figure 2. Receiver operating characteristic curve for probabilities of individual and overall cognitive models, discriminating FEP from HC and CHR-C from CHR-NC. BACS, Brief Assessment of Cognition in Schizophrenia Symbol Coding; BVMT-R, Brief Visuospatial Memory Test–Revised; CPT-IP, Continuous Performance Test–Identical Pairs; CHR, clinical high risk for psychosis; CHR-C, clinical high risk converters to psychosis; CHR-NC, clinical high risk nonconverters; FEP, first episode psychosis; HC, healthy control; HVLT-R, Hopkins Verbal Learning Test–Revised; NAB, Neuropsychological Assessment Battery mazes; WMS-3, Wechsler Memory Scale–Third Edition spatial span.
Discrimination models
In view of the differences in the performance of cognitive tests in distinguishing FEP from HC and CHR-C from CHR-NC, three different models were developed according to the purpose of discrimination. Table 3 shows that the cognitive tests of Trail Making A, BACS symbol coding, HVLT-R, NAB mazes, Category Fluency, and CPT-IP were significant factors in discriminating FEP from HC, and the MCD-H model achieved a classification accuracy rate of 82.3%. The NAB maze and BVMT-R cognitive tests were found to be significant factors in discriminating CHR-C from CHR-NC, and the MCD-M model achieved a classification accuracy rate of 79.4%. Cognitive tests of BACS symbol coding, HVLT-R, WMS-3 spatial span, and Category Fluency were found to be significant factors in discriminating CHR from HC, and the MCD-L model achieved a classification accuracy rate of 73.2%.
Table 3. Logistic regression (method: backward) models for discriminating FEP from HC (MCD-H), CHR-C from CHR-NC (MCD-M), and CHR from HC (MCD-L) of overall cognitive variables adjusted by age and sex

BACS, Brief Assessment of Cognition in Schizophrenia Symbol Coding; BVMT-R, Brief Visuospatial Memory Test–Revised; CPT-IP, Continuous Performance Test–Identical Pairs; CHR, clinical high risk for psychosis; CHR-C, clinical high risk converters to psychosis; CHR-NC, clinical high risk nonconverters; FEP, First Episode Psychosis; HC, Healthy Control; HVLT-R, Hopkins Verbal Learning Test–Revised; NAB, Neuropsychological Assessment Battery mazes; WMS-3, Wechsler Memory Scale–Third Edition spatial span. The MCD low-risk (MCD-L) model is a logistic regression model for discriminating CHR from HC, the MCD median-risk (MCD-M) model is the logistic regression model for discriminating CHR-C from CHR-NC, the MCD high-risk (MCD-H) model is the logistic regression model for discriminating FEP from HC, and PMCD−L, PMCD−M, and PMCD−H refer to probabilities generated from the MCD-L, MCD-M, and MCD-H models, respectively.
Note: Bate denotes the regression coefficient. SE is the standard error. The 95% CI was the estimated 95% confidence interval for the corresponding parameter. β is the standardized regression coefficient.
Models application for MCD
To further interpret the risk probabilities generated from the MCD-L, MCD-M, and MCD-H models, Fig. 3 provides ROC curves based on the values of PMCD−L, PMCD−M, and PMCD−H. These curves provide insights into the discriminative performance of the models. In the MCD-L model, with a targeted sensitivity of 80% (prioritizing the screening of CHR from HC), the cut-off point for PMCD−L was determined at 0.57, achieving a specificity of 64.77%. Individuals with risk probabilities of MCD-L exceeding 0.57 are identified as CHR. For the MCD-M model, the optimal cut-off value of PMCD−M, calculated according to the Yoden index, was found to be 0.21, with a sensitivity of 63.45% and a specificity of 60.54%. In the MCD-H model, targeting a specificity of 80% (prioritizing the screening of FEP from HC), The cut-off point of PMCD−L was established at 0.56, with a sensitivity of 83.94%. Detailed statistics for discrimination across several thresholds of PMCD−L, PMCD−M, and PMCD−H are provided in Table-s18–20.

Figure 3. MCD models application, discriminating FEP from HC, CHR-C from CHR-NC and CHR from HC. AUC, area under the receiver operating characteristic curve; CHR, clinical high risk for psychosis; CHR-C, clinical high risk converters to psychosis; CHR-NC, clinical high risk nonconverters; FEP, first episode psychosis; HC, healthy control. The MCD-L model is the logistic regression model for discriminating CHR from HC; the MCD low-risk (MCD-L) model is the logistic regression model for discriminating CHR from CHR-NC; MCD high-risk (MCD-M) model is the logistic regression model for discriminating CHR-C from CHR-NC; MCD high-risk (MCD-H) model is the logistic regression model for discriminating FEP from HC; and PMCD−L, PMCD−M, and PMCD−H refer to probabilities generated from the MCD-L, MCD-M, and MCD-H models.
Discussion
Although various neurocognitive assessments have been applied to improve the effectiveness of prediction or discrimination in populations with high-risk or first-episode psychosis, very few studies have been conducted specifically for comparisons of cognitive performance between the two stages. To our knowledge, this study has one of the largest sample sizes in which both the FEP and CHR groups were matched to the HC group, respectively. This study was based on a drug-naïve cohort sample at their first contact with mental health services, which is another strength. This avoided a significant impact on neurocognition due to the confounding factors of the medications. Furthermore, the current sample excluded individuals with substance abuse-induced psychotic symptoms, such as methamphetamine, which can better reflect the neurocognitive functions of primary psychotic disorders. To exclude the possible confounding effects of demographic characteristics, we adjusted our results for sex and age in all analyses.
Key findings
In this study, we aimed to analyze differences in neurocognitive profiles between the FEP and HC, CHR-C and CHR-NC, and CHR and HC groups, but at different stages of early psychosis. As expected, the clinical sample performed worse than the HC group in almost all domains, and the CHR-C group performed worse than the CHR-NC group in some domains; however, subgroups varied in the difference level of effect size and the affected domains. The neurocognitive model based on distinguishing FEP from HC cannot effectively predict CHR-C from CHR-NC. We discourage the use of a single neurocognitive model to characterize psychotic risk across diverse early stages. Instead, to enhance the effectiveness of psychotic risk assessment using the neurocognitive model, we recommend evaluating distinct risk levels in three early scenarios: employing the MCD-H model to differentiate FEP from HC, utilizing the MCD-M model to predict psychosis and distinguish CHR-C from CHR-NC, and applying the MCD-L model to identify CHR from HC. The MCD models were designed following a staging strategy to help better identify and predict psychosis risk and improve effectiveness in terms of accurate early identification measures.
MCD-H
The MCD-H model was designed to discriminate FEP from HC with stratification of high-level MCD. This model obtained an AUC of 0.893, indicating good discrimination ability. More specifically, for MCD-H individuals with model estimates (PMCD−H) higher than 0.56, these estimates had excellent sensitivity (83.9%) and specificity (80.0%). In this model, six neurocognitive tests were significant discriminators of FEP, in which BACS symbol coding, CPT-IP, HVLT-R, and category fluency tests were the top four factors (p < 0.001). Notably, the BACS symbol coding and category fluency tests belong to the cognitive domain of ‘processing speed’ and HVLT-R belongs to ‘verbal memory,’ which have been proven to be associated with poor multi-domain outcomes on symptoms, social functioning, and a higher number of episodes in patients with FEP (Cowman et al., Reference Cowman, Holleran, Lonergan, O'Connor, Birchwood and Donohoe2021; Cuesta et al., Reference Cuesta, Sanchez-Torres, Moreno-Izco, Garcia de Jalon, Gil-Berrozpe, Zarzuela and Zandio2022; Stouten, Veling, Laan, van der Helm, & van der Gaag, Reference Stouten, Veling, Laan, van der Helm and van der Gaag2014). Compared to the MCD-M model for predicting CHR-C, the MCD-H model included a broader range of neurocognitive dysfunction and varied in neurocognitive domains, and none of the top four tests in the MCD-H model contributed significantly to the MCD-M model. This inconsistency implies that the closer relationship between processing speed and verbal memory impairment is not a predictor of psychosis onset but rather in the discriminator factors of patients with FEP.
MCD-M
The MCD models varied in the level of risk and the affected neurocognitive domains, especially in the MCD-M model, which was designed for the prediction of conversion to psychosis, with stratification of median level MCD. This model yielded an AUC of 0.656, which is an acceptable prediction ability. More specifically, for MCD-M individuals with model estimates (PMCD−M) higher than 0.21, these estimates had acceptable sensitivity (63.5%) and specificity (60.5%) for the prediction of CHR-C from CHR-NC. In this model, only the BVMT-R (domain of ‘visual learning’) and NAB mazes (domain of ‘reasoning and problem solving’) tests were significant predictors of CHR-C. The MCD-M model suggested that declined performance in the BVMT-R test may be considered a particularly important marker for predicting psychosis in the CHR stage. This result was consistent with the results of the NAPLS-2 (Seidman et al., Reference Seidman, Shapiro, Stone, Woodberry, Ronzio, Cornblatt and Woods2016), suggesting a central role for visual learning abilities in the development of psychosis from the CHR stage. The results were also consistent with our recent findings that the BVMT-R test is a significant independent predictor of psychosis when included in a risk calculator (Cui et al., Reference Cui, Giuliano, Zhang, Xu, Wei, Tang and Stone2020; Zhang et al., Reference Zhang, Xu, Li, Woodberry, Kline, Jiang and Wang2021, Reference Zhang, Cui, Wei, Tang, Xu, Hu and Wang2022).
Previous studies have evaluated sex differences in the cognitive performance of patients with psychosis (Li et al., Reference Li, Hui, Lee, Chang, Chan and Chen2019). Interestingly, our results only suggested the sex effects of neurocognition on psychosis prediction (MCD-M) but not on FEP discrimination (MCD-H). This may be due to the fact that patients with FEP generally are older than CHR individuals. Younger age of onset in men is a robust finding in psychosis research (Hafner et al., Reference Hafner, an der Heiden, Behrens, Gattaz, Hambrecht, Loffler and Stein1998; Vazquez-Barquero et al., Reference Vazquez-Barquero, Cuesta Nunez, de la Varga, Herrera Castanedo, Gaite and Arenal1995), which is generally associated with more genetic loading, a more serious course of illness, and poorer prognosis. In addition, previous studies (Li et al., Reference Li, Hui, Lee, Chang, Chan and Chen2019; Penn, Mueser, & Spaulding, Reference Penn, Mueser and Spaulding1996) have found higher levels of correlation between female outcomes, negative symptoms, and cognition, suggesting that these aspects are more interrelated than in men. Compared with FEP, the CHR population has more non-specific symptoms, such as emotional symptoms, which are more commonly reported in females than in males (Abel, Drake, & Goldstein, Reference Abel, Drake and Goldstein2010), which may lead to sex effects on neurocognitive performance during episodes of psychosis.
MCD-L
Given that it is already well-known that neurocognitive impairments have been observed in every stage of psychosis, even during the prodromal phase (Bora et al., Reference Bora, Lin, Wood, Yung, McGorry and Pantelis2014; Fusar-Poli et al., Reference Fusar-Poli, Deste, Smieskova, Barlati, Yung, Howes and Borgwardt2012), the novelty of the MCD-L model is that with the application of neurocognitive assessments, we can effectively identify people at risk of psychosis by identifying attenuated psychotic symptoms. The MCD-L model was designed to discriminate CHR from HC with stratification of low-level MCD. This model obtained an AUC of 0.802, which has good discrimination ability, with model estimates (PMCD−L) higher than 59.0%, which had good sensitivity (80.0%) and acceptable specificity (64.8%). Similar to MCD-H, the domains of ‘processing speed’ and ‘verbal memory’ were significant discriminators of the CHR. Additionally, the working memory represented by the WMS-3 spatial span test was a significant factor in the MCD-L model. Working memory has been described as the ability to gate sensory information, and has repeatedly been shown to be impaired in CHR studies (Luo et al., Reference Luo, Zhang, Zhang, Chen, Hong, Luo and Jiang2021; Ramyead et al., Reference Ramyead, Kometer, Studerus, Baumeler, von Rotz and Riecher-Rossler2017; Zhang et al., Reference Zhang, Li, Stone, Woodberry, Seidman, Tang and Wang2015a). The effectiveness of the MCD-L model suggests that neurocognitive dysfunction may not only be a consequence of psychotic symptoms but also precedes psychotic development and can be used to identify psychosis risk groups in the general population.
Age was a significant factor in the MCD-L model. Consistent with converging studies, there was a significant impact on neurocognitive performance due to age effects (Fagerlund et al., Reference Fagerlund, Pantelis, Jepsen, Raghava, Rostrup, Thomas and Glenthoj2021; Rajji, Ismail, & Mulsant, Reference Rajji, Ismail and Mulsant2009). Our previous study (Zhang et al., Reference Zhang, Cui, Wei, Tang, Xu, Hu and Wang2022) found that adolescents and adults with CHR varied in the level of severity and affected domains when compared with HC adolescents and adults. This finding revealed the development of neurocognitive functions, which were dynamic (Reichenberg et al., Reference Reichenberg, Caspi, Harrington, Houts, Keefe, Murray and Moffitt2010) and not balanced over all domains (Smelror et al., Reference Smelror, Jorgensen, Lonning, Kelleher, Cannon, DeRosse and Agartz2019) at different ages. A specific model considering age effects could improve strategies for early identification of psychosis risks in non-clinical settings.
Clinical implications:
Our study carries important clinical implications for the early identification and intervention in individuals at risk for psychosis. The tailored application of MCD models is crucial for optimizing accuracy in early identification efforts. Therefore, we recommend selecting specific MCD models based on the clinical goals. For instance, the MCD-M model may be particularly relevant for predicting conversion, while the MCD-L model may be more suitable for identifying CHR individuals in a broader population. The distinct cognitive domains that discriminate between groups offer insights into potential areas for personalized interventions. Notably, impairments in processing speed and verbal memory, which discriminate HC from both FEP and CHR, suggest deficits in attention and memory functions. This implies that interventions targeting these more general cognitive functions may be valuable for this population. Moreover, the distinct cognitive profile observed in CHR-C compared to CHR-NC, particularly in visual learning, highlights the potential for personalized interventions. Given the association of visual learning with aspects of reality testing and insight (Kim et al., Reference Kim, Jung, Moon, Jeon, Seo, Jung and Kim2021; Xu et al., Reference Xu, Cui, Wei, Qian, Tang, Hu and Wang2022a, Reference Xu, Hao, Wei, Cui, Qian, Wang and Wang2022b), interventions addressing this specific cognitive domain may have implications for enhancing insight and mitigating the risk of progression to overt psychosis. As part of the ongoing efforts to translate these findings into clinical practice, we have initiated clinical trial exploring personalized transcranial magnetic stimulation therapy. This innovative approach targets visual learning deficits, with a focus on left parieto-hippocampal network associated with visuospatial learning (Tang et al., Reference Tang, Xu, Zhu, Cui, Qian, Kong and Wang2023).
Limitations
The present study has some limitations that should be considered. First, although the CHR group in the current study was followed up longitudinally, participants in the FEP and HC groups were evaluated with neurocognition only at enrollment. A longitudinal assessment of cognition is ongoing for further dynamic verification of MCD models. Future studies will explore the cognitive performance of CHR individuals at various time points, including the 2-year follow-up, to provide a more nuanced understanding of cognitive trajectories. Second, it is essential to note that the CHR cohort was monitored in a naturalistic manner, allowing for flexibility in treatment approaches. This means that individuals within the CHR group, while initially enrolled without a history of medication use, may have been prescribed medications during the follow-up period. The diverse medication exposures, encompassing variations in timing, types, doses, and adherence among different individuals, present a potential confounding factor. The complex and non-standardized nature of medication data introduces challenges in objectively quantifying and adjusting for these factors in our analysis. Therefore, it is important to consider that the observed psychotic trajectory during clinical outcome assessments in the CHR cohort may have been influenced by these various medication exposures. Third, no objective intelligence quotient (IQ) test was performed in this study. Whether the differences in neurocognitive performance across groups were attributed to different IQ remains unknown. Fourth, the exclusion of individuals with substance abuse-induced psychosis, while allowing for a more targeted examination of primary psychotic disorders, may limit the generalizability of our findings to the broader population of individuals with psychosis, given the known association between cannabis misuse and the onset of psychosis. Fifth, it is important to acknowledge the potential influence of a floor effect, particularly in individuals with FEP, who may exhibit lower cognitive performance compared to CHR individuals. The presence of a floor effect could impact the discriminative capacity of neurocognitive models, potentially contributing to the observed differentiation in results between FEP and CHR groups. Further research addressing and mitigating floor effects is warranted to enhance the robustness of findings.
Conclusion
This study demonstrated that MCD in early psychosis development varies with stage and population. Based on the different purposes of risk assessment, we proposed three MCD models to define the stage of psychotic risk severity. Therefore, clinicians may need to be particularly vigilant to perform risk assessment based on early signs of cognitive decline, that is, for high-risk suspected psychotic patients, applying MCD-H to discriminate FEP; for moderate-risk CHR individuals, applying MCD-M to predict psychosis onset; and for low-risk general population, applying MCD-L to screen potential at-risk groups. Additionally, it is crucial for clinicians to consider potential environmental factors, such as social withdrawal and substance abuse, when interpreting early signs of cognitive decline in individuals with CHR and FEP.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291724000382.
Acknowledgements
This study was supported by National Key R&D Program of the Ministry of Science and Technology of China (2023YFC2506800), National Natural Science Foundation of China (82171544, 82371505, 82151314, 82101623, 82101582) and Clinical Research Plan of SHDC (SHDC2022CRD026, SHDC2020CR4066).
Authors’ contributions
Dr TH.Z. and JJ.W. conceptualized the study, wrote the first draft of manuscript and conducted the statistical analyses. LH.X., HR.C., and YY.W. interviewed participants and collected and organized the primary data. Z.X.W., T.C., HC.L., YG.H., and XC.T. managed the literature searches, statistical analyses and edited the manuscript. CB.L., TH.Z., and JJ.W. designed the study and provided supervision in the implementation of the study.
Competing interests
None.









