Background
Improvements in healthcare in the past century have contributed to people living longer, thus resulting in an increase of non-communicable diseases in the world’s aging population.Reference Lunenfeld and Stratton 1 The number of people living with dementia worldwide was estimated by the World Health Organization at 47.5 million in 2015 and is projected to increase to 75.6 million by 2030. 2 Dementia prevalence is known to double with every 5-year increment in age after the age of 65 years. 2 Two-thirds of all first ever strokes also occur in patients >65 years of age.Reference Feigin, Forouzanfar and Krishnamurthi 3 Older stroke patients have higher risk-adjusted mortality, greater disability, longer hospital stays and are less likely to be discharged to their original place of residence when compared with their younger counterparts,Reference Benjamin, Blaha and Chiuve 4 and some of these poorer outcomes may be related to concurrent dementia. We sought to understand the prevalence of concurrent dementia in hospitalized patients with ischemic stroke (as the most common stroke subtype), as well as their characteristics and differences in their outcomes.
Methods
We performed a population-based retrospective cohort study of all ischemic stroke patients admitted to hospital using data from the Canadian Institutes for Health Information (CIHI), a non-profit organization that collects universally available healthcare information from 28 pan-Canadian databases. The CIHI’s Discharge Abstract Database (DAD) contains demographic, administrative, and clinical data (including deaths and transfers) on all inpatient hospital discharges. Health services are administered by each of Canada’s ten provinces and three territories. All hospitals in those provinces and territories (with the exception of Quebec) are required to report to the DAD; Quebec reports their data through DAD but does not allow it to be shared. 5 Because all acute care hospitals in Canada provide universal access to the Canadian population, the data are nationally comprehensive.Reference Kokotailo and Hill 6
We analyzed a large administrative data set with anonymized record-level data from April 2003 to March 2015. Individual patient consent is not required, as broad consent is provided by patients at the time of hospitalization. These secondary analyses do not require full ethics review and have been waived by ethics reviews boards. Patients were identified using International Classification of Diseases 10 (ICD-10) codes for ischemic stroke (I63.x, I64.x), excluding transient ischemic attack (TIA) (G45.x), aneurysmal subarachnoid hemorrhage (I60.x) and intracerebral hemorrhage (I61.x). Coding of stroke in the DAD is known to have high sensitivity and specificity.Reference Kokotailo and Hill 6 Administrative data such as these are commonly termed secondary use research data, but when they are applied to an appropriate question with validated case definitions high-quality and reliable conclusions can be inferred.Reference Yu, Holodinsky and Zerna 7 Stroke risk factors (hypertension, diabetes mellitus and atrial fibrillation, congestive heart failure, previous stroke/TIA) and comorbid illness were identified by ICD-10 code and by Charlson-Deyo Index (grouped 0-1 vs. 2+).Reference Deyo, Cherkin and Ciol 8 The Charlson-Deyo index is a score derived from the sum of each present comorbid illness according to the Charlson Comorbidity Score Mapping Table using either ICD-9 or ICD-10 codes. Dementia was recorded as comorbidity at the time of hospital admission, using ICD-10-CA diagnostic codes (codes used are provided in Table 1). The diagnosis of dementia thus probably reflects a chronic outpatient diagnosis; however, we cannot exclude that in some cases the stroke admission may have brought the dementia to medical attention. Discharge location categories were home without services; home with homecare services; inpatient rehabilitation; other acute care facility; complex continuing care; long-term care; died; and other. Discharge locations were used as outcome parameters.
Table 1 Diagnostic codes used for diagnosis of concurrent dementia at the time of hospital admission
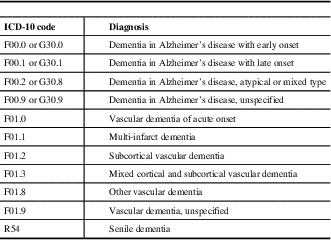
ICD=International Classification of Diseases.
There were no missing data. Measures of central tendency and measures of variability were calculated according to standard descriptive statistics. We used adjusted negative binomial regression models to determine the independent association of dementia with length of stay and adjusted Poisson regression models for mortality and discharge destination, controlling for the following covariates: age, sex, and pre-stroke history of cardiovascular risk factors (namely atrial fibrillation, coronary artery disease, congestive heart failure, diabetes mellitus, hypertension, previous stroke/TIA). All analyses were conducted with the use of SAS software (SAS Institute Inc, Cary, NC, USA). All reported p-values are two-sided.
Results
Between April 2003 and March 2015, 464,741 patients were admitted to the hospital for any stroke syndrome. Ischemic stroke was the most common diagnosis with a prevalence of 67.4%, followed by TIA and subarachnoid hemorrhage both with a prevalence of 16.6% and intracerebral hemorrhage with a prevalence of 11.1%. Concurrent dementia was diagnosed in 29,812 (6.4%) of all of these stroke patients.
Amongst the 313,138 patients with ischemic stroke, 21,788 (7.0%) patients had concurrent dementia. Complete baseline characteristics of ischemic stroke patients with and without concurrent dementia are displayed in Table 2. The proportion of ischemic stroke patients with dementia increased markedly with age, such that 11.1% of >75-year-old stroke patients had dementia.
Table 2 Clinical baseline characteristics of ischemic stroke patients

IQR=interquartile range; TIA=transient ischemic attack.
Patients with concurrent dementia had significantly higher proportions of mortality and discharge to care facilities (Table 3). They had significantly lower proportions of discharge to rehabilitation facilities or home.
Table 3 Univariate outcome analysis of ischemic stroke patients

LOS=length of stay.
A multivariable negative binomial regression model showed that length of stay was more than 40% longer in ischemic stroke patients with concurrent dementia compared with those without dementia after controlling for the effects of age, sex, and pre-stroke cardiovascular risk factors (adjusted risk ratio [RR] 1.439, 95% confidence interval [CI] 1.420-1.458, p-value<0.0001). Multivariable Poisson regression models showed that ischemic stroke patients with dementia had a 14% higher risk of dying in hospital (adjusted RR 1.145, 95% CI 1.112-1.179, p-value<0.0001) and three times higher risk of discharge to a long-term care facility (adjusted RR 3.089, 95% CI 2.992-3.188, p-value<0.0001) compared with ischemic stroke patients without dementia. The patients with dementia were also significantly less likely to return home (adjusted RR 0.756, 95% CI 0.737-0.776, p-value<0.0001).
Discussion
The prevalence of concurrent dementia in hospitalized ischemic stroke patients in our study was low overall (7.0%), but those patients were significantly different in regard to their baseline characteristics and outcomes compared with non-demented ischemic stroke patients. Our study shows that in-hospital mortality was increased and the ability to be discharged to home was reduced in patients coded to have dementia at the time of stroke hospitalization.
Few previous studies have examined the relationship between dementia and death after ischemic stroke, and dementia information is often missing from large stroke registries. A prior review of risk adjustment for stroke mortality failed to identify dementia as a significant factor, although this may be related to lack of ascertainment of dementia status.Reference Katzan, Spertus and Bettger 9 Other single-center studies examining ischemic stroke mortality found concurrent dementia to predict a four-fold increase within 6 months to 2 years during adjusted analysis.Reference Barba, Morin, Cemillan, Delgado, Domingo and Del Ser 10 , Reference Gao, Lian and Zhang 11 However, these studies had sample sizes well below 1000 patients and might reflect low compliance with prescribed treatment regimens in the catchment area or local approaches of treating such patients less aggressively in both the acute stage and regarding their secondary prophylaxis. A risk prediction score for post-stroke mortality rates at 1 year derived from the Registry of the Canadian Stroke Network (RCSN) in Ontario found an approximately 1.5-fold increase during adjusted analysis of data from all provincial stroke centers.Reference Saposnik, Kapral and Liu 12 To our knowledge, ours is the largest population-based study to examine the association between dementia and stroke outcomes so far. We only found a 14% relative risk increase for in-hospital mortality in our study. The lower estimate compared with the other studies could be caused by a shorter follow-up period, as well as the fact that stroke mortality is also common in patients without concurrent dementia, leading to odds ratios exaggerating the relative risk.
The reason for higher mortality in ischemic stroke patients with concurrent dementia cannot be ascertained from our study, other than that it was not explained by older age or a greater number of vascular risk factors. Potential reasons could include pre-existing disabilities including gait impairment and dysphagia that can accompany dementia, more severe strokes, or a greater propensity for next of kin to pursue palliative care. Because of limitations inherent to administrative health data, we were unable to analyze pre-stroke living status, stroke severity, or palliative care orders.
Among ischemic stroke survivors, our study documented a 40% increase in risk for prolonged length of stay in the hospital. Reasons could include barriers in disposition planning owing to patient’s physical functionality and orientation, as well as higher risk of complications, including risk of falls and pressure ulcers, which need prolonged medical care.Reference Motzek, Junge and Marquardt 13 A study of concurrent dementia among patients on an internal medicine ward admitted for acute illness revealed that the prolonged stay caused excess costs of 19% compared with other admissions.Reference Motzek, Junge and Marquardt 13 Therefore, early planning for discharge seems warranted in patients with dementia to try to reduce length of stay.
Hospitalized ischemic stroke patients were also significantly more often discharged to long-term care facilities and significantly less likely to return to their home. A high proportion of people with dementia need some care, ranging from support with (instrumental) activities of daily living to full personal care and round-the clock supervision. In many high-income countries, such as Canada, one-third to one-half of people with dementia live in resource- and cost-intensive residential or nursing homes.Reference Macdonald and Cooper 14 Dementia is imposing huge economic burdens, both through direct (medical and social care) and indirect costs (unpaid caregiving by families and friends). 2 Although ischemic stroke patients with dementia were at a higher risk for discharge to long-term care, it was possible to discharge almost one in five to home. An early focus on discharge planning and home services may facilitate discharge to home rather than to a resource-intensive long-term care facility.
Strengths of the study include the large sample size and the population-based data collection, as Canada has a single-payer health system. Limitations include those common to administrative health databases: no information on pre-stroke living status, the presenting stroke severity, or the modalities of treatment provided at the receiving hospital including the use of palliative care. A prior study in the Netherlands showed high validity of diagnosis of overall dementia using ICD-9 in a nationwide register, but no such analysis has been conducted for Canada and current coding standards.Reference van de Vorst, Vaartjes, Sinnecker, Beks, Bots and Koek 15 In addition, if stroke and TIA are under-recognized or misdiagnosed in patients with dementia leading to fewer hospitalizations coded for stroke and TIA are, then the true prevalence of dementia in stroke patients could be underestimated by administrative data. It is unclear how this research undertaken in the Canadian health system might be extrapolated to other systems.
Conclusion
Approximately 1 in 13 hospitalized ischemic stroke patients have concurrent dementia as per diagnostic coding. Ischemic stroke patients with dementia have higher mortality, face significantly more dependence and utilize greater healthcare resources. Early care planning and coordination could possibly optimize the outcomes of these patients and minimize societal costs, but further research needs to be conducted in this area.
Acknowledgments
Parts of this material are based on data and information provided by the Canadian Institute for Health Information. However, the analyses, conclusions, opinions and statements expressed herein are those of the author and not those of the Canadian Institute for Health Information. Funding for the data analysis was provided by the Heart and Stroke Foundation of Canada.
Disclosures
CZ, MPL, RHS and JF have nothing to disclose. EES reports other from Heart and Stroke Foundation of Canada, during the conduct of the study, as well as grants from Canadian Institutes of Health Research and grants from Brain Canada, outside the submitted work.
Statement of Authorship
CZ contributed to the study concept and design, interpretation of the data, as well as drafting and revising the manuscript. MPL contributed to the study concept and design, data acquisition and management, data interpretation and revising the manuscript. JF contributed to the data analysis and interpretation, as well as revising the manuscript. RHS contributed to the study concept and design, and revising the manuscript. EES contributed to the study concept and design, interpretation of the data, revising the manuscript and functioned as the senior author for this work.





