In the last 50 years, there has been a shift in the structure of the diet towards a higher-energy density one, characterised by higher intakes of fat and proteins (mostly from animal sources) and added sugars present in foods and lower intakes of complex carbohydrates, fruits and vegetables. At the same time, chronic diseases have become the main cause of CVD and cancer mortality, leading in the list of mortality causes in Western countries( Reference Roger, Go and Lloyd-Jones 1 ). Thus, the knowledge about the effect that nutrients and foods might have on health is of great importance for public health management. The intake of meat, specifically red and processed, has increased in industrialised countries, resulting in it becoming the basic component of meals. The effect of meat consumption on health is being studied in depth by nutritional epidemiologists( Reference Sacks and Campos 2 – Reference Cocate, Natali and Oliveira 5 ). General meat consumption has been reported to be associated with all-cause and specific-cause mortality. However, when considering the type of meat consumed, different associations have been observed. Systematic reviews and meta-analyses have found a higher incidence of CVD, diabetes and some types of cancers to be related to higher red and processed meat consumption( Reference Aune, Ursin and Veierød 6 – Reference Chan, Lau and Aune 10 ), while no association or a tendency towards an inverse association between white meat consumption and total mortality has been observed in some cases( Reference Sinha, Cross and Graubard 11 ). Large prospective studies have found a higher incidence of CVD and a higher risk of all-cause mortality among greater meat eaters( Reference Sinha, Cross and Graubard 11 – Reference Rohrmann, Overvad and Bueno-de-Mesquita 13 ). Very recently, results obtained from another meta-analysis on red and processed meat consumption have shown that the consumption of processed meat and total red meat is positively associated with all-cause mortality( Reference Larsson and Orsini 14 ). However, there is considerable scientific debate regarding the association between meat consumption and CVD and IHD mortality( Reference Sinha, Cross and Graubard 11 – Reference Rohrmann, Overvad and Bueno-de-Mesquita 13 , Reference Nagao, Iso and Yamagishi 15 – Reference Takata, Shu and Gao 18 ). Most of the positive associations found between meat consumption and CVD mortality have been observed in studies conducted in North America( Reference Sinha, Cross and Graubard 11 Reference Pan, Sun and Bernstein 12 ) and Europe( Reference Rohrmann, Overvad and Bueno-de-Mesquita 13 , Reference Chang-Claude, Hermann and Eilber 19 ), while results obtained from Asian studies do not indicate a clear association( Reference Nagao, Iso and Yamagishi 15 , Reference Lee, McLerran and Rolland 16 ). As the evidence from prospective cohort studies on the association of white, red and processed meat consumption with all-cause, CVD and IHD mortality has not been summarised yet, we carried out a meta-analysis to quantitatively summarise the existing published evidence from cohort studies on the association between the consumption of total meat and three types of meats (white, red and processed) and the risk of death from any cause, CVD and IHD.
Methods
Search strategy
We searched the PubMed and ISI Web of Knowledge databases to identify published prospective cohort studies in which dietary intake was measured at baseline (through August 2013). Keywords included, either in the title or in the abstract (without restrictions), the following: meat; red meat; white meat; processed meat; ham; sausages; hamburger; bacon; luncheon meats; beef; poultry; pork; rabbit; turkey; lamb; duck; all combined with mortality; total mortality; death; fatal coronary heart disease; fatal event and CVD; IHD; myocardial infarction; heart attack; heart failure. Death from CVD included mortality cases due to diseases of the circulatory system, IHD and cerebrovascular diseases. The reference lists of the selected studies and systematic reviews and meta-analyses were examined to identify further studies.
‘Red meat’ was defined as fresh meat from beef, veal, lamb, or pork, hamburgers and meatballs. In the study carried out by Sinha et al. ( Reference Sinha, Cross and Graubard 11 ), red meat included processed and unprocessed meats; therefore; the analysis was repeated by excluding this study. ‘White meat’ was defined as poultry (chicken and turkey) and rabbit. In one study( Reference Sinha, Cross and Graubard 11 ), fish was combined with the white meat consumption group; thus, the analysis was repeated by excluding this study. ‘Processed meat’ was defined as any meat preserved by smoking, curing or salting or addition of chemical preservatives, such as bacon, salami, sausages, hot dogs or luncheon meats. ‘Total meat’ was defined as the total of these three categories.
We contacted the authors of four studies( Reference Rohrmann, Overvad and Bueno-de-Mesquita 13 , Reference Nagao, Iso and Yamagishi 15 – Reference Kappeler, Eichholzer and Rohrmann 17 ) to obtain missing data needed to conduct dose–response analyses. Only two authors( Reference Nagao, Iso and Yamagishi 15 , Reference Kappeler, Eichholzer and Rohrmann 17 ) provided the requested information.
Study selection
We selected prospective cohort studies in which the relationship between the intake of total meat and/or red meat and/or white meat and/or processed meat and total mortality and/or mortality from CVD and/or mortality from IHD was investigated. Studies comparing only vegetarians and non-vegetarians( Reference Fraser and Shavlik 20 – Reference Crowe, Appleby and Travis 22 ) were excluded, but three studies that reported the comparison of vegetarians and non-vegetarians also analysed dietary variables (including meat) regardless of the group (vegetarian and non-vegetarian) and were therefore included in the analysis( Reference Chang-Claude, Hermann and Eilber 19 , Reference Fraser 23 , Reference Mann, Appleby and Key 24 ).
Risk ratios had to be available with 95 % CI either in the publication or on being requested from the authors. To be included in the dose–response analysis, a quantitative measure of intake had to be presented in the article or be obtainable from the authors. When several publications of the same study were identified, only the most recent or most detailed publication was used. The Shanghai Women's Health Study was included in two articles( Reference Lee, McLerran and Rolland 16 , Reference Takata, Shu and Gao 18 ); therefore, for the comparison of the highest v. the lowest consumption category, only the study carried out by Lee et al. ( Reference Lee, McLerran and Rolland 16 ) was considered, and for the dose–response meta-analysis, only the study carried out by Takata et al. ( Reference Takata, Shu and Gao 18 ) was considered.
Data extraction
The following information was extracted from each article: country; sample size and number of total, CVD or IHD deaths; method used for the identification and verification of the cause of death; duration of follow-up; method used for dietary intake assessment (FFQ, or diet history, only at baseline or updated during follow-up and whether the method had been validated); meat type; highest and lowest intake amounts; relative risks (RR) and 95 % CI; variables included in the adjusted model (Table 1). The articles were independently reviewed by two researchers (A. R. V. and I. A. G.) and information was extracted.
Table 1 Characteristics of the selected prospective cohort studies on the association between meat (total, white, red and processed) consumption and mortality (all-cause, CVD or IHD) (Hazard ratios (HR) and 95 % confidence intervals and number of participants)

M, male; F, female; TM, total meat; RM, red meat; PA, physical activity; HBP, hypertension; WM, white meat; AC, alcohol consumption; PM, processed meat; EL, education level; MI, myocardial infarction; TIA, transient ischaemic attack; DM, diabetes mellitus; NIH-AARP, National Institutes of Health-American Association of Retired Persons; TEI, total energy intake; JACC, Japan Collaborative Cohort; HPFS, Health Professional Follow-up Study; NHS, Nurses' Health Study; HRT, hormone-replacement therapy; NHANES III, Third National Health and Nutrition Examination Survey; SCE, socio-economic status; EPIC, European Prospective Investigation into Cancer and Nutrition; BW, body weight; SWHS, Shanghai Women's Health Study; SMHS, Shanghai Men's Health Study.
* Hamburgers are included in this group.
† This red meat group includes processed and unprocessed red meats.
‡ The white meat group includes fish consumption.
Statistical analyses
We conducted two types of meta-analyses. First, we combined the RR for the highest v. the lowest category of meat (red, white, processed and total) consumption using a random-effects model, which considers both within-study and between-study variations( Reference DerSimonian and Laird 25 ). Second, we conducted a dose–response meta-analysis using the methods proposed by Greenland & Longnecker( Reference Greenland and Longnecker 26 ) and Orsini et al. ( Reference Orsini, Bellocco and Greenland 27 ) to derive the log-linear dose–response slope within each study from categorical data. The method requires that the distribution of cases and person-years and the RR with the variance estimates be given for at least three quantitative exposure categories. The reported median or mean level of meat intake in each category of consumption was assigned to the corresponding RR for each study. For studies that reported intake by ranges( Reference Kappeler, Eichholzer and Rohrmann 17 , Reference Whiteman, Muir and Jones 28 ), we estimated the mid-point in each category by calculating the average of the lower and upper bounds. When the highest or lowest category of consumption was open-ended, the open-ended interval length was assumed to be of the same length as the adjacent interval. When studies reported the intake in servings and time/d per week or g/4184 kJ (g/1000 kcal)( Reference Sinha, Cross and Graubard 11 , Reference Pan, Sun and Bernstein 12 , Reference Kappeler, Eichholzer and Rohrmann 17 , Reference Chang-Claude, Hermann and Eilber 19 , Reference Fraser 23 , Reference Mann, Appleby and Key 24 , Reference Fortes, Forastiere and Farchi 29 , Reference Jamrozik, Broadhurst and Forbes 30 ), we converted the intakes to grams of intake per d using standard units of 120 g for total, red and white meats and 50 g for processed meat( Reference Norat, Lukanova and Ferrari 31 ). The results are presented per 100 g/d for total, red and white meats and per 50 g/d for processed meat. For studies that reported results stratified by sex but not results for men and women together, a combined estimate of the association was calculated using fixed-effects models before including the studies in the overall analysis. Overall risk estimates were calculated for men and women separately and combined.
Statistical heterogeneity among the studies was assessed using I 2, which is the amount of total variation that is explained by the between-study variation, and the Q test( Reference Higgins and Thompson 32 ), and values of 25, 50, 75 and >75 % were considered to indicate low, moderate, high and very high heterogeneity, respectively. We conducted subgroup analyses by duration of follow-up ( < 20 years or ≥ 20 years), number of cases ( < 5000 or ≥ 5000), dietary intake assessment, consumption categories (predefined or quintiles) and differences in adjustment variables. We assessed publication bias using Egger's test( Reference Egger, Davey Smith and Schneider 33 ) and Begg's test( Reference Begg and Mazumdar 34 ); the results were considered to indicate publication bias when P< 0·10( Reference Aune, Ursin and Veierød 6 ). To ensure that the results obtained were not simply due to the inclusion of one large study or a study with an extreme result, we carried out sensitivity analyses by excluding one study at a time to determine whether the results were robust. All statistical analyses were conducted using Stata, version 12, software (StataCorp). A two-tailed P< 0·05 was considered statistically significant.
Results
Study selection
A total of thirteen cohort studies including 1 674 272 individuals, 163 524 cases of total mortality, 44 340 cases of CVD mortality and 1370 cases of IHD mortality were identified (Fig. 1). The characteristics of the thirteen studies are summarised in Table 1. Of these studies, five were carried out in Europe, four in the USA, one in Australia and three in Asia.

Fig. 1 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart( Reference Moher, Liberati and Tetzlaff 40 ). Screening and selection of studies analysing the association between meat (red/white/processed) consumption and CVD mortality. For more information, visit http://www.prisma-statement.org
In the analysis of all-cause mortality, ten cohort studies could be included: five for total meat( Reference Pan, Sun and Bernstein 12 , Reference Lee, McLerran and Rolland 16 , Reference Mann, Appleby and Key 24 , Reference Fortes, Forastiere and Farchi 29 , Reference Jamrozik, Broadhurst and Forbes 30 ) consumption; seven for red meat( Reference Sinha, Cross and Graubard 11 – Reference Rohrmann, Overvad and Bueno-de-Mesquita 13 , Reference Lee, McLerran and Rolland 16 – Reference Takata, Shu and Gao 18 , Reference Whiteman, Muir and Jones 28 ) consumption; six for white meat( Reference Sinha, Cross and Graubard 11 , Reference Rohrmann, Overvad and Bueno-de-Mesquita 13 , Reference Lee, McLerran and Rolland 16 – Reference Takata, Shu and Gao 18 , Reference Whiteman, Muir and Jones 28 ) consumption; five for processed meat( Reference Sinha, Cross and Graubard 11 – Reference Rohrmann, Overvad and Bueno-de-Mesquita 13 , Reference Kappeler, Eichholzer and Rohrmann 17 , Reference Whiteman, Muir and Jones 28 ) consumption.
In the analysis of CVD mortality, nine cohort studies could be included: five for total meat( Reference Pan, Sun and Bernstein 12 , Reference Nagao, Iso and Yamagishi 15 , Reference Lee, McLerran and Rolland 16 , Reference Chang-Claude, Hermann and Eilber 19 , Reference Jamrozik, Broadhurst and Forbes 30 ) consumption; seven for red meat( Reference Sinha, Cross and Graubard 11 – Reference Rohrmann, Overvad and Bueno-de-Mesquita 13 , Reference Nagao, Iso and Yamagishi 15 – Reference Takata, Shu and Gao 18 ) consumption; six for white meat( Reference Sinha, Cross and Graubard 11 , Reference Rohrmann, Overvad and Bueno-de-Mesquita 13 , Reference Nagao, Iso and Yamagishi 15 – Reference Takata, Shu and Gao 18 ) consumption; six for processed meat( Reference Sinha, Cross and Graubard 11 – Reference Rohrmann, Overvad and Bueno-de-Mesquita 13 , Reference Nagao, Iso and Yamagishi 15 , Reference Kappeler, Eichholzer and Rohrmann 17 , Reference Chang-Claude, Hermann and Eilber 19 ) consumption.
In the analysis of IHD mortality, six cohort studies could be included: three for total meat( Reference Nagao, Iso and Yamagishi 15 , Reference Chang-Claude, Hermann and Eilber 19 , Reference Mann, Appleby and Key 24 ) consumption; four for red meat( Reference Nagao, Iso and Yamagishi 15 , Reference Takata, Shu and Gao 18 , Reference Fraser 23 , Reference Whiteman, Muir and Jones 28 ) consumption; three for white meat( Reference Nagao, Iso and Yamagishi 15 , Reference Takata, Shu and Gao 18 , Reference Whiteman, Muir and Jones 28 ) consumption; three for processed meat( Reference Nagao, Iso and Yamagishi 15 , Reference Chang-Claude, Hermann and Eilber 19 , Reference Whiteman, Muir and Jones 28 ) consumption.
All-cause mortality
In the meta-analysis combining the risk estimates for the highest v. the lowest consumption category, the consumption of processed meat but not of total, red and white meats was found to be positively associated with all-cause mortality (RR 1·22; 95 % CI 1·16, 1·29; I 2= 44·4, P= 0·126) (Figs. 2(a) and 3(a); Table 2). There was very high and significant heterogeneity among the studies, with the I 2 ranging from 86·9 to 95·4 %. In sensitivity analyses, the heterogeneity was substantially decreased for total meat consumption when the studies carried out by Lee et al. ( Reference Lee, McLerran and Rolland 16 ) and Jamrozik et al. ( Reference Jamrozik, Broadhurst and Forbes 30 ) were excluded (I 2= 55·8 %, P= 0·104); thus, the RR increased and the CI moved to the right with a trend towards a positive association with all-cause mortality (RR 1·23; 95 % CI 0·98, 1·53). For red meat consumption, the heterogeneity remained when each study was excluded one by one, and a positive association was confirmed (RR 1·14; 95 % CI 1·01, 1·29) when an Asian study( Reference Lee, McLerran and Rolland 16 ) was excluded. For white meat consumption, between-study heterogeneity decreased (I 2= 0 %, P= 0·630) when a large American study( Reference Sinha, Cross and Graubard 11 ) was excluded, but no association with all-cause mortality was observed (RR 0·92; 95 % CI 0·84, 1·05).

Fig. 2 Association between highest v. lowest processed meat consumption and (a) all-cause, (b) CVD and (c) IHD mortality risk. The relative risk (RR) of each study is represented by a ■ and the size of the ■ represents the weight of each study to the overall estimate. 95 % CI are represented by ![]() and the ◇ represents the overall estimate and its 95 % CI.
and the ◇ represents the overall estimate and its 95 % CI.
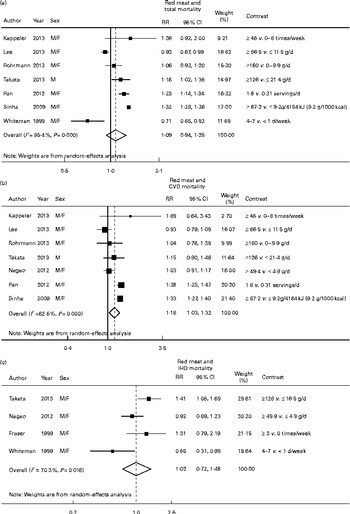
Fig. 3 Association between highest v. lowest red meat consumption and (a) all-cause, (b) CVD and (c) IHD mortality risk. The relative risk (RR) of each study is represented by a ■ and the size of the ■ represents the weight of each study to the overall estimate. 95 % CI are represented by the ![]() and the ◇ represent the overall estimate and its 95 % CI.
and the ◇ represent the overall estimate and its 95 % CI.
Table 2 Summary of the estimated relative risks (RR) and 95 % confidence intervals

P h, heterogeneity P value; TM, total meat; RM, red meat; WM, white meat; PM, processed meat; NC, not calculable.
* Dose–response analysis: RR/100 g per d increase for total and red meats and 50 g/d increase for processed meat.
The analysis stratified by sex showed that processed meat consumption was positively associated with an increased risk of all-cause mortality in both men (RR 1·22; 95 % CI 1·13, 1·31; I 2= 60·9, P= 0·053) and women (RR 1·23; 95 % CI 1·19, 1·27; I 2= 0, P= 0·670). Red meat consumption was associated with a 17 % higher risk of all-cause mortality in men (RR 1·17; 95 % CI 1·04, 1·32; I 2= 89·3, P< 0·001), but not in women (RR 1·13; 95 % CI 0·96, 1·34; I 2= 94·1, P< 0·001). White meat consumption was associated with a 5 % lower risk of all-cause mortality only in women (RR 0·95; 95 % CI 0·91, 0·99; I 2= 0, P= 0·805).
Among the selected studies, two studies could not be included in the dose–response meta-analysis because the number of deaths and subjects for the consumption categories of each type of meat were not reported( Reference Lee, McLerran and Rolland 16 ) and meat consumption was divided into two categories( Reference Jamrozik, Broadhurst and Forbes 30 ). The dose–response analysis showed that the RR for a 50 g/d increase in processed meat intake was 1·25 (95 % CI 1·07, 1·45; I 2= 95·7 %, P< 0·001). In the analysis stratified by sex, the positive association was confirmed in both men and women. On the other hand, a 100 g/d increase in total, red and white meat intake was not associated with all-cause mortality (Table 2). However, when the analysis was stratified by sex, a positive association was found between red meat consumption and mortality risk in both men (RR 1·21; 95 % CI 1·15, 1·26; I 2= 47·7 %, P= 0·137) and women (RR 1·14; 95 % CI 1·00, 1·30; I 2= 91·4 %, P< 0·001). There was no evidence of publication bias (P>0·10) in any of the analyses.
CVD mortality
Risk estimates for the comparison of the highest v. the lowest consumption category of processed meat (RR 1·18; 95 % CI 1·05, 1·32; I 2= 73·5, P= 0·002) and red meat (RR 1·16; 95 % CI 1·03, 1·32; I 2= 82·5, P< 0·001) showed positive associations with CVD mortality. There was very high and significant heterogeneity in both cases (Figs. 2(b) and 3(b)). In the analysis of processed meat consumption, the heterogeneity ranged from I 2= 68·5 % (P= 0·013 and a RR of 1·23 (95 % CI 1·09, 1·38)) when a Japanese study( Reference Nagao, Iso and Yamagishi 15 ) was excluded to I 2= 89·4 % (P< 0·001 and a RR of 1·20 (95 % CI 1·07, 1·35)) when a US study( Reference Kappeler, Eichholzer and Rohrmann 17 ) was excluded. In the sensitivity analysis of red meat consumption, the heterogeneity decreased substantially (I 2= 14·7 %, P= 0·319) when Asian studies( Reference Nagao, Iso and Yamagishi 15 , Reference Lee, McLerran and Rolland 16 , Reference Takata, Shu and Gao 18 ) were excluded and the association was strengthened (RR 1·33; 95 % CI 1·26, 1·40). When the analysis was stratified by sex, the association between processed and red meat consumption and CVD mortality was slightly strengthened in women but not in men (Table 2).
Total meat (RR 1·08; 95 % CI 0·85, 1·36; I 2= 90·6, P< 0·001) and white meat (RR 1·01; 95 % CI 0·96, 1·07; I 2= 10·6, P= 0·348) consumption was not associated with CVD mortality in the analysis of the highest v. the lowest consumption category. Similar associations were observed when the analysis was stratified by sex (Table 2).
The same two studies mentioned in the All-cause mortality section could not be included in the dose–response meta-analysis( Reference Lee, McLerran and Rolland 16 , Reference Jamrozik, Broadhurst and Forbes 30 ). In the dose–response meta-analysis, the RR per 50 g/d increase in processed meat intake (RR 1·24; 95 % CI 1·09, 1·40; I 2= 76·4 %, P= 0·001) and the RR per 100 g/d increase in red meat intake (RR 1·15; 95 % CI 1·05, 1·26; I 2= 76·6 %, P< 0·001) were positively associated with CVD mortality. In the analysis stratified by sex, the association between red meat consumption and CVD mortality was strengthened in both sexes, while the association between processed meat consumption and CVD mortality was strengthened only in women (Table 2).
No associations were observed between total and white meat consumption and CVD mortality in the dose–response meta-analysis, and similar associations were observed in the analysis stratified by sex (Table 2 and Supplementary Figs. 4–5). There was no evidence of publication bias in any of the analyses.
IHD mortality
In the meta-analysis of the highest v. the lowest consumption category, processed meat consumption was found to be not associated with IHD mortality (RR 1·52; 95 % CI 0·50, 4·66; I 2= 81·7, P= 0·004), but the 95 % CI was broad and shifted to the right (Fig. 2(c)). Red meat consumption was not associated with IHD mortality (RR 1·02; 95 % CI 0·72, 1·46; I 2= 70·3, P= 0·018) (Fig. 3(c)). Similarly, total meat (RR 1·52; 95 % CI 0·68, 3·40; I 2= 82·7, P= 0·030) and white meat (RR 1·00; 95 % CI 0·82, 1·21; I 2= 0, P= 0·780) consumption was not associated with IHD mortality. Only the analysis of red meat consumption could be stratified by sex. No association was observed between red meat consumption and IHD mortality either in men (RR 1·30; 95 % CI 0·66, 2·55; I 2= 82·5, P= 0·003) or in women (RR 1·17; 95 % CI 0·89, 1·53; I 2= 0, P= 0·447).
Similar associations were observed in the dose–response meta-analysis for all types of meats analysed (Table 2).
There was no evidence of publication bias determined by Begg's (P>0·10) and Egger's tests (P>0·10) in any of the analyses.
Subgroup analyses
Stratified analyses were carried out for red and processed meat consumption and total and CVD mortality risk to examine the sources of heterogeneity. Most results were consistent across the strata (Tables 3 and 4). Larger studies ( ≥ 5000 cases) and studies with longer follow-up periods ( ≥ 20 years) reported, on average, stronger associations of red and processed meat consumption with total and CVD mortality compared with the other studies. In general, studies that included adjustment variables such as total energy intake, fruits and vegetables, smoking history, physical activity, cardiovascular risk factors, vitamin supplements and BMI, on average, in the model reported stronger associations of red and processed meat consumption with total and CVD mortality, but this did not lead to a reduction of the heterogeneity. Studies that adjusted for socio-economic status reported, on average, weaker associations of red and processed meat consumption with total and cardiovascular mortality compared with studies that did not adjust for it (Tables 3 and 4).
Table 3 Results of the subgroup analyses (for the highest v. the lowest consumption) of studies evaluating red meat consumption and all-cause and CVD mortality as clinical outcomes (Relative risks (RR) and 95 % confidence intervals)

P h, heterogeneity P value; NC, not calculable.
Table 4 Results of the subgroup analyses (for the highest v. the lowest consumption) of studies evaluating processed meat consumption and all-cause and CVD mortality as clinical outcomes (Relative risks (RR) and 95 % confidence intervals)

P h, heterogeneity P value; NC not calculable.
Discussion
In the present meta-analysis, processed meat consumption was found to be associated with an increased risk of mortality from any cause and CVD. Subjects in the highest category of processed meat consumption had 22 and 18 % higher mortality risk from any cause and CVD, respectively, than those in the lowest category of consumption. On the other hand, red meat consumption was associated only with an increased risk of CVD mortality. In the analysis stratified by sex, the association of processed and red meat consumption with CVD mortality remained significant in women but not in men. It is unclear whether these differences in the association are due to physiological differences between the sexes or simply due to differences in the selected studies. Only one study reported sex differences in the association between red meat consumption and IHD mortality, showing a significant association in men but not in women( Reference Takata, Shu and Gao 18 ).
Overall, the results of this meta-analysis indicate that the consumption of both red meat and processed meat might have an adverse effect on health, increasing the risk of CVD mortality. When all types of meats were considered together, no association was found to emerge, which highlights the importance of considering each type of meat separately. These findings are in agreement with those of a very recent meta-analysis on the relationship between red and processed meat consumption and all-cause mortality, in which subjects in the highest category of processed and total red meat consumption were found to have an increased all-cause mortality risk of 23 and 29 %, respectively, compared with those in the lowest consumption category. Previous meta-analyses on the association between red and processed meat consumption and CVD incidence, type 2 diabetes and certain types of cancers, such as colorectal cancer, have also found positive associations( Reference Aune, Ursin and Veierød 6 – Reference Chan, Lau and Aune 10 ). It has been suggested that the consumption of red meat, especially processed meat, may increase the risk of all-cause mortality as well as CVD mortality by means of several components that boost cardiovascular alterations. Saturated fat, cholesterol and haeme Fe contents in meats seem to be the key factors involved in atherosclerotic processes that promote the appearance of cardiovascular risk factors and chronic diseases such as hypertension, hypercholesterolaemia, endothelial dysfunction, insulin resistance and type 2 diabetes( Reference Zhao, Li and Liu 35 , 36 ). On the other hand, preservatives such as Na and nitrates in processed meats might explain the positive associations observed for processed meat but not for red meat( Reference Micha, Michas and Lajous 9 ). High Na consumption is a well-recognised factor for the development of hypertension; nitrates and their derivatives have been reported to be associated with oxidative stress processes promoting metabolic disturbances in main organs and tissues, resulting in insulin resistance, endothelial dysfunction, type 2 diabetes and some types of cancers( Reference Aune, Ursin and Veierød 6 , Reference Aune, Chan and Vieira 37 ). Inflammatory mechanisms have also been proposed as intermediary processes promoting atherosclerosis, CVD and type 2 diabetes. In a recent cross-sectional study conducted in the Nurses' Health Study, increased C-reactive protein levels have been observed in women consuming higher quantities of red and processed meat than in those consuming lower quantities( Reference Ley, Sun and Willett 38 ).
The association between red meat consumption and CVD mortality became stronger when the Asian studies( Reference Nagao, Iso and Yamagishi 15 , Reference Lee, McLerran and Rolland 16 , Reference Takata, Shu and Gao 18 ) were excluded from the analysis. Meat consumption in Asian countries is considerably lower than that in Western countries( Reference Lee, McLerran and Rolland 16 ), which could explain in part the weak associations observed in the cohort studies. In a pooled analysis of eight Asian cohorts, the association between red meat consumption and CVD mortality was found to be inverse and statistically significant( Reference Lee, McLerran and Rolland 16 ). The authors indicated that dietary factors, lifestyle, socio-economic status and disease distribution are changing in Asian countries and, thus, other factors may be stronger predictors of mortality than meat consumption. On the other hand, the food preparation technique, which is not considered in observational prospective cohort studies, might also have a role.
Very little has been reported on the effect of white meat consumption on mortality risk. In the analysis of the highest v. the lowest consumption category, a weak inverse association was observed in women for all-cause mortality. Previously, Sinha et al. ( Reference Sinha, Cross and Graubard 11 ) had observed a small decrease in total and cancer mortality risk in men and women consuming higher quantities of white meat. Recently, Lee et al. ( Reference Lee, McLerran and Rolland 16 ) have also found an inverse association between poultry intake and total mortality in men and women. However, the interpretation of the effect of white meat consumption on health is a difficult task, as subjects consuming more white meat are, at the same time, consuming less red meat. Findings obtained in the present meta-analysis are weak and not conclusive. More studies assessing the effect of white meat consumption on mortality are required.
The present meta-analysis has several strengths. The large number of total and CVD mortality cases provided the statistical power to detect meaningful associations with the exposure. We summarised the RR estimates for the highest v. the lowest level of intake in the studies and used generalised least-squares models for trend estimation and dose–response assessments. The analyses were conducted by types of meats (total, red, white and processed), and only two studies classified red meat( Reference Sinha, Cross and Graubard 11 ) and processed meat( Reference Whiteman, Muir and Jones 28 ) differently. An analysis excluding these studies was also carried and the association was found to not change (data not shown). On the other hand, although in almost all analyses there was no evidence of publication bias determined by Begg's and Egger's tests, such tests have limited statistical power in the setting of relatively few studies. We contacted authors and included unpublished results to reduce the potential impact of publication bias.
The limitations of the meta-analysis should also be mentioned. Long-term prospective cohorts are limited by misclassification and residual confounding( Reference Alexander 39 ); thus, each of these studies has potential limitations, and our findings should be interpreted in that context. It is possible that the observed positive association between red and processed meat consumption and all-cause and CVD mortality could be due to unmeasured or residual confounding. Most of the studies used models adjusted for several factors; however, residual confounding could still be present as a result of imperfect covariate measurement. Measurement of dietary intake data is imperfect, and measurement error would likely lead to an underestimation of the true effect of the exposures with the outcome. Only two studies updated dietary intake data during follow-up or corrected their estimates for the effect of measurement error( Reference Pan, Sun and Bernstein 12 , Reference Chang-Claude, Hermann and Eilber 19 ). Similarly, higher consumption of processed meat is often associated with other unhealthy lifestyles including physical inactivity, overweight, smoking, and low fruit and vegetable intake. Although several studies included some food groups as adjustment variables, none of the studies adjusted by dietary patterns, leading to possible residual confounding by an overall dietary pattern.
Socio-economic status could be an important confounder. Studies that did not adjust for socio-economic status tended to show stronger RR. Finally, heterogeneity was apparent in many of the models, which could be partly explained by differences between the studies with regard to the amount of meat consumed (mean or median from the highest and lowest categories) and the type of meat items considered in each meat group and the duration of follow-up, as well as the method used for dietary intake assessment.
Because of the possibility of residual confounding and there is significant heterogeneity in many of the models, the summary risk estimates should be interpreted with caution.
In conclusion, we found that processed meat consumption could increase the risk of any-cause and CVD mortality, while red meat consumption is only positively but weakly associated with CVD mortality. These findings highlight the importance of differentiating the meat types as the impact of processed meat consumption seems to be stronger than that of unprocessed meat consumption, but policy efforts should focus on limiting red meat and processed meat intake. More studies assessing the impact of meat consumption on IHD mortality are required. On the other hand, white meat consumption might be the ‘healthy’ alternative to red and processed meat consumption; however, more studies assessing the specific role of white meat consumption in CVD are essential.
Overall, the results of this meta-analysis should be interpreted with caution due to the high heterogeneity obtained in most of the analyses as well as the possibility of residual confounding.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S000711451400124X
Acknowledgements
The authors thank Nagao Masanori and Sabine Rohrmann for providing supplementary data from the Japan Collaborative Cohort Study and the Third National Health and Nutrition Examination Survey.
This research received no specific grant from any funding agency or commercial or not-for-profit sectors. I. A. G. received financial support from the Carlos III Health Institute of the Spanish Ministry of Health for her ‘Sara Borrell’ postdoctoral fellowship (CD11/00196). The Carlos III Health Institute had no role in the design and analysis of the research or in the writing of this article.
The authors' contributions are as follows: I. A. G., D. R. and T. N. were responsible for the study design; I. A. G. and A. R. V. were responsible for literature search, study selection, data extraction, and table and figure preparation; I. A. G. and A. R. V. analysed the data; I. A. G. wrote the manuscript; A. L. d. M. critically revised the manuscript. All authors contributed to the interpretation of the results, critically reviewed the manuscript for important intellectual content and approved the final version of the manuscript.
None of the authors has any conflicts of interest to declare.









