INTRODUCTION
Decades of research have examined the association between recreational cannabis use and cognitive outcomes; however, few studies have examined the relationship between cognitive function and medical cannabis (MC) use, defined as using cannabis specifically to treat symptoms of a medical condition. Although recreational and MC products are derived from the same plant species (Russo, Reference Russo2007), inherent differences typically exist between those who use cannabis recreationally and those who use for medical purposes.
Recreational cannabis use often begins during adolescence (Substance Abuse and Mental Health Services Administration, 2019), a period marked by critical neurodevelopment (Casey, Galvan, & Hare, Reference Casey, Galvan and Hare2005; Giedd et al., Reference Giedd, Blumenthal, Jeffries, Castellanos, Liu, Zijdenbos and Rapoport1999; Gogtay et al., Reference Gogtay, Nugent, Herman, Ordonez, Greenstein, Hayashi and Thompson2006; Houston, Herting, & Sowell, Reference Houston, Herting and Sowell2014; Lebel & Deoni, Reference Lebel and Deoni2018). Many studies assessing recreational cannabis users have demonstrated that earlier cannabis onset is related to poorer cognitive performance (Gruber & Sagar, Reference Gruber and Sagar2017; Lisdahl, Gilbart, Wright, & Shollenbarger, Reference Lisdahl, Gilbart, Wright and Shollenbarger2013; Sagar & Gruber, Reference Sagar and Gruber2018), likely the result of cannabis exposure during this period of neurodevelopmental vulnerability (Casey et al., Reference Casey, Galvan and Hare2005; Giedd et al., Reference Giedd, Blumenthal, Jeffries, Castellanos, Liu, Zijdenbos and Rapoport1999; Gogtay et al., Reference Gogtay, Nugent, Herman, Ordonez, Greenstein, Hayashi and Thompson2006). As the endocannabinoid system impacts growth, differentiation, positioning, and connectivity among neurons, exposure to exogenous cannabinoids, specifically delta-9-tetrahydrocannabinol (THC), the primary intoxicating constituent of cannabis, may disrupt neural development, particularly during adolescence. In contrast, the majority of MC patients initiate MC use during adulthood (Gruber et al., Reference Gruber, Sagar, Dahlgren, Gonenc, Smith, Lambros and Lukas2018; Ilgen et al., Reference Ilgen, Bohnert, Kleinberg, Jannausch, Bohnert, Walton and Blow2013), when they are beyond vulnerable developmental periods; later initiation of MC is likely due to increased prevalence of chronic conditions as individuals age (Atella et al., Reference Atella, Mortari, Kopinska, Belotti, Lapi, Cricelli and Fontana2019) and MC laws which generally apply to adults. Accordingly, recreational users and MC patients may experience different cognitive effects of cannabis given potential differences in age of onset of use.
In addition, recreational users and MC patients may have access to the same products, but recreational users typically choose products with considerable amounts of THC as they desire “high” or mood-altering effects (Wachtel, ElSohly, Ross, Ambre, & de Wit, Reference Wachtel, ElSohly, Ross, Ambre and de Wit2002; Zeiger et al., Reference Zeiger, Haberstick, Corley, Ehringer, Crowley, Hewitt and Rhee2010). MC patients, whose primary goal is symptom alleviation (Nunberg, Kilmer, Pacula, & Burgdorf, Reference Nunberg, Kilmer, Pacula and Burgdorf2011), may also use products containing THC which can confer therapeutic effects such as analgesia (De Vita, Moskal, Maisto, & Ansell, Reference De Vita, Moskal, Maisto and Ansell2018), antiemesis, and appetite stimulation (Abrams, Reference Abrams 2016; Walsh, Nelson, & Mahmoud, Reference Walsh, Nelson and Mahmoud2003). However, they often want to avoid feeling intoxicated, and frequently seek products with varied cannabinoid profiles, particularly those containing cannabidiol (CBD), a non-intoxicating cannabinoid touted for its therapeutic potential.
Additional research studies, particularly clinical trials, are needed to determine the potential efficacy of CBD for various conditions (Black et al., Reference Black, Stockings, Campbell, Tran, Zagic, Hall and Degenhardt2019; NASEM, 2017). Although data are currently mixed, various factors including dosing and CBD product type (e.g., single, purified vs. full- or broad-spectrum) likely impact findings (Gallily, Yekhtin, & Hanuš, Reference Gallily, Yekhtin and Hanuš2015; Millar et al., Reference Millar, Stone, Bellman, Yates, England and O’Sullivan2019), and several studies have highlighted promising results (Bonaccorso, Ricciardi, Zangani, Chiappini, & Schifano, Reference Bonaccorso, Ricciardi, Zangani, Chiappini and Schifano2019). Studies suggest CBD may mitigate or decrease negative effects often associated with THC exposure, including cognitive decrements, as noted in a study examining the effects of smoked cannabis with higher versus lower amounts of CBD (Morgan, Schafer, Freeman, & Curran, Reference Morgan, Schafer, Freeman and Curran2010), and a study in which frequent cannabis users were administered 200 mg oral CBD for 10 weeks (Solowij et al., Reference Solowij, Broyd, Beale, Prick, Greenwood, van Hell and Yucel2018). Although some studies found THC induces psychotic-like symptoms or anxiogenic effects at high doses (Hunault et al., Reference Hunault, Bocker, Stellato, Kenemans, de Vries and Meulenbelt2014; Martin-Santos et al., Reference Martin-Santos, Crippa, Batalla, Bhattacharyya, Atakan, Borgwardt and McGuire2012), these effects may be mitigated by CBD, as demonstrated in a study assessing the impact of smoked cannabis with varied cannabinoid levels (Schubart et al., Reference Schubart, Sommer, van Gastel, Goetgebuer, Kahn and Boks2011). Additionally, Bergamaschi et al. (Reference Bergamaschi, Queiroz, Chagas, de Oliveira, De Martinis, Kapczinski and Crippa2011) reported that pre-administration of 600 mg oral CBD prior to a Simulated Public Speaking Test reduced anxiety, discomfort, and cognitive impairment relative to a placebo group. Similarly, Crippa et al. (Reference Crippa, Derenusson, Ferrari, Wichert-Ana, Duran, Martin-Santos and Hallak2011) reported reduced anxiety and altered limbic activity following oral administration of 400 mg CBD (Crippa et al., Reference Crippa, Derenusson, Ferrari, Wichert-Ana, Duran, Martin-Santos and Hallak2011). Overall, THC and CBD have been shown to have opposing neural effects, particularly in regions rich in cannabinoid receptors (Lorenzetti, Solowij, & Yucel, Reference Lorenzetti, Solowij and Yucel2016).
It is possible that if MC treatment reduces physical or psychological symptoms, cognitive function may actually improve as patients feel better. For example, chronic pain, the most common indication for MC use (Park & Wu, Reference Park and Wu2017), has been shown to adversely impact cognitive performance, specifically tasks requiring attention and executive function (Moriarty, McGuire, & Finn, Reference Moriarty, McGuire and Finn2011). Sleep has also been associated with cognitive function; good sleep quality promotes better cognitive function and protects against age-related cognitive decline and dementia (Minakawa, Wada, & Nagai, Reference Minakawa, Wada and Nagai2019; Scullin & Bliwise, Reference Scullin and Bliwise2015; Shi et al., Reference Shi, Chen, Ma, Bao, Han, Wang and Lu2018). Additionally, studies note that anxiety, another common indication for MC (Grella, Rodriguez, & Kim, Reference Grella, Rodriguez and Kim2014), interferes with attention and executive function (Vytal, Cornwell, Letkiewicz, Arkin, & Grillon, Reference Vytal, Cornwell, Letkiewicz, Arkin and Grillon2013). Certain cannabinoids, particularly CBD, appear to have anxiolytic properties, demonstrated by acute administration studies in healthy volunteers (Zuardi, Cosme, Graeff, & Guimaraes, Reference Zuardi, Cosme, Graeff and Guimaraes1993) and individuals with anxiety disorders (Bergamaschi et al., Reference Bergamaschi, Queiroz, Chagas, de Oliveira, De Martinis, Kapczinski and Crippa2011). A large-case series also reported a 79% improvement in retrospective anxiety ratings after 1 month of CBD treatment (Shannon, Lewis, Lee, & Hughes, Reference Shannon, Lewis, Lee and Hughes2019). MC treatment could therefore be associated with better concentration and enhanced cognitive performance if primary medical symptoms are reduced.
Given inherent differences between medical and recreational cannabis use, it is likely that MC patients will not exhibit the same pattern of decrements on neuropsychological measures traditionally observed in young, heavy recreational users (Crean, Crane, & Mason, Reference Crean, Crane and Mason2011; Jacobus & Tapert, Reference Jacobus and Tapert2014; Lisdahl, Wright, Kirchner-Medina, Maple, & Shollenbarger, Reference Lisdahl, Wright, Kirchner-Medina, Maple and Shollenbarger2014). Interestingly, in the first study to directly assess patients using “real world” MC products pre- versus post-MC treatment, we collected pilot data in 11 patients at baseline (prior to initiating MC use), and following 3 months of regular MC treatment (Gruber et al., Reference Gruber, Sagar, Dahlgren, Racine, Smith and Lukas2016). In contrast to executive function decrements typically observed in recreational cannabis users, MC patients exhibited improved performance on the Stroop Color Word Test and Trail Making Test, reflected by faster response time without loss of accuracy. Further, MC patients experienced cognitive improvements in the context of moderate improvements on measures of depression, sleep, and quality of life.
Additionally, Olla et al (Reference Olla, Rykulski, Hurtubise, Bartol, Foote, Cutler and Erdodi2019) recently examined the short-term effects of THC in MC patients at baseline (not intoxicated), immediately after using high THC (20%) cannabis products, and several hours later. Although the authors hypothesized poorer performance during intoxication, findings revealed stable or improved performance across several cognitive domains. Of note, findings may have been significantly impacted by practice effects, as alternate test versions were not used, and only a short period of time elapsed between assessments. However, as several studies report poorer cognitive performance following acute cannabis administration (Desrosiers, Ramaekers, Chauchard, Gorelick, & Huestis, Reference Desrosiers, Ramaekers, Chauchard, Gorelick and Huestis2015; Hart et al., Reference Hart, Ilan, Gevins, Gunderson, Role, Colley and Foltin2010; Hart, van Gorp, Haney, Foltin, & Fischman, Reference Hart, van Gorp, Haney, Foltin and Fischman2001), results from Olla and colleagues provide further evidence that patients using cannabis medically may not experience the same decrements often observed in recreational consumers.
In order to more thoroughly examine the long-term impact of MC treatment on cognition, the current, ongoing study expanded our pilot investigation (Gruber et al., Reference Gruber, Sagar, Dahlgren, Racine, Smith and Lukas2016), utilizing a longitudinal design where MC patients completed baseline cognitive and clinical assessments prior to initiation of MC use and were reassessed at multiple time points following initiation of MC treatment (3, 6, and 12 months). Information regarding MC treatment regimens, including frequency of use and exposure to THC and CBD were also quantified to determine if MC use variables contributed to cognitive and clinical changes.
Hypotheses
Based on pilot work as well as the fact that MC patients are often older and may choose non-intoxicating products or those with varied cannabinoid profiles, we hypothesized that MC patients would not demonstrate decrements and might instead exhibit improvements in cognitive function after initiating regular MC use for up to 1 year. Further, we predicted that MC patients would report improved clinical ratings following MC treatment. Additional analyses examined potential relationships between changes in cognitive function and self-reported clinical ratings as well as measures of cannabinoid use (e.g., frequency of use, THC, and/or CBD exposure).
METHODS
This research was completed in accordance with the Declaration of Helsinki.
Participants
To date, 54 MC patients have been successfully enrolled and completed at least one follow-up assessment (3, 6, and/or 12 months of MC use). Of the 54 MC patients included in the current analyses, 51 completed a 3-month follow-up, 44 completed a 6-month follow-up, and 32 returned after 12 months (ns = 50, 41, and 29, respectively, for completion of neuropsychological assessments). More specifically, 27 patients completed all four visits, and five missed an interim visit(s). Ten participants remain enrolled but are awaiting their next follow-up timepoint. Only 12 patients (22%) were discontinued or withdrew from the study because they either stopped MC use (n = 3) or were lost to follow-up (n = 9). As not all patients completed all four timepoints, we compared baseline data between completers and non-completers to assess the potential impact of missing data. Completers and non-completers did not differ significantly on any variable with the exception of Trails B errors (p =.03); however, both groups made less than one error. Therefore, missing data should be considered missing completely at random (MCAR) and unlikely to influence results.
Participants were recruited for the study via ads targeting individuals interested in using MC; ads were posted on our research volunteer portal, social media, and at MC certification centers. Study staff did not facilitate MC certification. To qualify for entry, participants had to be 21 or older and have either a valid certification for MC or report a desire to use hemp-based products, which do not currently require certification, in an attempt to enroll only those interested in using cannabis for medical purposes. Patients could plan to use MC for a variety of indications, including chronic pain, anxiety, mood, sleep, or other medical/psychiatric conditions. MC patients must not have begun regular MC treatment or endorsed recent recreational cannabis use prior to baseline assessments. Specifically, participants were either required to be cannabis naïve (≤15 lifetime uses) or, if they reported a history of cannabis use, they could not have regular recreational use (>1x/month) within the last year to limit effects of recent exposure. To help confirm this entry criterion, all patients were required to test negative for urinary THC metabolites at baseline. In addition, all patients completed the two-factor Wechsler Abbreviated Scale of Intelligence (WASI; (Wechsler, Reference Wechsler1999) at baseline and were required to have an IQ of ≥75.
Study Design
Upon arrival, study procedures were explained, and patients were required to read and sign an informed consent form approved by the Partners Healthcare Institutional Review Board. Prior to initiating MC treatment, all patients completed a neurocognitive battery and measures of clinical state and sleep quality. Following 3, 6, and 12 months of MC treatment, patients returned to repeat study measures. The neuropsychological test battery was designed to assess executive function and memory, as studies of recreational users have shown these domains to be most vulnerable to cannabis use (Sagar & Gruber, Reference Sagar and Gruber2018). To examine various aspects of executive function, patients completed the Stroop Color Word Test (MacLeod, Reference MacLeod1991), Trail Making Test (Lezak, Howieson, Bigler, & Tranel, Reference Lezak, Howieson, Bigler and Tranel2012), computerized Wisconsin Card Sorting Test (WCST-64; Berg, Reference Berg1948; Heaton & PAR Staff; Lezak et al., Reference Lezak, Howieson, Bigler and Tranel2012), Letter-Number Sequencing (LNS) subtest of the Wechsler Adult Intelligence Scale-Revised (WAIS-R; Wechsler, Reference Wechsler1987), and the Controlled Oral Word Association Test (COWAT; Strauss, Sherman, & Spreen, Reference Strauss, Sherman and Spreen2006). Verbal learning and memory were assessed using the Rey Auditory Verbal Learning Test (RAVLT; Schmidt, Reference Schmidt2016). To limit practice effects, alternate test forms were used at each follow-up visit for all tasks except Stroop and WCST.
To examine potential clinical changes related to MC use which could impact cognitive performance, various aspects of clinical state were assessed using the Profile of Mood State (POMS; Pollock, Cho, Reker, & Volavka, Reference Pollock, Cho, Reker and Volavka1979), which provides a score reflecting Total Mood Disturbance (TMD); the Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, Reference Beck, Ward, Mendelson, Mock and Erbaugh1961); the Beck Anxiety Inventory (BAI; Beck & Steer, Reference Beck and Steer1990); and the State Trait Anxiety Index (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, Reference Spielberger, Gorsuch, Lushene, Vagg and Jacobs1983), which provides subscores for state (how one currently feels) and trait anxiety (how one generally feels). For these scales, higher scores are indicative of increased symptomatology. MC patients also completed the Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, Reference Buysse, Reynolds, Monk, Berman and Kupfer1989); higher scores indicate poorer sleep.
Upon completion of baseline procedures, MC patients were provided with drug diaries to track MC use. Diaries recorded product information, route of administration, frequency, and amount of MC used. Between visits, patients were contacted monthly by phone to corroborate diary entries using a modified timeline follow-back procedure (TLFB) (Robinson, Sobell, Sobell, & Leo, Reference Robinson, Sobell, Sobell and Leo2014), which utilizes cannabis-specific follow-up queries. During phone check-ins, discrepancies with diary information were rectified using additional queries to ensure accurate information was recorded.
To gather information about cannabinoid content within each product, each patient was asked to provide a sample of their most frequently used MC product(s) for cannabinoid constituent profiling, completed by an outside laboratory (ProVerde Laboratories, Inc.). Although reports provided information on 12 cannabinoids, specific focus was placed on THC and CBD. When patients were unable to supply a sample for analysis, constituent information was gathered from certificates of analyses (COAs) from manufacturers/dispensaries, or from product labels when COAs were unavailable. Using constituent information along with use data from diary entries and/or TLFB queries, a standard metric of cannabinoid exposure, measured in mg of THC and CBD used per week, was calculated for each patient at each follow-up visit.
Statistical Analyses
For all analyses, raw scores were utilized for cognitive performance data and self-report ratings, as methods for standardization were not uniform across assessments. Repeated Measures Analyses of Variance (rmANOVAs, 2-tailed) were used to assess within-subject changes for cognitive and clinical variables. As changes from baseline were the contrasts of interest, analyses compared data at each follow-up visit relative to baseline ratings (i.e., baseline vs. 3 months, baseline vs. 6 months, and baseline vs. 12 months); these methods maximized study sample size and statistical power. Results from each of these analyses are reported with effect sizes (partial eta squared) and 95% confidence intervals. Expected power for all contrasts was calculated as ≥86.96% for medium effect sizes and ≥97.23% for large effect sizes.
For cognitive and clinical measures demonstrating consistent, statistically significant patterns of change at follow-up visits relative to baseline, bivariate correlation analyses (Pearson’s r, 2-tailed) were used to assess the relationship between changes in cognitive performance and clinical ratings as well as the association between these variables and cannabinoid exposure (frequency, mg of THC/week, CBD/week). For these correlations, difference scores comparing baseline to 3 months were utilized in order to maximize sample sizes and statistical power.
RESULTS
Demographics
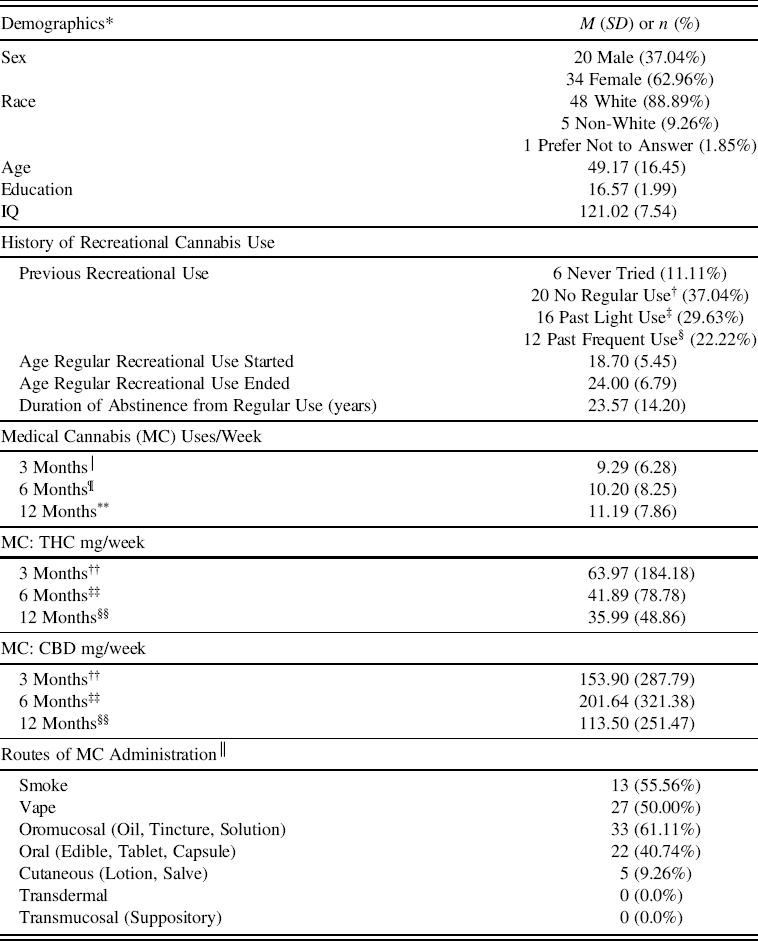
To date, 54 MC patients (20M, 34 F) between the ages of 23–78 completed baseline assessments and returned for at least one follow-up visit (Table 1). Of the 54 patients in this sample, 26 were cannabis naive (≤15 lifetime uses), and 28 reported a history of past recreational cannabis use with at least 12 months of abstinence from regular use (defined as >1x/month). See Table 1 for information regarding initiation/cessation and duration of abstinence from recreational use. All participants tested negative for THC metabolites at baseline.
Table 1. MC patient demographics & medical cannabis/cannabinoid use

THC = delta-9-tetrahydrocannabinol; CBD = cannabidiol.
* n = 54, unless otherwise noted.
† No regular use was defined as <1x/month and a maximum of 15 lifetime uses.
‡ Light use was defined as regular use of at least 1x/month but <2x/week.
§ Frequent use was defined as regular use of at least 2x/week.
│ n = 50.
¶ n = 44.
** n = 31.
†† n = 38.
‡‡ n = 32.
§§ n = 24.
││ Participants could report multiple modes of use.
Patients used MC to treat a variety of symptoms and conditions, including pain (n = 36), anxiety or PTSD (n = 31), sleep (n = 22), mood (n = 14), attention (n = 4), and other medical conditions (n = 4); 36 patients reported using MC to treat more than one condition. Over the course of the study, patients reported using MC 9–11 times/week on average. Interestingly, cannabinoid exposure calculations revealed that overall, THC exposure (mg/week) was notably lower than CBD exposure (mg/week) at each visit; this difference was only statistically significant for MC use after 6 months of treatment (F (1, 31) = 7.73, p =.01).
Cognition
Over the course of 12 months of MC treatment, patients exhibited significant changes on several measures of executive functioning (see Table 2). On the Stroop Interference condition, MC patients exhibited significantly faster times at all follow-up visits relative to baseline (all ps<.01), suggesting improved inhibitory processing. Overall, MC patients exhibited high levels of accuracy (Range: 97.86–98.76%) across visits. Although a statistically significant reduction in accuracy was noted at 3 months relative to baseline (p<.01), given the high levels of accuracy attained and very small change from baseline to 3 months (<1%), this does not appear clinically significant. Further, as this pattern was not observed at later visits, results suggest that improvements in Stroop completion times did not come at the expense of lower task accuracy.
Table 2. Changes in cognitive performance over the course of 3, 6, and 12 months of MC treatment

COWAT = Controlled Oral Word Association Test; LNS = Letter-Number Sequencing; MC = medical cannabis; RAVLT = Rey Auditory Verbal Learning Test; WCST = Wisconsin Card Sorting Test
Significant values (p≤.05) are bolded.
* Degrees of freedom (df) = 1, 49.
† df = 1, 40.
‡ df = 1, 28.
On the Trail Making Test Part B, MC patients demonstrated significantly faster times between baseline and 3 months (p = .04), but no significant differences were observed after 6 or 12 months compared to baseline. Although significantly higher error rates were observed after 6 and 12 months of MC use (p = .02 and .03, respectively), errors remained quite low at all visits (<1 error), suggesting differences in task performance were not clinically significant.
On the WCST, MC patients generally exhibited improved performance following initiation of MC treatment. Total categories achieved was higher at all follow-up visits relative to baseline, although this increase was only significant after 6 months of treatment (p = .02). In addition, perseverative errors generally decreased across visits; however, this was only statistically significant following 12 months of MC treatment (p = .01).
On the LNS, statistically significant improvements were detected at all three follow-up visits relative to baseline (all ps ≤ .01). Performance on the COWAT remained relatively stable after 3 and 12 months of treatment, but statistically significant improvements were noted following 6 months of treatment (p = .01).
On the RAVLT, verbal learning and memory performance were generally maintained during follow-up visits relative to baseline, as few statistically significant changes were noted. While patients recalled fewer words on the long delay condition after 12 months of treatment relative to baseline (p = .04), closer analysis revealed that MC patients remembered approximately 11 total words at both visits (baseline = 11.38; 12 months = 10.66), suggesting that while this difference is statistically significant, it does not appear to be clinically significant.
Clinical Ratings: Mood, Anxiety, and Sleep
Clinical ratings are reported in Table 3. On the POMS, patients exhibited significantly decreased total mood disturbance (TMD) at each follow-up visit relative to baseline (all ps≤.05). Further, BDI scores indicated significant reductions in self-reported symptoms of depression across all follow-up visits relative to baseline (all ps < .01). Additionally, anxiety ratings generally decreased over time. BAI scores were significantly lower after 6 and 12 months of MC treatment (both p = .02). On the STAI, trait anxiety significantly decreased at follow-up visits relative to baseline (all ps < .01), while state anxiety generally showed a pattern of decreased anxiety; this reached significance for only the baseline to 6-month comparison (p < .01). On the PSQI, patients reported better sleep quality at all follow-up visits after initiating MC treatment (all ps < .01).
Table 3. Changes in self-reported mood, anxiety, and sleep over the course of 3, 6, and 12 months of MC treatment

MC = medical cannabis.
Significant values (p ≤ .05) are bolded.
* Degrees of freedom (df) = 1, 50 for all scales except PSQI where df = 1, 47.
† df = 1, 43 for all scales except PSQI where df = 1, 40.
‡ df = 1, 31 except PSQI where df = 1, 28.
Correlations: MC Use versus Cognitive Variables
Correlation analyses explored potential relationships between MC use variables (MC use episodes/week, THC mg/week, CBD mg/week) and select key cognitive variables demonstrating statistically significant changes between baseline and 3 months: Stroop Interference time, Trails B time, and LNS total. After controlling for outliers, no significant relationships were observed between any MC use variable and any cognitive performance variable.
Correlations: MC Use versus Clinical Variables
Correlation analyses examining relationships between MC use and clinical variables (POMS TMD, BDI, STAI trait anxiety, and PSQI) revealed that improvements in mood and anxiety were significantly associated with higher CBD exposure (mg/week). Specifically, improvement on POMS TMD (r (36) = .737, p < .001), BDI (r (36) = .521, p = .001), and STAI trait anxiety (r (36) = .634, p < .001) correlated with increased CBD use. Additionally, improved trait anxiety on the STAI was significantly correlated with greater MC use episodes per week (r (48) = .327, p = .021). Importantly, THC exposure was not associated with any clinical variables, and no other relationships were observed between MC use and clinical changes.
Correlations: Cognitive versus Clinical Variables
As exposure to individual cannabinoids did not directly impact cognitive function, correlations assessed whether the observed cognitive improvements may be related to clinical improvements, suggestive of an indirect effect of MC use on cognition. Results revealed that faster Stroop Interference times were significantly associated with clinical improvement: POMS TMD (r (48) = .411, p = .003), BDI (r (48) = .437, p = .002), STAI trait anxiety (r (48) = .487, p < .001), and PSQI (r (45) = .288, p = .049). No significant relationships were observed between any other cognitive and clinical variables.
DISCUSSION
In contrast to studies reporting decrements in cognitive performance among recreational cannabis consumers, particularly those with adolescent onset (Crean et al., Reference Crean, Crane and Mason2011; Lisdahl et al., Reference Lisdahl, Wright, Kirchner-Medina, Maple and Shollenbarger2014; Sagar & Gruber, Reference Sagar and Gruber2018), data from the current investigation suggest that 3 to 12 months of MC use does not appear to be associated with poorer cognitive performance. These findings extend pilot findings which indicated improvement on some measures of executive function following 3 months of MC treatment (Gruber et al., Reference Gruber, Sagar, Dahlgren, Racine, Smith and Lukas2016). The current longitudinal, observational study examined a larger sample of MC patients over a longer time course and found that MC patients demonstrated improvements on several executive tasks. Specifically, over time, patients exhibited faster time on the Stroop Interference condition while maintaining high levels of accuracy. Performance on the Trail Making Test suggested faster psychomotor speed, but more variable performance in terms of cognitive flexibility and set-shifting across visits. On the computerized version of the WCST, MC patients achieved more categories while making fewer perseverative errors. MC patients generally exhibited improved performance on the LNS at all follow-up visits, and phonemic fluency remained relatively stable with transient evidence of improvement over time. Interestingly, these improvements may not extend to measures of verbal memory; although verbal learning performance remained stable over time, some evidence for slightly decreased verbal memory following a long delay after 12 months of MC treatment emerged. While this finding was statistically significant, it is unlikely that there is clinical significance between recalling 11.38 words compared to 10.66 words. These results stand in contrast to findings in recreational cannabis users which frequently note decrements on measures of verbal learning and memory (Lisdahl et al., Reference Lisdahl, Wright, Kirchner-Medina, Maple and Shollenbarger2014; Sagar & Gruber, Reference Sagar and Gruber2018; Schwartz, Gruenewald, Klitzner, & Fedio, Reference Schwartz, Gruenewald, Klitzner and Fedio1989; Solowij et al., Reference Solowij, Jones, Rozman, Davis, Ciarrochi, Heaven and Yucel2011).
Few studies have examined cognitive performance in those using cannabis for medical purposes. A recent 12-month longitudinal study utilized a single self-report measure of cognition, the Cognitive Failures Questionnaire, to examine individuals using cannabis to self-medicate for chronic medical conditions; although no improvements were reported over time, no evidence of cognitive deterioration was reported over 12 months (Bouso et al., Reference Bouso, Jimenez-Garrido, Ona, Woznica, Dos Santos, Hallak and Farre2020). However, in contrast to the current investigation, study participants were not cannabis-naïve at baseline, which likely impacted baseline performance. As data from the current study suggest that improvement often occurs within 3 months of initiating treatment, cognitive improvements occurring in the early stages of MC initiation may not have been detected in the Bouso et al. (Reference Bouso, Jimenez-Garrido, Ona, Woznica, Dos Santos, Hallak and Farre2020) sample, given no cannabis-naïve baseline assessment.
Importantly, improvements in cognitive performance in the current study appear to occur in the context of significant improvements on measures of mood, anxiety, and sleep. These findings are supported by retrospective reports of clinical improvements secondary to MC use among various patient populations. For example, a survey study of California residents found that of the 5% who reported having tried MC, 92% reported MC helped treat a serious medical condition (Ryan-Ibarra, Induni, & Ewing, Reference Ryan-Ibarra, Induni and Ewing2015). Another study of MC patients in Arizona reported that among those endorsing symptoms of anxiety, 83% reported “a lot or almost complete relief” from anxiety when using MC (Troutt & DiDonato, Reference Troutt and DiDonato2015). In addition to these surveys, acute administration studies, observational studies, a handful of clinical trials, and several reviews have reported improvements in medical and psychiatric symptoms secondary to medical cannabis or cannabinoid use across a range of conditions and symptoms, including chronic pain (NASEM, 2017; Pawasarat et al., Reference Pawasarat, Schultz, Frisby, Mehta, Angelo, Hardy and Kim2020; Poli, Crestani, Salvadori, Valenti, & Sannino, Reference Poli, Crestani, Salvadori, Valenti and Sannino2018), anxiety (Bergamaschi et al., Reference Bergamaschi, Queiroz, Chagas, de Oliveira, De Martinis, Kapczinski and Crippa2011; Masataka, Reference Masataka2019; Shannon et al., Reference Shannon, Lewis, Lee and Hughes2019; Zuardi et al., Reference Zuardi, Cosme, Graeff and Guimaraes1993; Zuardi, Shirakawa, Finkelfarb, & Karniol, Reference Zuardi, Shirakawa, Finkelfarb and Karniol1982), and sleep (Kuhathasan et al., Reference Kuhathasan, Dufort, MacKillop, Gottschalk, Minuzzi and Frey2019).
Several factors likely contribute to findings in the current study, the first to directly assess the longitudinal impact of MC on cognition and clinical variables in “real world” MC patients, and to quantify THC and CBD exposure. On average, MC patients in the current study reported higher CBD exposure relative to THC at all follow-up visits following initiation of MC use. As higher amounts of THC are often linked to cognitive decrements in recreational cannabis users (Kowal et al., Reference Kowal, Hazekamp, Colzato, van Steenbergen, van der Wee, Durieux and Hommel2015; Morgan et al., Reference Morgan, Gardener, Schafer, Swan, Demarchi, Freeman and Curran2012; Ramaekers et al., Reference Ramaekers, Kauert, van Ruitenbeek, Theunissen, Schneider and Moeller2006), and CBD has demonstrated efficacy in mitigating or preventing THC-related negative effects on cognition (Morgan et al., Reference Morgan, Gardener, Schafer, Swan, Demarchi, Freeman and Curran2012; Solowij et al., Reference Solowij, Broyd, Beale, Prick, Greenwood, van Hell and Yucel2018), this pattern of cannabinoid exposure may have influenced results. However, current findings also demonstrate that neither THC nor CBD use was directly correlated with observed improvements on measures of executive function. Rather, increased CBD exposure was associated with improved mood and anxiety symptoms. Correlation analyses suggest that improved cognitive performance (particularly faster Stroop Interference time) was associated with improvements in clinical symptoms. Accordingly, improvements in cognition may not be directly attributed to MC; instead MC treatment may indirectly improve cognition secondary to clinical improvements. In fact, symptoms commonly endorsed by MC patients, including anxiety and pain (the two most common indications reported by study participants), have been associated with reduced cognitive performance (Moriarty et al., Reference Moriarty, McGuire and Finn2011; Vytal et al., Reference Vytal, Cornwell, Letkiewicz, Arkin and Grillon2013). Although larger sample sizes are needed to perform mediation analyses, the current study provides preliminary evidence that clinical symptom improvement may result in improved performance, as patients may think more clearly if they feel better overall. Additionally, while the current study quantified THC and CBD exposure, other cannabinoids in patients’ products may have also impacted study findings. As laboratory analyses of products quantified twelve cannabinoids, future studies will explore the relative contributions of additional constituents.
Further, it is imperative to assess age as a potential moderating variable. Unlike most studies of recreational cannabis users which assess young adults, who often initiate use during adolescence, those enrolled in the current study were significantly older (average age of 49). This is an important distinction, both because of neuromaturational changes during adolescence, and because marked changes occur in the endocannabinoid system in older relative to younger individuals. For example, preclinical studies reveal decreased levels of the endocannabinoid 2-arachidonoylglycerol (2-AG) in the aging mouse brain, and report that CB1 receptor binding peaks in puberty, remains stable early to mid-adulthood, and ultimately declines in older adulthood (Piyanova et al., Reference Piyanova, Lomazzo, Bindila, Lerner, Albayram, Ruhl and Bilkei-Gorzo2015). Human studies have similarly revealed higher CB1 receptor binding in younger individuals relative to older adults (Di Marzo, Stella, & Zimmer, Reference Di Marzo, Stella and Zimmer2015). In addition, although few studies have examined cognition secondary to MC use in older adults, several animal studies highlight the potential for cannabis to improve cognition in this population (Weinstein & Sznitman, Reference Weinstein and Sznitman2020), including one study demonstrating that administration of low-dose THC reversed age-related decline in older adult mice (Bilkei-Gorzo et al., Reference Bilkei-Gorzo, Albayram, Draffehn, Michel, Piyanova, Oppenheimer and Zimmer2017)
While results from the current study are promising, findings must be considered given several limitations. Although the demographic makeup of the current sample reflects Massachusetts, this sample of patients has limited diversity; thus, findings may not be generalizable to all populations. In future, larger analyses, a more diverse sample will facilitate examination of the potential influence of sex and race.
It is also of note that MC patients were examined over time but were not directly compared to patients with similar conditions who do not use cannabis. Accordingly, we have enrolled treatment-as-usual (TAU) group; once this sample is large enough, cognitive and clinical changes will be examined within and between both groups.
Another limitation is the potential confounding effects of treatment expectancy or the degree to which patients believe cannabis use can positively impact symptoms. Although no measures currently exist to assess treatment expectancies specifically related MC use, to begin to address this issue, patients completed the modified Marijuana Effect Expectancy Questionnaire-Brief (MEEQ-B; (Torrealday et al., Reference Torrealday, Stein, Barnett, Golembeske, Lebeau, Colby and Monti2008), which assesses positive and negative expectancies related to cannabis use in general. Interestingly, neither positive nor negative cannabis expectancies at baseline correlated with cognitive changes from baseline to 3 months of treatment, suggesting that cognitive and clinical improvements are not directly related to patients’ preconceived beliefs regarding cannabis use. Further, as cognitive measures are objective, validated assessments, it is unlikely that MC patients would actually perform better simply as a result of perceived improvements.
Additionally, study results were not corrected for multiple comparisons; instead, we utilized a targeted approach by pre-selecting only the most relevant variables to limit the number of comparisons made, as done in previous studies (Fontes et al., Reference Fontes, Bolla, Cunha, Almeida, Jungerman, Laranjeira and Lacerda2011). This method avoids an overly conservative approach (e.g., Bonferroni corrections), which has the potential to increase the risk of Type II errors; results should therefore be considered accordingly.
Finally, the contribution of practice effects should be considered. However, utilizing alternate forms (for all tasks except Stroop and WCST) combined with the 3–6 month period between task administrations reduces the likelihood of practice effects. Studies examining practice effects reported no impact on LNS or Trail Making tasks, even with weekly administration (Beglinger et al., Reference Beglinger, Gaydos, Tangphao-Daniels, Duff, Kareken, Crawford and Siemers2005), and studies noting practice effects on the Stroop typically utilized daily to weekly administration (Gul & Humphreys, Reference Gul and Humphreys2015). Further, in the current study, patients exhibited patterns of improvement on tasks of executive function but consistent, stable performance on tasks of memory, providing additional evidence that study findings are not likely attributable to practice effects. Future inclusion of a TAU group will further address this concern.
CONCLUSIONS
In a 12-month longitudinal, observational study, patients using MC for various medical conditions exhibited improved executive function and stable verbal learning and memory within the context of improvements on measures of mood, anxiety, and sleep relative to baseline. While greater improvement of clinical state over time was significantly associated with increased CBD exposure (mg/week), improved cognitive performance over time did not correlate with MC use. Future investigations examining the impact of individual cannabinoids and age of onset of use are warranted to clarify the implications of MC use. Ultimately, for MC patients, it is imperative to understand the relationship between these variables in order to maximize the therapeutic potential of cannabis while minimizing potential risk and harms.
FUNDING
This work was supported by private donations to the Marijuana Investigations for Neuroscientific Discovery (MIND) program at McLean Hospital.
CONFLICTS OF INTEREST
All authors have no conflicts of interest to disclose.