Maternal and child undernutrition is the underlying cause of 3·5 million deaths annually, with over half of mortalities among children under 5 years of age occurring in sub-Saharan Africa( Reference Black, Allen and Bhutta 1 , Reference Murray, Laakso and Shibuya 2 ). Widespread impacts of undernutrition include undernourished women giving birth to low-weight infants, reduced volume and quality of breast milk, and limited caring and working capacity( Reference Black, Allen and Bhutta 1 , 3 ). The typical high-starch plant-based diets usually lack sufficient diversity and this places women, infants and school-aged children (ages 5–14 years) at risk of multiple micronutrient deficiencies( Reference Arimond, Wiesmann and Becquey 4 – Reference Best, Neufingerl and van Geel 6 ). Inadequate intakes of Fe, Zn, Ca, vitamin A, vitamin B12 and riboflavin( 7 – Reference Neumann, Bwibo and Murphy 9 ) are of concern for infants and young children, and recently eleven micronutrients of concern for women in developing countries were identified( 10 ). In Kenya, 35 % of children under the age of 5 years are stunted( 11 ), which is associated with limited cognitive development( Reference Grantham-McGregor, Cheung and Cueto 12 ). In addition, a high proportion of Kenyan children and women experience Zn (50 %, both), Fe (89 and 56 %, respectively) and vitamin A (84 and 12 %, respectively) deficiencies( 13 ).
The majority of the world's poor live in rural areas and many depend on small farms for food and income. In sub-Saharan Africa, including Kenya, smallholder farmers are challenged with limited agricultural resources( 3 ). Improving the productivity of small farms is considered one of the best and most sustainable means for reducing hunger and nutrition in poor rural communities( 14 – Reference Berti, Krasevec and FitzGerald 16 ).
Wakulima Dairy Ltd (WDL), a community-based dairy group in Kenya, buys and sells members’ raw milk, and provides them with regular payments and goods and services on credit. WDL member training to enhance dairy farm productivity has been conducted for more than 8 years through a partnership with Farmers Helping Farmers (FHF), a Canadian non-governmental organization, the Atlantic Veterinary College at the University of Prince Edward Island (UPEI) and the Canadian International Development Agency. Early in the training programme, FHF volunteers were concerned that farmers may opt to sell all of their milk, to the detriment of household nutrition. The training programme was then expanded to encourage households to provide children with two cups of milk each day for better school performance and health. This message, delivered by the WDL milk quality specialist, was accompanied by a two-page ‘fact sheet’ that reiterated the messages and included recipes to expand milk use.
Integrated participatory agriculture and nutrition interventions have demonstrated positive nutritional outcomes( Reference Berti, Krasevec and FitzGerald 16 , Reference Bezner Kerr, Berti and Shumba 17 ); however, there is limited evidence of the effects of agricultural production interventions on individuals’ nutritional outcomes( Reference Berti, Krasevec and FitzGerald 16 , Reference Leroy JL & Frongillo 18 , Reference Bhutta, Ahmed and Black 19 ). This is important for women and children, who are often disadvantaged in household food distribution. Dairy farm enhancement initiatives reported higher milk production, household income, average per-capita milk consumption and intakes of energy, total fat, protein, retinol and Fe( Reference Mullins, Wahome and Tsangari 20 – Reference Huss-Ashmore 25 ). Our previous results found WDL membership duration longer than 3 years was positively associated with higher milk production and that members had higher average per-capita milk availability compared with non-members (0·6 v. 0·3 l/person per d)( Reference Walton, VanLeeuwen and Yeudall 26 ). Longer duration and intensity of involvement in an integrated agricultural and nutrition project had positive impacts on height and weight of children (under 3 years of age) in Malawi( Reference Bezner Kerr, Berti and Shumba 17 ) but no other reports detailing the duration impact of agricultural intervention, including enhanced dairy farming, on health and nutrition were identified.
The objectives of the present study were to examine associations among dairy group membership, membership duration and non-member status with diet quality indicators and prevalence of inadequate nutrient intakes for women and school-aged children in rural Kenya.
Methods
Setting
WDL is located in Mukurweini Division, Central Province, Kenya. Farmer members live along four rural routes and around a central town. Non-member farmers live among the dairy group members throughout the division. The 6000 dairy group member households represent about 28 % of the division's population of 83 932 (2009).
Study design and sampling method
A cross-sectional survey of eighty-eight WDL member households, evenly distributed over four membership duration ‘study groups’ (1–3, 4–6, 7–9 and 10+ years), and twenty-three non-member households was conducted in August 2009. The sample size was established to generate data with reasonable power, balanced with limited resources, and included 10 % additional participants to maintain numbers in case of spoiled surveys.
Study group members in the four duration groups were identified using chain referral sampling since there was no available list of members with duration status or contact information to use in selecting a stratified random sample( Reference Biernacki and Waldorf 27 , Reference Penrod, Preston and Cain 28 ). Eight WDL members were selected to initiate the referrals. These initiators represented a wide range of age, geographic distribution and involvement within the dairy group. Each initiator referred farmer members that represented the four membership-duration groups. The research team contacted referred members to confirm membership duration. This procedure was repeated until sufficient numbers of members in each membership-duration group were identified. Referred WDL members were asked to identify non-members to generate a non-member list (n 50). The non-member participants (n 23) were randomly selected from this list. Directors and managers of WDL and teachers were excluded from the study in order to focus the research on households with farming as their primary livelihood strategy.
Survey and dietary assessments
The interview was conducted in person by two trained interviewers, including the first author, using translators as needed. Demographic information was collected from the household adult(s) and women were interviewed for food preparation and consumption using a four-pass 24 h recall( Reference Gibson 29 ). Dietary intake was recorded for the women and for a randomly selected child in households with at least one daily resident child (biological, grandchild or adopted) aged between 5 and 14 years. Children do not attend school in August, which was the time of the survey; when available, children verified food intake reported by the mother and provided information on additional foods consumed. In two households, the father was the main food preparer and was similarly interviewed, but only data for the child's food intake were analysed. Interviews were conducted to ensure each day of the week was evenly represented in the recalls for each membership group.
Women's weight was measured using a ‘Salter-Speedo’ dial mechanical scale (model 148; 0·5 kg accuracy). Height was measured using a rigid measuring tape (0·1 cm accuracy) and a square angle. Whenever possible, women stood on level ground next to a vertical surface. Height and weight measurements were used to compute BMI for each woman. Children's weights and heights were not measured, as not all children were always available during data collection.
Ingredients in mixed dishes obtained from the 24 h recall were quantified by: (i) weighing directly; (ii) using home measures (e.g. scoop, heaped tin) with standard food densities; or (iii) estimated from locally purchased items (e.g. tomato, cabbage). The volume of cooked mixed dishes was computed using the pot circumference or diameter and food height. When no recipe was provided (e.g. ate at neighbours) or the recipe and yield were contradictory, an average recipe, based on ten randomly selected household recipes within the survey, was used to estimate nutrient intakes.
Portions served were estimated by the women placing dried beans into the individual's bowl to represent the serving. When leftover food was reported, the leftover volume was estimated using the dried beans and removed, leaving the portion consumed. The beans representing the portion consumed were weighed and the bean density used to estimate the volume of food consumed. The same dried beans were used throughout. The quantity of mixed dish ingredients consumed was computed as a fraction of the recipe yield. Liquid food intakes, mainly tea and thin porridge (uji), were estimated from the mass of water in the individual's cup or from the commercial volume for purchased beverages. Food and beverage intakes were used to compute diet quality indices and to estimate nutrient intakes.
Data analysis
Milk intake and dietary diversity were estimated from results of the 24 h recall. Dietary diversity was computed using a nine food-group indicator, with a 15 g minimum intake, validated for use in resource-poor settings( 10 ). The mean dietary diversity (number of different food groups consumed) was computed for each membership group.
Energy, distribution of dietary energy and nutrient intakes were computed using the World Food Dietary Assessment System Version 2·0 (Wfood2) and the Kenyan food composition database( 30 ). Foods not found in this database were imported from other databases within Wfood2 or imputed from US Department of Agriculture or Canadian Nutrient File values. Median (first quartile (Q1), third quartile (Q3)) nutrient intakes were computed. Wfood2 estimates bioavailable Fe and Zn intake using adjustments for enhancing and interfering factors in the same meal. Both Fe and Zn status was assumed to be basal for these availability adjustments.
Women's energy intakes were examined for over- and under-reporters, using a high physical activity level (3·0) and the Goldberg method( 10 , Reference Black 31 ). Data for women with energy intake outside the range 5230–17 400 kJ (1250–4160 kcal) were examined for errors and plausibility and excluded from analysis as applicable. Data from nursing women were also excluded.
Women's milk consumption, energy, percentage energy sources and dietary diversity were analysed to examine associations with membership status and membership duration, using the t test or ANOVA with Bonferroni multiple comparison adjustment. Associations of diet quality indicators with membership duration were examined using linear regression. Children's milk consumption, energy and percentage energy distribution were compared between members and non-members using the t test for normally distributed variables and the Mann–Whitney test for variables not following a normal distribution. Normal distribution was assessed using the Shapiro–Wilk test and transformations were applied to some variables to achieve a normal distribution.
Estimates of the prevalence of inadequate intakes (PII) were computed using the Estimated Average Requirement cut-point method( 32 ), with the following exceptions: (i) women's energy intake was compared with 9414 kJ (2250 kcal), the average daily energy requirement for Kenya( 33 ); (ii) Ca was assessed using Adequate Intake values; (iii) protein and available Zn and Fe requirements were estimated using women's ages, weights (or median study group weight, 61·4 kg, for twenty women without measured weight) and mean requirements (and for women under 50 years of age, 0·48 mg Fe/d requirement was added for menstrual losses); and (iv) children's energy, protein and available Fe and Zn requirements were compared with the children's mean requirements( 32 ) based on mean age and 25th percentile weight-for-age( 34 , Reference Murphy, Beaton and Calloway 35 ).
Significant associations of PII among membership groups (women only) and between members and non-members were examined using χ 2 analysis.
Recipe calculations were performed using Microsoft® Excel v 6. Statistical analysis was conducted using the Stata 10 statistical software package. Significance was assessed at P < 0·05 for women and, with the small sample size, at P < 0·10 for children.
Approval to conduct the study was obtained through FHF, WDL and the UPEI Research Ethics Board prior to conducting the study. Written consent was obtained from all participants after the nature of the study had been fully explained to them.
Results
All dairy group members approached agreed to participate in the survey (100 % response). Women in two non-member households refused to participate, and the next household on the list was approached. Energy intakes were examined for over- and under-reporters. As a result, data from two women were excluded from the analysis. Intakes from other women with high or low energy were retained as data seemed probable, given other information. One woman being treated for anaemia was excluded.
Women's age increased with membership duration, as expected, and ranged from 35 years in the groups with membership duration of 1–3 and 4–6 years, to 43 and 53 years respectively in the membership-duration groups of 7–9 and 10+ years. The average age of women members (41·5 years) and non-members (43·9 years) was not different. Age and the proportions of girls and boys were not different between member and non-member children (age 8·6 and 8·4 years, respectively).
Diet quality indicators
Milk intake was higher for member children and women compared with non-members (Table 1). Member women with lactating cow(s) had significantly higher milk intake than those without lactating cow(s). A similar trend was observed for non-member women with and without lactating cow(s). Women's milk intake was also positively associated with membership duration (data not shown; R 2 = 0·24).
Table 1 Median (Q1, Q3) milk consumption (g/d) by dairy group membership, cattle ownership and cow lactation status among women and school-aged children, Mukurweini Division, Central Province, Kenya, August 2009
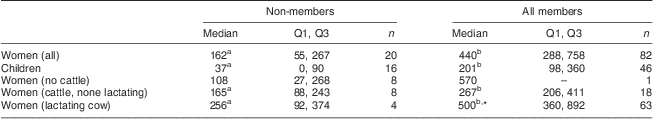
Q1, first quartile; Q3, third quartile.
a,bMedian values within a row with unlike superscript letters were significantly different (P < 0·05).
*Member women with a lactating cow consumed more milk than member women without a lactating cow (t test): P < 0·05.
Member women had higher energy intakes and weight status (BMI) than non-members (Table 2). Women's overweight status (BMI ≥ 25 kg/m2) was associated with dairy group membership, with 54 % of members and 29 % of non-members classified as overweight. In addition, members had a higher percentage of energy from animal-source foods (ASF) and from saturated fat compared with non-members. Membership duration was negatively associated with the percentage of energy from carbohydrates (R 2 = 0·11) and positively associated with the percentage of energy from ASF (R 2 = 0·17) and percentage of energy from saturated fat (R 2 = 0·13).
Table 2 Median (Q1, Q3) dietary energy, weight status and energy distribution by dairy group membership and membership-duration groupFootnote † among women, Mukurweini Division, Central Province, Kenya, August 2009
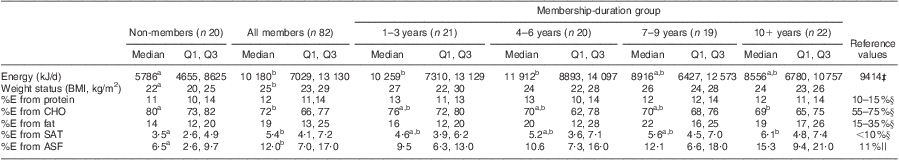
Q1, first quartile; Q3, third quartile; %E, percentage of energy; CHO, carbohydrate; SAT, saturated fat; ASF, animal-source foods.
a,b Median values within a row with unlike superscript letters were significantly different (P < 0·05).
† Variables were transformed before analysis.
‡ 9414 kJ/d (2250 kcal/d) average daily energy requirement for Kenya( 33 ).
§ WHO guidelines( 36 ).
|| Kenya food supplies’ %E from ASF( 13 ).
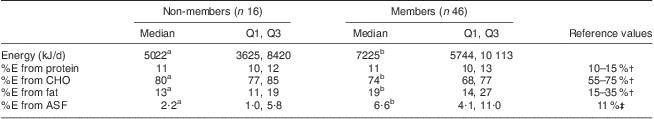
Similarly, member children consumed more energy than non-member children, and had a lower percentage of energy from carbohydrates, a higher percentage of energy from ASF and a higher percentage of energy from fat than non-members (Table 3). Energy from flesh foods (meat, fish and poultry) was negligible for all women and children.
Table 3 Median (Q1, Q3) dietary energy and energy distribution by dairy group membership among school-aged children, Mukurweini Division, Central Province, Kenya, August 2009
Dietary diversity was greater for member women than non-members but not different among membership-duration groups (Table 4). Few women, in any group, consumed organ meat, eggs or flesh foods.
Table 4 Percentage of women consuming each food group and mean (standard error) dietary diversity score by dairy group membership, Mukurweini Division, Central Province, Kenya, August 2009
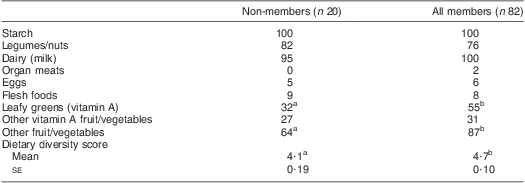
a,bValues within a row with unlike superscript letters were significantly different (P < 0·05).
Nutrient intake and prevalence of inadequate intake
Member women had higher median intakes of macronutrients and most micronutrients (except thiamin, niacin, folate and vitamin C) than non-members (data not shown). Women's micronutrient intakes per kilojoule were positively associated with membership duration for riboflavin, vitamin B12, vitamin A from ASF, Ca and available Zn. Phytate densities of women's diets were not different between members and non-members.
Member women had lower PII for energy, protein and micronutrients (except thiamin, niacin, Zn and Fe) compared with non-member women (Table 5). Longer WDL membership duration was associated with lower PII for riboflavin, vitamin B12 and Ca and higher PII for niacin (data not shown).
Table 5 Prevalence (%) of inadequate intake by dairy group membership among women and school-aged children, Mukurweini Division, Central Province, Kenya, August 2009
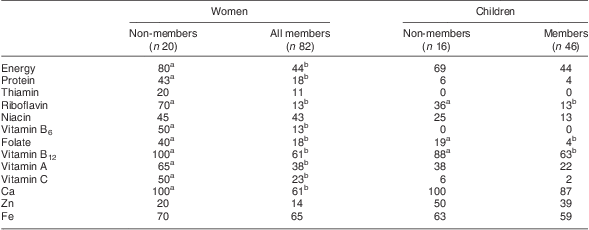
a,bValues within a row with unlike superscript letters were significantly different (P < 0·05), women and children analysed separately.
Children from member households had significantly higher intakes of protein, fat, vitamin A from ASF, Ca, riboflavin, vitamin B6 and vitamin B12 compared with non-member children (data not shown). The PII for member children was significantly lower for riboflavin, folate and vitamin B12, and marginally lower for energy (P = 0·11; Table 5). The PII for vitamin B12, Ca, Zn and Fe was >40 % for all children.
Women's median Na intake was 1155 mg (Q1, Q3 670, 1725 mg). Na intake was not different among groups but was positively associated with membership duration. One-quarter of member women exceeded the recommended population average Na intake (2000 mg/d)( 36 ).
Discussion
Diet quality indicators
Previous studies have reported higher household milk consumption with higher dairy farm productivity( Reference Mullins, Wahome and Tsangari 20 , Reference Kisusu, Mdoe and Turuka 23 , 37 , Reference Nicholson, Thornton and Muinga 38 ). Our study revealed that WDL member women and children consumed significantly more milk. Median milk intake for member children (201 g/d) indicated that 50 % of member children had the potential for the health, growth and cognitive benefits associated with the daily consumption of 200 ml of milk( Reference Siekmann, Allen and Bwibo 8 , Reference Grillenberger, Neumann and Murphy 39 – 41 ). Member women's milk consumption was significantly higher with a lactating cow than without a lactating cow. A similar trend was observed for non-members, although the low numbers limited the statistical power. Farm management to reduce the ‘dry period’ for cows, and/or ownership of more than one cow with varied lactations, is therefore likely to have nutritional benefits for women and children.
Milk in Kenya is consumed primarily in tea, and the proportion of milk in the tea can vary widely. Tannins in tea reduce Fe bioavailability( Reference Zijp, Korver and Tijburg 42 ), and if member women and children are drinking more tea, there may be a negative impact on Fe status; this merits further investigation. Consumption of less milk than promoted (‘two cups daily’) by member children may be due, in part, to limits in the amount of tea a child can consume, and reinforces the need to promote milk consumption. Milk intake from the 24 h recall was lower than our reported average per-capita household milk availability (0·6 l/person per d)( Reference Walton, VanLeeuwen and Yeudall 26 ), which suggests that average per-capita household milk availability may overestimate individual milk consumption when assessing the impact of dairy intensification. This difference may be explained by uneven intra-household allocation, sharing milk or tea with visitors and neighbours, or using milk for calf rearing.
Our survey was conducted in August 2009 prior to harvest and following a drought and crop failure, when food shortages occurred( 43 ). Member women had a lower PII for energy that corresponded with their higher median weight status, which may relate to staple foods (e.g. maize, flour) being available on credit to members. Despite higher median energy intake for member children, the PII for energy was high for members and non-members. Some instances of under-reported energy may have occurred. In one study, when children were not available to modify food intakes reported by their mothers, energy intakes were under-reported by 13–19 %( Reference Gewa, Murphy and Neumann 44 ). However, comparisons between groups would still likely be valid, with non-differential under-reporting, although the PII figures for children may be slightly inflated. Children's protein intake was mostly adequate, as expected with the traditional diet( Reference Beaton, Calloway and Murphy 45 ).
Members’ energy distribution was in line with WHO recommendations, in contrast to that of non-members( 36 ). Members had a higher percentage of energy from ASF and dietary diversity, that indicated higher diet quality compared with non-members( 10 ). Women members’ median percentage of energy from ASF reflected Kenyan food availability (11 %)( 13 ). A wide range of percentage of energy from ASF has been reported: from 40 % for Egyptian women( 46 ), to 26–32 % for dairy farm women in Rift Valley, Kenya( Reference Huss-Ashmore 25 ), 3–12 % for women in resource-poor settings( Reference Arimond, Wiesmann and Becquey 4 ) and 0–4 % for poor Ethiopian women( Reference Ayele and Peacock 47 ). Three-quarters of member children exceeded 4 % of energy from ASF observed for Kenyan schoolchildren( Reference Grillenberger, Neumann and Murphy 39 ), but few reached the 11 % available ASF in Kenya( 13 ) or the 9·8–12 % reported for urban Ugandan children( Reference Yeudall, Sebastian and Cole 48 ).
The positive association of women's percentage of energy from ASF with membership duration suggests nutritional benefits of sustained WDL membership. The higher percentage of energy from ASF, even for long-term members, was explained by milk, not flesh food, consumption. ASF consumption is often limited by resources. Our 24 h recall method may not have been sufficiently sensitive to detect an increase in consumption of flesh foods, if there was one. Using an FFQ, 86 % of Tanzanian dairying households reported consuming meat/fish more than five times per month compared with 56 % of non-dairying households( Reference Lwelamira, Binamungu and Njau 24 ). Future studies could benefit from inclusion of food frequency data which may reflect broader changes to the dietary pattern associated with dairy group membership duration. Modestly higher dietary diversity may reflect access to rented land( Reference Walton, VanLeeuwen and Yeudall 26 ) that enabled members to grow a greater variety of food crops and/or that dairy income enabled the purchase of additional (non-ASF) foods.
Micronutrients
Members had significantly higher micronutrient intakes and lower PII values that reflected higher energy intake, percentage of energy from ASF and dietary diversity. Milk nutrients figured prominently in associations of women's nutrient intakes and PII with membership duration. Benefits to members’ health and productivity, and specifically children's vitamin B12 status, are expected( Reference Siekmann, Allen and Bwibo 8 , Reference Murphy, Gewa and Liang 49 ). Lower PII for vitamin C and folate can be attributed to higher dietary diversity (leafy greens, other fruits and vegetables). Despite positive change, PII for micronutrients remained high for women and children and reflected relatively low dietary diversity and intake of flesh foods( Reference Arimond, Wiesmann and Becquey 4 , Reference Arimond and Ruel 5 , Reference Murphy, Gewa and Grillenberger 40 ). Inadequate intakes have negative inter-generational impacts that include poor pregnancy outcomes, reduced or delayed infant growth and development, and reduced personal health and productive capacity.
Children's high PII for Zn, in contrast with women's, may be related to children's lower energy and milk intakes and their high Zn requirement. High seasonal consumption of mangoes, by children but not women, may explain children's lower PII for vitamins A and C( 10 , Reference Gewa, Murphy and Neumann 44 ). Seasonal differences in food patterns and dietary diversity of Ghanian, Malawian and rural Benin children have also been reported( Reference Ferguson, Gibson and Opare-Obisaw 50 , Reference Mitchikpe, Dossa and Ategbo 51 ).
Nutrition transition
Women's weight status and intakes of saturated fat and Na were examined, as even small income increases have been associated with a transition from under- to overnutrition. This transition is characterized by the consumption of a ‘Western diet’ (high in saturated fat, sugar and refined foods, and low in fibre) and is associated with a higher prevalence of chronic disease( Reference Popkin 52 ). The higher proportion of overweight member women reflects this transition and is an area of concern. Energy from saturated fat remained below the recommended level (10 %) and median intake was lower than that reported previously for rural Kenyan women (17·2 g/d)( Reference Steyn, Nel and Parker 53 ). However, energy from saturated fat and Na were positively associated with membership duration.
Study limitations
Limitations in the present research need to be considered in the interpretation of the results. First, the cross-sectional design limits conclusions concerning causality between dairy group membership (or duration) and specific outcomes. Stratified random sampling, although preferable, was not possible, necessitating the use of chain referral sampling. To reduce potential bias from chain referral sampling, the survey list was generated using multiple chains, initiated through individuals with a wide range of age, geographic location and involvement within the dairy group. Further research, using a longitudinal study design and a randomized sample, would help fulfill the criteria for causality needed to conclude the hypothesized ‘impacts’ of WDL membership.
Second, the use of a single 24 h recall, and inability to adjust for usual intake, leads to greater variability in the nutrient intake distributions. The resulting intake estimates are valid for comparing medians across groups( Reference Gibson 54 , Reference Willett 55 ). Using unadjusted intakes to estimate PII may over- or underestimate these values. In addition, pregnant women were not identified systematically and their higher nutritional requirements were not factored into analyses. This could lead to an underestimation of women's PII values, although this would be a non-differential bias among both members and non-members. As a result, our PII figures are valid for comparison between groups in the study, but may have limited comparability to other situations. A four-pass 24 h recall, with trained interviewers, was used to maximize the food recall and local measures were used to maximize the accuracy of measurements( Reference Kigutha 56 ); however, random error may still be involved in these estimates. For example, women may deliberately over- or under-report intakes if motivated by pride or seeking aid. This potential error was addressed, in part, by clearly explaining the nature and purpose of the study in advance and through appropriate exclusion of observations with unlikely high and low energy intakes. All reasonable efforts were made to accurately measure heights and weights, although it was sometimes a challenge to find a suitable level surface. The non-differential error resulting from small inaccuracies in height measurement should not detract from the importance of the BMI findings.
Third, we were unable to assess membership duration for children's intakes due to lower than expected numbers of children. Consequently, member and non-member schoolchildren were compared. Children's intakes may be under-reported when children were not available to contribute to the intake estimation( Reference Gewa, Murphy and Neumann 44 ). This may limit comparability of our results, but comparisons between groups within the study remain valid.
Finally, the comparison of our Zn, Fe and vitamin A results may be limited. Intakes reported here were corrected for absorption-enhancing (e.g. vitamin C) and -inhibiting factors (e.g. phytate, tannins). As well, the bioconversion factors for β-carotene (1:12) and other carotenoids (1:24) to vitamin A (retinol equivalents) were half that previously used, based on more recent evidence( 30 ).
Conclusions
Our study presents positive dietary associations with semi-commercial dairy farming, within a dairy group, that can potentially improve member women's and schoolchildren's health and capacities. Our results contrast those of cash-cropping schemes in six developing countries in Africa, Asia and Latin America that had no short-term positive or adverse nutritional effects( Reference Kennedy, Bouis and von Braun 57 ) and others that suggested the higher prevalence of malnutrition among children in Western Kenya was due to parents being involved in cash-crop farming rather than mixed farming( Reference Kwena and Baliddawa 58 ). Duration of membership was associated with lower prevalence of inadequate intake for some micronutrients, particularly those in milk. Micronutrient deficiencies were not, however, fully addressed by WDL membership and women and children remain at risk of undernutrition, even with long-term dairy group membership. In addition, member women's weight status and trends in saturated fat and Na consumption represent areas of concern.
These results highlight the need to modify the typical diet of rural Kenyan women and children, and support assertions that increased income from dairy was used for purchases other than nutritious food( Reference Mullins, Wahome and Tsangari 20 , Reference Nicholson, Thornton and Muinga 38 ). To enhance positive and mitigate negative nutritional effects of dairy projects, activities should be expanded to include nutrition education, numeracy, literacy and complementary agricultural activities. These additions were associated with positive nutrition impacts in a dairy goat production project( Reference Ayele and Peacock 47 ). A review of agricultural interventions revealed that investing in more than three of the recognized livelihood assets, in particular nutrition education of women, had the greatest nutritional impact( Reference Berti, Krasevec and FitzGerald 16 , 59 ), which is in keeping with the recommendation for more productive collaborations between agricultural and nutritional sciences( Reference Demment, Young and Sensenig 60 ).
Specific strategies for dietary modification, within this resource-limited setting, include strengthening efforts to increase children's milk consumption, initiating complementary interventions to enable the consumption of nutrient-dense foods (e.g. flesh and organ meats, fruit and vegetables) and promoting the use of food-based strategies that improve Zn and Fe bioavailability in plant foods( Reference Gibson 54 ). In addition, there is a need to monitor the nutritional transition within development initiatives.
Acknowledgements
Sources of funding: This research was supported by the Veterinarians without Borders and the XXIII World Veterinary Congress Foundation. Conflicts of interest: None of the authors have any conflicts of interest. Authors’ contributions: C.W. wrote the manuscript with input from the other authors. J.V. and C.W. developed the study hypothesis and proposal and secured the funding. C.W. and S.M. were the field leaders and coordinators, and participated in data collection. C.W. performed data analysis, and J.T. and F.Y. assisted in interpretation of the data. Acknowledgements: The cooperation of FHF and WDL, and the openness and honesty of member and non-member farmers, are truly appreciated. The efforts of Regina Gitau, Lisa Wolff and Ruth Wanjiro were paramount for locating the many rural households, providing translation, and patiently and persistently conducting the survey.







