The prevalences of overweight and obesity are continuing to increase in developed as well as developing countries(Reference Das1). Obesity leads to a state of chronic, low-grade inflammation(Reference Das2), characterised by increased levels of circulating inflammatory biomarkers including C-reactive protein (CRP), TNF-α and IL-6(Reference Luc, Bard and Juhan-Vague3). This chronic inflammatory state is believed to contribute to the pathophysiology of a wide range of life-threatening diseases, such as CVD, diabetes and cancer(Reference Das4). Hence, therapeutic strategies that target obesity-induced inflammation have the potential to greatly reduce disease burden.
Carotenoids are a class of non-enzymatic fat-soluble antioxidants(Reference El-Agamey, Lowe and McGarvey5) that can be found in tomatoes and tomato products(Reference Canene-Adams, Campbell and Zaripheh6). Lycopene is the most potent antioxidant among the carotenoids and protects the body from oxidative stress by scavenging free radicals(Reference Chaiyasit, McClements and Decker7). Lycopene also acts as an anti-inflammatory agent(Reference Bignotto, Rocha and Sepodes8, Reference Saedisomeolia, Wood and Garg9). Antioxidants, such as lycopene, inhibit the activation of NF-κB, thereby reducing expression of inflammatory cytokines such as IL-6 and IL-8(Reference Kim, Sanders and Siekierski10, Reference Kim, Kim and Ahn11). Lycopene also acts in human adipose tissue(Reference Walfish, Walfish and Agbaria12), on cytokine pathways and reduces the production of inflammatory biomarkers(Reference Herzog, Siler and Spitzer13). Therefore, lycopene has the potential to alleviate the damaging effects of obesity-induced inflammation.
There are limited clinical data regarding the effects of tomato juice, or more specifically lycopene, on inflammation in obese or overweight individuals. We hypothesised that the consumption of a lycopene-rich food would reduce inflammation in people who are overweight or obese. The aim of the present study was to determine the effect of tomato juice consumption on the level of inflammatory biomarkers (IL-6, IL-8, high-sensitivity CRP and TNF-α) in overweight and obese female students of the Tehran University of Medical Sciences.
Experimental methods
Study population
A total of 106 overweight or obese female students were recruited from the Tehran University of Medical Sciences via advertisement. Participants were aged between 20 and 40 years and had a BMI of 25 kg/m2 or higher. Exclusion criteria were inflammatory diseases, including diabetes, CVD, multiple sclerosis, rheumatoid arthritis, cancers, asthma and allergies, smoking and daily usage of any anti-inflammatory drugs such as aspirin or vitamin/mineral supplements. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Ethics Committee of Human Experimentation of Tehran University of Medical Sciences (no. 10 492). Written informed consent was obtained from all subjects/patients.
Study design
Subjects were randomly allocated to the intervention or control group. The group allocation for subjects was determined by computer-generated random allocation, derived and held by an independent statistician. Initial diet allocation was concealed from the clinical recruitment staff until each subject had entered the trial and received a randomisation code. Neither participants nor investigators were blinded to the treatment allocation. The intervention group (n 53) and control group (n 53) received 330 ml of tomato juice (Takdaneh Company) and water daily for 20 d, respectively. The tomato juice provided 37·0 mg lycopene. Subjects were asked to consume the juice with their main meals (110 ml, three times a day = 330 ml). Subjects were advised to maintain their usual diet and physical activity levels. Analysis of background diet indicated that participants were consuming between one and three tomatoes (average two) and one to three other vegetables and up to two fruit servings daily. The average background lycopene intake of participants was 8·2 (sem 1·2) mg/d. Adherence to the study was maintained by using telephone calls every 3 d.
Participant flow
A total of 106 subjects were recruited for the study. No adverse events were reported. In the intervention group, all the fifty-three subjects completed the trial and in the control group, fifty-one of the fifty-three subjects completed the study. The progress of subjects through the trial is described in Fig. 1.

Fig. 1 Study plan.
Clinic visits
Subjects visited the clinic at baseline and at day 20. At each visit, height and weight were measured (Seca) and BMI was calculated by dividing weight (kg) by height2 (m2). Blood (10 ml) was collected at baseline and at day 20. Samples were centrifuged at 3000 g for 10 min at 4°C. Serum was separated and frozen at − 70°C in an ultra-freezer (Sanyo) until analysis.
Biochemical analysis
Serum concentrations of IL-6, IL-8 and TNF-α were measured using commercial ELISA kits (Bender MedSystems Diagnostics GmbH) according to the manufacturer's instructions. High-sensitivity CRP was measured using the turbidometry method (Roche).
Dietary assessments
At baseline and at day 20, dietary intake was estimated using three 24-h food recalls (two working days and one weekend day) (Table 2). The intakes of energy and macronutrients were estimated using Food Processor II Software (ESHA Research). Carotenoid intake (α-carotene, β-carotene, lutein, zeaxanthin and lycopene) was estimated at baseline and at day 20 using a FFQ. Values were converted to mg per d using manual calculations compared to standard tables of the United States Department of Agriculture–Nutrition Coordinating Center Carotenoid Database for US Foods.
Statistical analysis
The Statistical Package for Social Sciences (SPSS) (version 16 for windows; SPSS, Inc.) was used to compare variables at baseline and at day 20 (paired t test), as well as the change variables between the two groups (independent t test). The Kolmogorov–Smirnov test confirmed that the data were normally distributed. In order to observe the relationship between independent and dependent variables, covariance analysis was performed. P values < 0·05 were considered as statistically significant. Values were presented as mean values with their standard errors. Pearson's correlation coefficients were used to examine associations between change variables.
Results
Baseline characteristics were similar for subjects who were randomised to the control v. the intervention groups (Table 1). There were also no differences in serum levels of IL-6, IL-8, high-sensitivity CRP and TNF-α between the two groups at baseline (Table 1). Analysis of dietary records indicated that there was no difference between the groups in dietary intake at baseline (Table 2). There were also no changes in dietary intake of nutrients at day 20, compared with baseline, in either group (Table 2). Following the treatment phase, in the intervention group, tomato juice consumption led to significantly decreased serum concentrations of TNF-α and IL-8 compared with baseline and with control group (Table 3). In the control group, there was an increase in TNF-α and IL-8 concentrations compared with baseline (Table 3). Additional subgroup analysis was performed, with subjects classified as overweight or obese, according to their baseline BMI. In the overweight intervention group, serum concentrations of TNF-α and IL-8 decreased compared with baseline and with overweight control group (Table 3). Among obese subjects, serum IL-6 concentration was decreased in the intervention group compared with the control group, with no differences in IL-8, high-sensitivity CRP and TNF-α observed (Table 3).
Table 1 Subject characteristics at baseline (Mean values with their standard errors)

hsCRP, high-sensitivity C-reactive protein.
Table 2 Dietary intake at baseline and change in dietary intake between baseline and day 20, in the intervention and control groups (Mean values with their standard errors)

* Percentage of energy derived from each nutrient. There were no differences in baseline values between the control and the intervention groups. There were also no differences in the change in dietary intake between baseline and day 20 and between the control and intervention groups.
Table 3 Change in systemic inflammatory markers between baseline and day 20, in the intervention and control groups (Mean values with their standard errors)
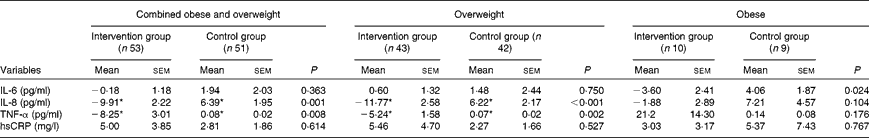
hsCRP, high-sensitivity C-reactive protein.
* Mean values were significantly different for day 20 compared to baseline (P< 0·05).
Discussion
The present study is the first to demonstrate that tomato juice reduces systemic inflammation in overweight and obese females. Following supplementation, we observed that tomato juice significantly decreased serum concentrations of IL-8 and TNF-α. Subgroup analysis demonstrated that subjects who were overweight were more responsive to the effects of the tomato juice, with decreases in serum concentrations of TNF-α and IL-8, while obese subjects showed a decrease in serum IL-6 concentration.
The results that we observed in the present study are in general agreement with a study by Riso et al. (Reference Riso, Visioli and Grande14), which showed modest effects on the production of inflammatory mediators, such as TNF-α, following the regular intake of a tomato drink by young healthy volunteers. Similarly, Watzl et al. (Reference Watzl, Bub and Briviba15) demonstrated that the highest production of TNF-α was associated with a low lycopene intake. Hazewindus et al. (Reference Hazewindus, Haenen and Weseler16) in their study on lycopene, ascorbic acid and α-tocopherol, also found that lycopene considerably diminished inflammation by suppressing the release of TNF-α and stimulating IL-10 production. Cell culture(Reference Saedisomeolia, Wood and Garg9) as well as experimental studies(Reference Wang, Ausman and Greenberg17) also reported similar findings. In vitro (Reference Saedisomeolia, Wood and Garg9), lycopene decreased the release of IL-6 and interferon-induced protein of 10 kDa by airway epithelial cells following exposure to lipopolysaccharide. It has also been shown that lycopene reduced oxysterol-induced pro-inflammatory cytokine (IL-1β, IL-6, IL-8 and TNF-α) production and expression in human macrophages(Reference Palozza, Simone and Catalano18). Ex vivo studies revealed that inflammatory biomarkers such as IL-6, monocyte chemotactic protein-1 and IL-1β decreased (at both the mRNA and protein level) when epididymal adipose tissue of mice was incubated with lycopene(Reference Gouranton, Thabuis and Riollet19). The same effect was reported when adipose tissue was pre-incubated with lycopene and then stimulated using TNF-α(Reference Gouranton, Thabuis and Riollet19). Likewise, Gouranton et al. (Reference Gouranton, Thabuis and Riollet19) reported similar findings with human adipocyte primary cultures. In a clinical setting(Reference Wang, Ausman and Greenberg17), lycopene and tomato extract inhibited non-alcoholic steatohepatitis-promoted hepatocarcinogenesis, mainly as a result of reduced oxidative stress, which could modify various pathways involved in carcinogenesis.
The nature of obesity as a chronic inflammatory condition(Reference Eckel20) highlights the potential beneficial role of nutrients such as lycopene, which have anti-inflammatory properties. As the level of inflammation increases with increasing BMI, we expected that obese subjects would experience a greater improvement in inflammation than the overweight subjects, following the tomato juice intervention, because higher fat mass leads to increased inflammation(Reference Mehta and Farmer21). However, subgroup analysis, with subjects classified according to their baseline BMI, determined that daily tomato juice consumption had a more pronounced effect in the overweight subjects. Indeed, there were decreases in the serum concentrations of both IL-8 and TNF-α in overweight subjects. In obese subjects, only serum concentration of IL-6 was decreased by tomato juice consumption. It is unclear why more significant changes were seen in the overweight, compared with the obese, group. It is possible that the anti-inflammatory actions of lycopene were not potent enough to dampen the heightened level of baseline inflammation that was present in the obese group. Alternatively, this observation may be due to study limitations. There was a disparity in the number of subjects in the overweight v. obese groups (overweight group, n 85 v. obese group, n 19). Hence, the obese group may have been underpowered to detect changes in other inflammatory mediators.
The anti-inflammatory effects that we observed may be attributable to the presence of lycopene in the tomato juice(Reference Maruyama, Imamura and Oshima22). Studies have shown that lycopene decreases the translocation of NF-κB, which leads to a decreased expression of inflammatory biomarkers including IL-8 as well as TNF-α(Reference Blackwell and Christman23). This anti-inflammatory effect of lycopene is related to its redox property(Reference Kim, Kim and Ahn11). Many studies have shown that lycopene decreases the translocation of NF-κB, via activation of I-κB, which is an inhibitor of NF-κB(Reference Palozza, Simone and Catalano18, Reference Huang, Fan and Lin24). Regarding the molecular mechanism, Gouranton et al. (Reference Gouranton, Thabuis and Riollet19) showed that the presence of lycopene suppresses the luciferase gene reporter under control of the NF-κB-responsive element. Furthermore, the involvement of the NF-κB pathway was shown by the modulation of Iκ kinase α/β phosphorylation by lycopene. Tomato juice also contains other important nutrients including the antioxidants β-carotene and vitamin C. β-Carotene(Reference Bai, Lee and Na25) and vitamin C(Reference Cindrova-Davies, Spasic-Boskovic and Jauniaux26) have been shown to decrease inflammation via their probable redox-based effect on inactivation of NF-κB. The individual effects of these other nutrients cannot be determined by the present study, but it is likely that they have also contributed to the anti-inflammatory effects (via their redox action) that we have observed. This highlights the benefit of supplementing with whole foods, rather than with individual nutrients, in order to maximise the benefits of nutritional interventions, a fact that has been shown by other researchers(Reference Basu and Imrhan27, Reference Shi, Kakuda and Yeung28). We conclude that tomato juice reduces the inflammatory mediators that are associated with overweight and obesity in females. Thus, increasing tomato intake may provide a useful approach for reducing the risk of inflammatory diseases, such as CVD and diabetes, which are associated with obesity.
Acknowledgements
The authors gratefully acknowledge the students and officials of Kooy and Vesal dormitories for their sincere cooperation. The present work was supported by the Vice-chancellor for Research of Tehran University of Medical Sciences (grant no. 10492), and the authors declare no conflict of interests. The authors would like to advise that all authors listed have contributed to the work. M. G. recruited the patients and collected the samples, performed laboratory tests, analysed the data and drafted the manuscript. A. S., G. S. and L. G. W. supervised the nutrition science experiments, contributed to the study design and reviewed the manuscript. M. D. supervised the biochemical experiments and contributed to the writing of the manuscript. M. R. E. contributed to the data analysis and the interpretation of results. A. M. M. performed laboratory tests and contributed to the writing of the manuscript.






