Butyrate is an important product of bacterial fermentation of dietary fibre in the colonic lumen, where it is consumed as a primary energy source by the mucosa(Reference Cummings1). It plays an active role in gut health by its anti-inflammatory properties(Reference Tedelind, Westberg, Kjerrulf and Vidal2), its ability to decrease paracellular permeability via modulation of the tight junction proteins(Reference Mariadason, Barkla and Gibson3) and promotion of the restoration and preservation of the gut barrier after damage(Reference Venkatraman, Ramakrishna and Pulimood4, Reference Kanauchi, Iwanaga, Mitsuyama, Saiki, Tsuruta, Noguchi and Toyonaga5).
The pathogenesis of a Campylobacter jejuni infection, currently one of the leading causes of food-borne bacterial gastroenteritis, is associated with bacterial invasion in and translocation over the intestinal epithelium(Reference Russell, O'Donnoghue, Blake, Zulty and DeTolla6, Reference Youssef, Corthier, Goossens, Tancrede, Henry-Amar and Andremont7) and disruption of tight junctions(Reference MacCallum, Hardy and Everest8). The mechanisms behind C. jejuni translocation are paradoxical: both transcellular and paracellular routes have been observed(Reference Bras and Ketley9, Reference Monteville and Konkel10). A drop in transepithelial resistance (TER) is noticed after prolonged infection times and high inoculation doses(Reference MacCallum, Hardy and Everest8, Reference Bras and Ketley9). This TER drop is associated with a re-localisation of occludin from an intercellular to an intracellular location(Reference MacCallum, Hardy and Everest8, Reference Chen, Ge, Fox and Schauer11) and thus a disruption of the tight junctions.
Because of the beneficial effects of butyrate on tight junction integrity and gut health, we investigated whether butyrate could play a protective role during C. jejuni infection by decreasing invasion and increasing tight junction integrity resulting in decreased bacterial paracellular translocation.
Materials and methods
Cell culture and reagents
Caco-2 cells were used in the present study, because this cell line is widely known for its ability to differentiate and its capacity to form high columnar epithelial cells, closely resembling intestinal epithelium(Reference Hidalgo, Raub and Borchardt12). Caco-2 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 1 % non-essential amino acids and 10 % fetal calf serum, penicillin (100 U/ml) and streptomycin (0·1 mg/ml) at 37°C in a 5 % CO2 atmosphere. All cell-culture reagents were from Gibco (Invitrogen, Merelbeke, Belgium). For all experiments twenty-four-well plates were used. Cells were seeded at 1 × 105 cells/well in 1 ml medium and allowed to attach and reach confluency for 3 d. Cells were subjected to experiments at two stages of differentiation: for undifferentiated cells, 3-d-old monolayers were used, while for differentiated cells, monolayers were used 17 d after seeding. Medium without butyrate supplement was refreshed every 72 h, while medium with butyrate was changed daily.
Bacterial strains and culture conditions
C. jejuni strain R-27456, kindly provided by Professor Dr Peter Vandamme (Ghent University, Ghent, Belgium), was isolated from a patient suffering from bloody diarrhoea. Bacteria were routinely cultured in Preston broth (Oxoid, Basingstoke, Hants, UK) supplemented with Campylobacter-specific growth supplements (SR117 and SR0232; Oxoid) at 42°C under microaerobic conditions (5 % O2; 5 % CO2; 5 % H2; 85 % N2). Escherichia coli DH5α and Salmonella enteritidis 76SA88 were routinely cultured in Luria broth at 37°C. For enumeration of bacteria, C. jejuni, E. coli and S. enteritidis were plated on modified charcoal cefoperazone deoxycholate agar (mCCDA) plates, Luria agar plates and brilliant green agar plates, respectively (Oxoid).
Cell number and cell viability assay
The number of Caco-2 cells available in the wells for bacterial invasion was quantified, since inhibition of proliferation and induction of apoptosis by butyrate could influence available Caco-2 cell number(Reference Siavoshian, Blottiere, Le Foll, Kaeffer, Cherbut and Galmiche13, Reference Ruemmele, Schwartz, Seidman, Dionne, Levy and Lentze14). Cells were seeded on glass coverslips in a twenty-four-well plate at 1 × 105 cells/well and allowed to adhere and grow for 3 or 17 d. Cell numbers of undifferentiated and differentiated cells, incubated with 0·0 mm-, 2·5 mm- or 5·0 mm-butyrate for 24, 48 or 72 h were enumerated microscopically after fixation with ice-cold methanol and staining with Hoechst 33342 (10 μg/ml; Sigma Aldrich) for 10 min at room temperature. Glass coverslips were mounted and were examined through a Leica DM CB microscope (Leica, Wetzlar, Germany) and cells of a minimum of seven fields were counted.
Cell viability was assessed by using the colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma, Bornem, Belgium) assay. Undifferentiated and differentiated cells were treated with butyrate as described above. MTT (0·5 mg/ml) was added to the wells and cells were further incubated for 3 h at 37°C and 5 % CO2. The reaction was stopped by aspirating the medium and the formed formazan crystals were dissolved by adding dimethylsulfoxide. Absorbance was measured at 570 nm with a reference filter at 690 nm.
Bacterial invasion assay
Caco-2 cells were seeded in twenty-four-well plates at 1 × 105 cells/well and allowed to adhere and reach confluency for 3 d. Because butyrate induces differentiation of the Caco-2 cell layer(Reference Siavoshian, Blottiere, Le Foll, Kaeffer, Cherbut and Galmiche13), we used undifferentiated and differentiated monolayers to investigate the effect of an increased cellular differentiation status on invasion susceptibility.
At 3 d and 17 d respectively, butyrate was added to the medium at 0·0, 2·5 or 5·0 mm for 24, 48 or 72 h. Thereafter, cell cultures were washed and exposed to 8 × 107 colony-forming units (cfu) of C. jejuni suspended in cell-culture medium without antibiotics and containing 0·0, 2·5 or 5·0 mm-sodium butyrate. Plates were centrifuged for 10 min at 600 g at 37°C to deposit bacteria to the surface of the monolayer. After 3 h, cell layers were washed and incubated with gentamicin (100 μg/ml) for 2 h to kill extracellular bacteria. To enumerate intracellular bacteria, cells were lysed using 0·25 % sodium deoxycholate and bacterial counts were performed by titration on modified charcoal cefoperazone deoxycholate agar (mCCDA) plates.
To examine the effect of butyrate on the C. jejuni ability to invade Caco-2 cells, bacteria were grown in Preston broth containing 5·0 mm-butyrate for 18 h at 42°C, under microaerobic conditions. The invasion assay was carried out on 3-d-old Caco-2 cells, not exposed to butyrate.
Bacterial translocation and epithelial permeability assays
For these experiments, 1 × 105 cells/well were seeded on the apical side of Transwell polyester membrane filters (6·5 mm diameter inserts, 3·0 μm pore size; Corning, Sigma Aldrich, Bornem, Belgium) and left to differentiate for 17 d, after which 0·0 mm- or 5·0 mm-sodium butyrate (Sigma Aldrich) was added to the apical and basolateral chamber. After 48 h, cells were washed and 2 × 107 cfu of C. jejuni were added to the apical chamber in cell culture medium without antibiotics and containing 0·0 mm- or 5·0 mm-sodium butyrate. Well plates were centrifuged for 10 min at 600 g at 37°C. E. coli DH5α and S. enteritidis 76SA88 served as negative and positive controls respectively for bacterial translocation and TER reduction(Reference Sugita-Konishi, Ogawa, Arai, Kumagai, Igimi and Shimizu15). To enumerate translocated bacteria, 20 μl samples were taken from the basolateral chamber at 0, 1, 3, 6 and 24 h after centrifugation and titrated. Resistance was measured before and after inoculation using the Endohm™ tissue resistance measurement chamber coupled to an EVOM™ epithelial voltohmmeter (World Precision Instruments, Stevenage, Herts, UK).
Statistical analysis
All statistics were performed on at least three independent assays. Results are given as mean values with their standard errors. Significance was tested by the two-tailed Student's t test and P < 0·05 was considered significant.
Results
The effect of butyrate on Caco-2 cell viability and cell number
Since butyrate is known to induce apoptosis in Caco-2 cells and cell invasion by C. jejuni is influenced by the available cell number in the monolayer, the effect of butyrate on cell viability and number was evaluated. Treatment of undifferentiated monolayers with butyrate resulted in decreased cell viability after 72 h incubation with 2·5 mm and 5·0 mm (P < 0·01) as shown in Fig. 1(A). Differentiated cells showed a decreased cell viability only after incubation with 5·0 mm-butyrate for 48 h and longer (Fig. 1(B)) (P < 0·05), while 2·5 mm had no effect.

Fig. 1 Effect of butyrate on cell viability and cell number after exposure to 2·5 mm- (![]() ) or 5·0 mm-butyrate (□) for 24, 48 and 72 h, compared with the untreated control (■) in (A) 3-d-old undifferentiated and (B) 17-d-old differentiated Caco-2 cells. Cell viability was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Results are expressed on the left axis as % of control (100 %). On the right axis, the cell number after incubation for 24, 48 and 72 h with concentrations of 2·5 mm (●) or 5·0 mm (○) is given as % of control (100 %). Values are means calculated from at least three different experiments, with standard errors represented by vertical bars. Mean value was significantly different from that of the untreated control: *P < 0·05, **P < 0·01.
) or 5·0 mm-butyrate (□) for 24, 48 and 72 h, compared with the untreated control (■) in (A) 3-d-old undifferentiated and (B) 17-d-old differentiated Caco-2 cells. Cell viability was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Results are expressed on the left axis as % of control (100 %). On the right axis, the cell number after incubation for 24, 48 and 72 h with concentrations of 2·5 mm (●) or 5·0 mm (○) is given as % of control (100 %). Values are means calculated from at least three different experiments, with standard errors represented by vertical bars. Mean value was significantly different from that of the untreated control: *P < 0·05, **P < 0·01.
The cell number in the monolayer was slightly affected by butyrate treatment. Cell number of undifferentiated monolayers (Fig. 1(A)) was only significantly reduced after 76 h incubation with 5·0 mm-butyrate and the percentage compared with the untreated control was 63 (sem 3) % (P < 0·01). For differentiated monolayers (Fig. 1(B)), 5·0 mm significantly reduced cell number after 24 and 48 h (86 (sem 2) and 79 (sem 3) % respectively compared with the untreated control (P < 0·01)).
Butyrate protects undifferentiated Caco-2 cells better against invasion of Campylobacter jejuni than differentiated cells
Treatment of the monolayer with butyrate caused a significant decrease in C. jejuni invasion and this effect was concentration dependent. This reduction was observed as soon as 24 h after pre-incubation of the monolayer with as little as 2·5 mm-butyrate. Data for undifferentiated monolayers are summarised in Fig. 2(A). No further decrease in invasion was found when monolayers where incubated longer than 48 h (P>0·05).
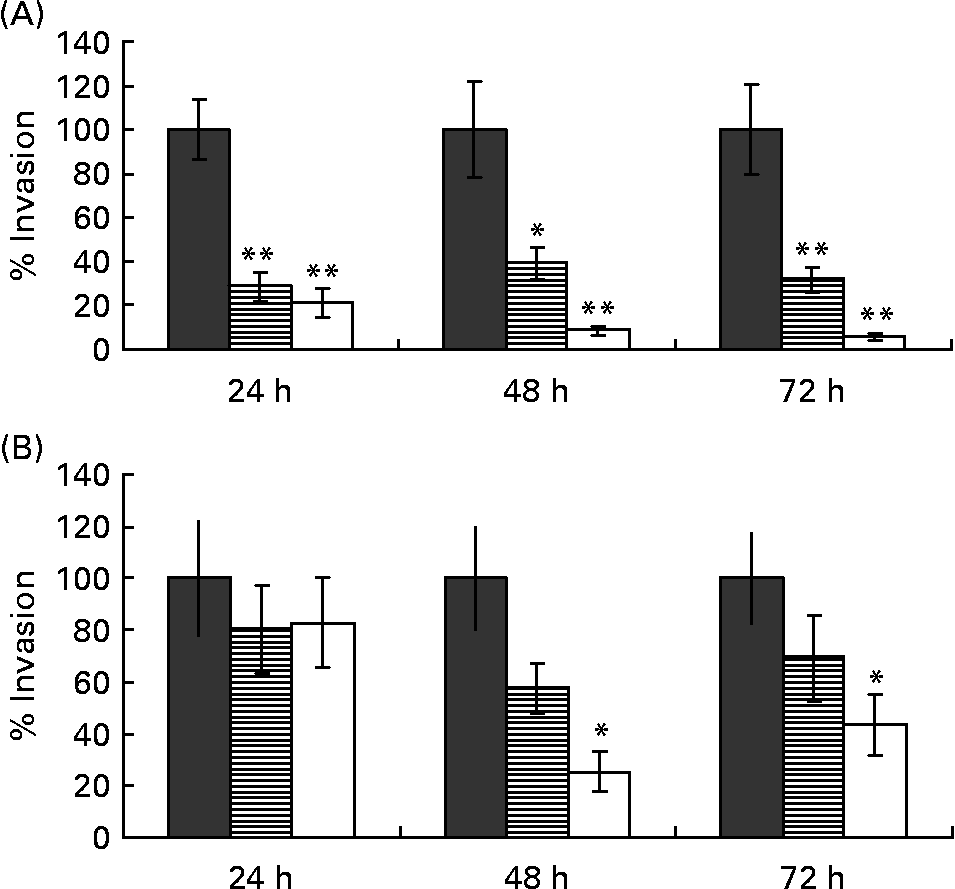
Fig. 2 The percentages of invaded Campylobacter jejuni bacteria in (A) 3-d-old undifferentiated and (B) 17-d-old differentiated Caco-2 cells, pre-treated with 2·5 mm- (![]() ) or 5·0 mm-butyrate (□) for 24, 48 or 72 h, compared with untreated control (100 %, ■). Values are means calculated from at least three different experiments, with standard errors represented by vertical bars. Mean value was significantly different from that of the untreated control: *P < 0·05, **P < 0·01.
) or 5·0 mm-butyrate (□) for 24, 48 or 72 h, compared with untreated control (100 %, ■). Values are means calculated from at least three different experiments, with standard errors represented by vertical bars. Mean value was significantly different from that of the untreated control: *P < 0·05, **P < 0·01.
Because butyrate enhances the differentiation of Caco-2 cells, we investigated the impact of the differentiation state alone on C. jejuni invasion. In absence of butyrate, undifferentiated 3-d-old Caco-2 monolayers were almost four times more susceptible for C. jejuni invasion than 17-d-old differentiated cells: 2·3 (sem 0·3) × 105 and 4·4 (sem 1·1) × 104 cfu/ml (P < 0·001) respectively, despite equal cell numbers in the monolayer (average 1·44 (sem 0·05) × 106 cells/well and 1·43 (sem 0·07) × 106 cells/well for differentiated and undifferentiated cells respectively; P>0·05). Even so, butyrate still had a protective effect on 17-d-old Caco-2 cells, although only concentrations of 5·0 mm and incubation times of 48 h or longer were needed. A butyrate concentration of 2·5 mm had no effect on C. jejuni invasion at all. Data are summarised in Fig. 2(B).
No significant differences were found in the ability of C. jejuni to invade the Caco-2 monolayer, when bacteria were grown in the presence of 5·0 mm-butyrate, compared with C. jejuni grown in Preston broth alone (data not shown).
Butyrate reduces translocation of Campylobacter jejuni over the Caco-2 cell monolayer, despite loss of transepithelial resistance
Because of the marked decrease of C. jejuni invasion due to butyrate treatment, we wondered if butyrate could also influence translocation of C. jejuni over Caco-2 cells. The addition of 5·0 mm-butyrate to the monolayer for a period of 48 h resulted in a slight but still significant drop in TER (0·0 mm-butyrate: 1056 (sem 34) Ω/cm2; 5·0 mm-butyrate: 841 (sem 78) Ω/cm2; P < 0·01).
When C. jejuni was added to the apical chamber of butyrate-treated and -untreated differentiated monolayers seeded in the Transwell system, bacteria were recovered from the basolateral chamber after centrifugation. However, 24 h after bacterial inoculation, approximately ten times less bacteria were recovered: 1·3 (sem 0·8) × 105 cfu/ml for monolayers treated with 5·0 mm-butyrate, compared with 3·0 (sem 1·5) × 106 cfu/ml for monolayers not treated with butyrate (P < 0·05). Results are given in Fig. 3.

Fig. 3 Campylobacter jejuni translocation over butyrate-pre-treated monolayers. Monolayers were incubated with 0·0 mm- (▲) or 5·0 mm-butyrate (△) for 48 h. Values are means calculated from three different experiments, with standard errors represented by vertical bars. * Mean value was significantly different from that of the untreated control (P < 0·05). cfu, Colony-forming units.
The addition of C. jejuni at 2 × 107 cfu to the apical chamber of untreated cells did not result in TER decrease (before inoculation: 1056 (sem 34) Ω/cm2; after inoculation: 1026 (sem 87) Ω/cm2; P>0·05). In contrast, when monolayers were inoculated with C. jejuni after pre-treatment of the Caco-2 cells with 5·0 mm-butyrate for 48 h, a pronounced drop of TER was observed 24 h after infection (before inoculation: 841 (sem 78) Ω/cm2; after inoculation: 425 (sem 53) Ω/cm2; P < 0·01).
No translocation or drop in TER was observed when both butyrate-pre-treated and -untreated monolayers were inoculated with E. coli DH5α, which served as a negative control. When S. enteritidis was added to the monolayers, a drop in TER was observed both in butyrate-treated and -untreated monolayers after 6 h, which was accompanied with a massive translocation of bacteria. Average TER loss for untreated cells and treated cells was, respectively, 78 (sem 4) and 90 (sem 37) Ω/cm2 (P>0·05), while translocation of bacteria over the monolayer was 22 (sem 4) × 107 cfu/ml and 8 (sem 1) × 107 cfu/ml for the untreated control and 5·0 mm-butyrate-treated monolayer respectively (P>0·05).
Discussion
The present study showed that butyrate can protect Caco-2 cells against the first steps of C. jejuni pathogenesis: invasion and translocation. These effects could be attributed to a protective effect of butyrate on Caco-2 cells and not to an effect on the pathogen itself regarding its invasive capabilities.
Butyrate protected Caco-2 cells from C. jejuni invasion in a concentration-dependent manner and an incubation time of 24 h was sufficient. It has been described that butyrate inhibits proliferation and induces apoptosis(Reference Siavoshian, Blottiere, Le Foll, Kaeffer, Cherbut and Galmiche13, Reference Ruemmele, Schwartz, Seidman, Dionne, Levy and Lentze14) and thus the observed decrease in invasion could be the result of a reduced cell number in the monolayer. However, in the case of undifferentiated cells, the effect of butyrate on cell number and cell viability could not explain these observations: the number and viability of undifferentiated Caco-2 cells was only significantly reduced from 72 and 48 h incubation with 5·0 mm-butyrate respectively, while the reduction of Campylobacter invasion was observed as soon as 24 h after treatment with butyrate.
The reduced bacterial invasion might be largely explained by the butyrate-induced cellular differentiation(Reference Siavoshian, Blottiere, Le Foll, Kaeffer, Cherbut and Galmiche13). This hypothesis is supported by the finding that, in the absence of butyrate, differentiated monolayers were four times less susceptible to C. jejuni invasion than undifferentiated cells. A possible mechanism of butyrate protection could be the decrease in tyrosine phosphorylation observed during Caco-2 cell differentiation(Reference Schwartz, Lamprecht, Polak-Charcon, Niv and Kim16). Indeed C. jejuni invasion has been shown to depend on tyrosine phosphorylation of the host cell(Reference Biswas, Niwa and Itoh17–Reference Wooldridge, Williams and Ketley19).
Butyrate still had a protective effect on differentiated monolayers, but only after a pre-treatment period of 48 h with 5·0 mm-butyrate. This is in accordance with earlier observations that the differentiating monolayer loses its sensitivity towards butyrate(Reference Mariadason, Velcich, Wilson, Augenlicht and Gibson20). The observed reduction of invasion of Campylobacter coincided with a decreased cell viability and it seems that, unlike undifferentiated cells, the protective effect of butyrate can be at least partly be attributed to a decrease in cell viability.
The second step in C. jejuni pathogenesis, namely translocation across the cell monolayer, was significantly reduced when cells were treated with butyrate, despite a marked decrease in TER. Although this drop suggests increased monolayer permeability, it seems that tight junctions still displayed sufficient integrity to prevent mass paracellular translocation of C. jejuni. The decrease in TER combined with a pronounced reduction of translocated bacteria thus favours the hypothesis that translocation of C. jejuni is transcellular in nature and that the observed reduction of translocation is caused by a decreased susceptibility of the cell for C. jejuni invasion.
Butyrate is frequently associated with various health-promoting functions, such as a decreased risk for colon cancer and irritable bowel syndrome. The present results suggest that butyrate might protect from campylobacteriosis. The concentration of butyrate can be increased by altering the composition of the diet. Resistant starch and dietary fibres that escaped digestion are the most important sources for fermentation by the colonic anaerobic microbiota. Various feeding studies demonstrate the ability of diet composition to alter SCFA concentrations in the intestine. Rats that were fed a wheat-bran diet had increased butyrate concentration compared with rats on a high-fermentable pectin diet(Reference Lupton and Kurtz21). The distribution of butyrate depends on the fermentability of fibres: the fermentation and absorption of SCFA is very fast and highly fermentable fibres such as pectin and oat bran are absorbed in the caecum and the proximal colon, while the consumption of slowly fermentable fibres, such as wheat bran, result in a consistently high butyrate level in the distal region of the rat colon(Reference McIntyre, Young, Taranto, Gibson and Ward22, Reference McIntyre, Gibson and Young23). It should be noted that fast fermentable fibres still are able to contribute to intestinal health and elevation in butyrate concentration, as demonstrated by Hallert et al. (Reference Hallert, Bjorck, Nyman, Pousette, Granno and Svensson24). In their study, patients suffering from ulcerative colitis were subjected to a diet consisting of 20 g dietary fibre from oat bran and their faecal butyrate concentration was significantly increased at the end of the trial. Since an elevation of butyrate concentration due to slow-fermenting fibres is most noticeable in the distal colon, and considering the topology of C. jejuni infections(Reference Peterson25, Reference Fauchere, Veron, Lellouch-Tubiana and Pfister26), dietary fibres could have a positive influence on C. jejuni-induced enteritis by reducing C. jejuni invasion and translocation over the gut epithelium.
In conclusion, the present study illustrates that butyrate is able to protect the Caco-2 monolayer from C. jejuni invasion and translocation, correlating with enhanced cellular differentiation. Since colonic butyrate concentrations depend on dietary fibre, diet formulation may influence the outcome of campylobacteriosis.
Acknowledgements
The study was financed by the Federal Public Service for Health, Food Chain Safety and Environment project R-04/002-CAMPY. The authors acknowledge that there is no conflict of interest.





