Insulin resistance (IR) is a condition in which normal amounts of insulin are inadequate to produce normal responses from fat, muscle (promote glucose uptake) and liver (inhibit glucose output) cells(Reference Moller and Flier1). It is a major hallmark in the development of type 2 diabetes mellitus and a number of associated metabolic disorders including non-alcoholic fatty liver disease (NAFLD), lipoatrophy and CVD(Reference Marchesini, Brizi, Morselli-Labate, Bianchi, Bugianesi, McCullough, Forlani and Melchionda2). Based on the National Health and Nutrition Examination Survey (NHANES) conducted between 1999 and 2002, 10 % of adult US men and women were diabetic and 35 % were hyperinsulinaemic (a surrogate marker for IR)(Reference Smith3, Reference Li, Ford, McGuire, Mokdad, Little and Reaven4). NAFLD is a clinicopathological term that encompasses a disease spectrum ranging from simple TAG accumulation in hepatocytes (hepatic steatosis) to hepatic steatosis with inflammation (steatohepatitis), fibrosis and cirrhosis. It is the most common chronic liver condition in the Western world, and affects 30 % of the adult population in the USA(Reference Adams and Lindor5, Reference Zivkovic, German and Sanyal6). IR and NAFLD can be found independently, but they often occur concurrently(Reference Vemuri, Kelley, Mackey, Rasooly and Bartolini7–Reference Angulo9). Frequently NAFLD and IR are associated with obesity, type 2 diabetes mellitus and dyslipidaemia(Reference Marchesini, Brizi, Morselli-Labate, Bianchi, Bugianesi, McCullough, Forlani and Melchionda2), but they are also associated with deficiency of depot fat(Reference Bloomgarden10).
Dietary factors that contribute to the development of IR and NAFLD include high-fat (saturated and trans-fatty acids) and high-sucrose diets(Reference Lee, Pinnamaneni, Eo, Cho, Pyo, Kim, Sinclair, Febbraio and Watt11–Reference Petersen and Shulman13). One of the trans-fatty acids in the diet is conjugated linoleic acid (CLA), which refers to linoleic acid isomers having conjugated double bonds. Although vaccenic acid (trans-11–18 : 1) is the most abundant natural trans-fatty acid found in ruminant meat and dairy products, and elaidic acid (trans-9–18 : 1) is the most abundant trans-fatty acid in industrially hydrogenated oils(Reference Kris-Etherton and Innis14), concentrations of CLA isomers from foods can vary greatly depending upon the method of hydrogenation. The average intake of CLA in the USA has been estimated to be less than 0·5 g/d(Reference Parodi, Sebedio, Christie and Adlof15); however, actual intakes by individuals consuming processed oils may be several folds greater than this, because the concentration of all CLA isomers in partially hydrogenated oil was 9·8 % of the total fatty acids and that of trans-10, cis-12-CLA was 2·6 %(Reference Jung and Ha16). Thus, the amounts of trans-10, cis-12-CLA consumed alone or along with other trans-fatty acids from partially hydrogenated or processed foods can reach levels beyond what is needed to induce IR and NAFLD. Supplementing animal diets with CLA has been reported to decrease body fat, while ectopically depositing fat in liver, muscle and other tissues(Reference Vemuri, Kelley, Mackey, Rasooly and Bartolini7, Reference Kelley and Erickson17); however, results from human studies regarding health benefits of CLA are quite inconsistent(Reference Tricon, Burdge, Williams, Calder and Yaqoob18, Reference Wahle, Heys and Rotondo19). Supplementing animal and human diets with CLA altered tissue lipids and their fatty acid composition, increased lipid peroxidation, inflammation, NAFLD, incidence of IR and diabetes(Reference Clement, Poirier, Niot, Bocher, Guerre-Millo, Krief, Staels and Besnard20–Reference Purushotham, Wendel, Liu and Belury29).
Trans-10, cis-12-CLA, and not cis-9, trans-11-CLA, is the isomer that reduces depot fats while causing fatty liver and IR, and these two isomers have contrasting effects on fatty acid and lipid metabolism(Reference Kelley and Erickson17–Reference Wahle, Heys and Rotondo19). Trans-10, cis-12-CLA increased the liver concentrations of oleic acid (18 : 1n-9) and decreased those of n-6 and n-3 PUFA(Reference Kelley, Bartolini, Warren, Simon, Mackey and Erickson26, Reference Kelley, Bartolini, Newman, Vemuri and Mackey30). NAFLD is associated with an increase in the hepatic n-6:n-3 ratio(Reference El-Badry, Graf and Clavien31). Animal diets usually have adequate amounts of n-6 PUFA, but many may be inadequate in n-3 PUFA. We therefore propose that the aggravation of n-3 PUFA inadequacy (increased ratio between n-6 and n-3 PUFA) by CLA exacerbates the development of fatty liver and IR; these CLA-induced pathologies could be prevented by the concomitant increase in the intake of n-3 PUFA. Fish oils that contain a mixture of EPA (20 : 5n-3) and DHA (22 : 6n-3) and purified DHA have been shown to prevent the development of IR and NAFLD induced by high-fat(Reference Delarue, LeFoll, Corporeau and Lucas32, Reference Flachs, Mohamed-Ali, Horakova, Rossmeisl, Hosseinzadeh-Attar, Hensler, Ruzickova and Kopecky33) or high-sucrose(Reference Rossi, Lombardo, Lacorte, Chicco, Rouault, Slama and Rizkalla34) diets and by diets containing a mixture of CLA isomers or purified trans-10, cis-12-CLA(Reference Vemuri, Kelley, Mackey, Rasooly and Bartolini7, Reference Ide35, Reference Winzell, Pacini and Ahren36). Supplementing diets of obese rats and mice with α-linolenic acid (ALA; 18 : 3n-3) prevented the development of NAFLD and IR(Reference Murase, Aoki and Tokimitsu37, Reference Mustad, Demichele, Huang, Mika, Lubbers, Berthiaume, Polakowski and Zinker38). It also prevented the sucrose-induced IR in non-obese rats(Reference Ghafoorunissa, Ibrahim, Rajkumar and Acharya39). To the best of our knowledge, none of the published reports have examined if concomitant supplementation with ALA will prevent the CLA-induced fatty liver and IR, which may involve different mechanisms from the obesity-related NAFLD and IR. The objective of the present study was to determine if ALA will prevent the development of fatty liver and IR induced by CLA. To address this question, we determined organ weights, liver and plasma lipids, and plasma concentrations of glucose and insulin in three groups of mice that were fed three different diets (control; control+CLA; control+CLA+flaxseed oil (FSO)). We also examined plasma concentrations of leptin, adiponectin, IL-6 and TNFα because of their claimed roles in IR and glucose homeostasis. In addition, we determined the fatty acid composition of the liver to support our hypothesis.
Research design and methods
Animals and diets
The animal protocol was approved by the Animal Use Committee at the University of California, Davis. Pathogen-free C57BL/6N female mice, aged 8 weeks, were purchased from Charles River (Raleigh, NC, USA). Species, sex, and strain of the animals were based on the use of this model by us and other investigators in a large number of published studies with CLA(Reference Kelley and Erickson17). The animals were maintained at the animal facility in the department of Nutrition, University of California, Davis. The temperature in the room was maintained at 25°C with dark and light cycles of 12 h each. Animals were fed the laboratory chow diet for week 1 and experimental diets for the subsequent 8 weeks. They were randomly divided into three groups (control; CLA; CLA+FSO) after feeding the laboratory chow diet for 1 week (ten animals per group).
An AIN 93G modified (soyabean oil replaced with maize oil) mouse diet was used as the basal diet as previously reported(Reference Kelley, Bartolini, Warren, Simon, Mackey and Erickson26). The fatty acid composition of the experimental diets is shown in Table 1. Highly enriched trans-10, cis-12-CLA (97·6 % purity) in the form of NEFA (Larodan Fine Chemicals, Malmö, Sweden) was used to provide the desired CLA concentration (0·5 g/100 g diet); it was incorporated into the diet by replacing an equivalent amount of maize oil. In the CLA+FSO group, FSO replaced an equivalent amount of maize oil to provide FSO at 0·5 g/100 g diet (ALA 0·3 g/100 g diet or 6·2 g/100 g dietary fatty acids). The n-6:n-3 ratios for the control, CLA and CLA+FSO diets were 53·0 (sem 1·27), 60·8 (sem 0·39) and 6·2 (sem 0·25), respectively. The amount of CLA supplemented was comparable with the amounts used in previous studies with rodent models, which ranged from 0·1 to 1·5 g/100 g diet of a mixture of CLA isomers(Reference Kelley and Erickson17–Reference Wahle, Heys and Rotondo19). The concentration of ALA was selected to mimic the linoleic acid (18 : 2n-6) and ALA ratios (7:1) found in the AIN-93 diet(Reference Reeves, Nielsen and Fahey40). Diets were constantly flushed with N2 gas to prevent oxidation of fatty acids while being gently mixed in a blender and packaged in 25 g samples. Packaged diets were stored at − 20°C until use. Fresh dietary packages were served each day and the food left in the cups from the previous day was weighed and recorded. Body weights were recorded every 7 d. After feeding the experimental diets for 8 weeks, animals were killed between 08.00 and 11.00 hours after withholding food for 10–12 h. They were anaesthetised with an intraperitoneal injection of ketamine HCl (100 mg/kg body weight) and xylazine (8 mg/kg body weight). Blood was collected by heart puncture into syringes containing EDTA. Livers were then removed, blotted dry on tissue paper, weighed, frozen in liquid N2, and stored frozen at − 80°C until processing. Since we did not perfuse the livers, the results reported for liver lipids may reflect concentrations both in the liver and blood. Further details regarding animal handling and diets can be found in our earlier reports(Reference Kelley, Bartolini, Warren, Simon, Mackey and Erickson26, Reference Kelley, Warren, Simon, Bartolini, Mackey and Erickson41).
Table 1 Fatty acid composition of the experimental diets
(Mean values (n 4) with their standard errors for each fatty acid measured)

CLA, trans-10, cis-12-conjugated linoleic acid; FSO, flaxseed oil.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05; ANOVA; a < b < c).
* Fatty acids with concentrations less than 0·5 g/100 g fatty acids are grouped as ‘minor’, unless their concentrations were significantly different between the three groups.
Liver and plasma lipids, plasma glucose and hormones
Liver total lipids were extracted using previously published methods(Reference Kelley, Bartolini, Warren, Simon, Mackey and Erickson26). Briefly, a portion of the frozen liver was weighed, freeze-dried and transferred into a 7 ml glass homogeniser. The sample was homogenised with 5 ml chloroform–methanol (2:1, v/v) containing 0·005 % butylated hydroxytoluene, and 1 ml of 1 m-sodium chloride. The homogenate was transferred to a screw-cap tube, centrifuged, and the lipid phase was removed into a 7 ml culture tube. The residual tissue lipids were extracted with an additional 5 ml chloroform–methanol (2:1, v/v) containing 0·005 % butylated hydroxytoluene. The two lipid extracts were combined and dried under N2. The lipid residue was dissolved in 1 ml chloroform, transferred to a tarred 2 ml vial, freeze-dried, and weighed.
Plasma total cholesterol, LDL-cholesterol, HDL-cholesterol, TAG and NEFA were analysed by using standard automated enzymic methods. Plasma glucose was measured using a commercial glucose assay kit (Sigma Aldrich, St Louis, MO, USA). Concentrations of insulin, leptin and adiponectin were determined by using multiplex/uniplex assay kits (Millipore, Billerica, MA, USA). IR and β cell function were calculated using the original homeostasis model assessment (homeostatic model assessment 1; HOMA1) of IR(Reference Wallace, Levy and Matthews42). The equations used were:
where FPI is fasting plasma insulin concentration (mU/l) and FPG is fasting plasma glucose (mm).
Statistical analysis
The Box–Cox approach was used to estimate appropriate transformations(Reference Box, Hunger and Hunter43). In most cases for which a power transformation did not stabilise the variances, the data were transformed by ranks. The one-way model was fitted with SAS PROC GLM (SAS Institute, Inc., Cary, NC, USA), along with Levene's test for heterogeneity of variance and multiple comparisons among the treatment means using Tukey's adjustment for multiplicity(44). The data shown are mean values with their standard errors, with P ≤ 0·05 considered significantly different. Weekly body weights were analysed using the repeated-measures model with a first-order autoregressive covariance structure among the repeated measures. The diet means were compared using Tukey's adjustment for multiplicity(Reference Littell, Milliken, Stroup, Wolfinger and Schabenberger45).
Results
Food intake and body and organ weights
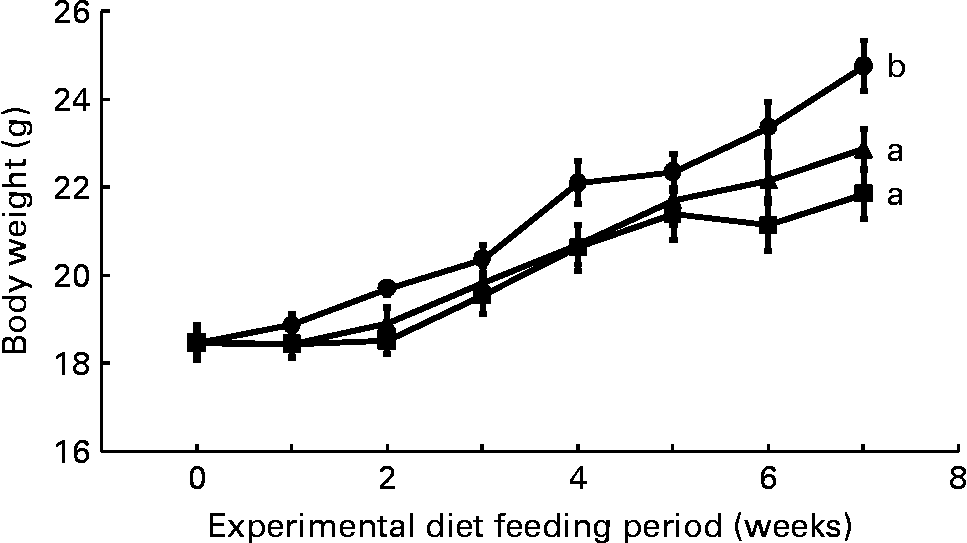
Mean daily food intake per mouse for the control, CLA and CLA+FSO groups was 4·53 (sem 0·10), 4·61 (sem 0·02) and 4·68 (sem 0·01) g, respectively; it did not differ significantly between the three dietary groups. Initial body weights did not differ between the three groups, but weight gained during the first 7 weeks of feeding the experimental diets was significantly lower in the CLA and CLA+FSO groups compared with the weight gained in the control group (P ≤ 0·05; Fig. 1). CLA also decreased the mean weights of perirenal and ovarian fat pads by 84 and 78 % and increased those of livers by 160 % compared with those in the control group (Table 2). Concomitant supplementation of FSO and CLA partially prevented the CLA-induced changes in body, liver and adipose tissue weights. Compared with the CLA group the decrease in liver (37 %) and increase in perirenal adipose tissue (60 %) weights were significant (P ≤ 0·05), while the body and ovarian adipose tissue weights did not differ significantly between the CLA and CLA+FSO groups. Weights of spleens, kidneys, hearts and brains did not differ between the three groups (Table 2).
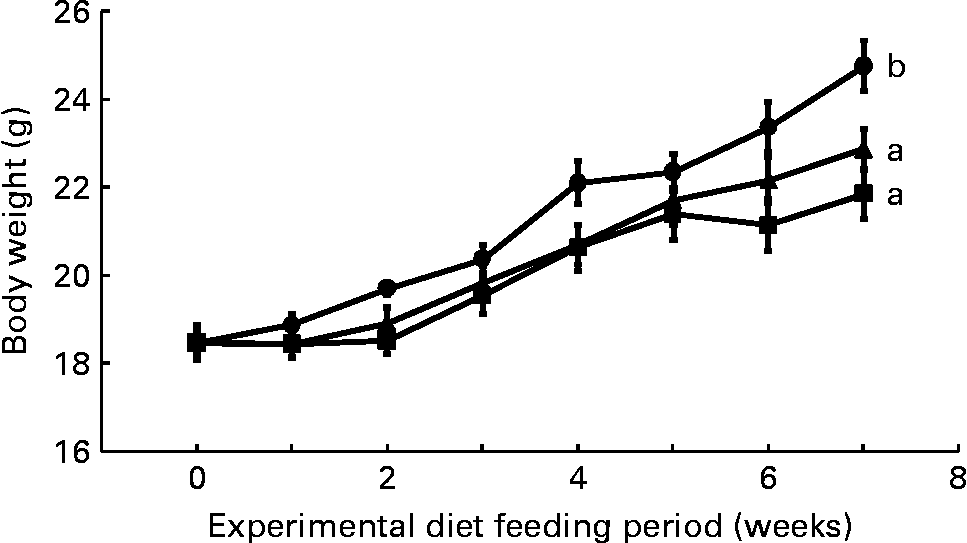
Fig. 1 Weekly body weights of mice fed control (●), trans-10, cis-12-conjugated linoleic acid (CLA; ■) or CLA+flaxseed oil (▲) diets. Data are means (n 10), with standard errors represented by vertical bars. Overall increases in body weights during the 7 weeks were compared and the lines with unlike letters are significantly different (P < 0·05), using a repeated-measures ANOVA and Tukey's test on the diet main-effect means (diet × time, P = 0·21).
Table 2 Effect of experimental diets on selected organ weights of mice
(Mean values (n 10) with their standard errors for each variable measured)

CLA, trans-10, cis-12-conjugated linoleic acid; FSO, flaxseed oil.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05; ANOVA; a < b < c).
Liver fatty acid composition
Wt% concentration of the liver lipid SFA did not differ between the mice fed the three different experimental diets, although there were minor and significant differences in the individual concentrations of 16 : 0 and 18 : 0 (Table 3). The total MUFA wt% concentration in liver lipids was significantly increased (35 %) by feeding the CLA diet when compared with the corresponding values in the control group. The CLA+FSO diet decreased the wt% of total MUFA by 9 % when compared with that in the CLA group (NS). The changes in the MUFA concentration were primarily due to changes in the concentration of 18 : 1n-9. CLA supplementation decreased the wt% concentration of n-6 PUFA by 57 % and that of n-3 PUFA by 73 % when compared with the corresponding values in the control group; it increased the n-6:n-3 ratio by 58 %. The decrease in n-6 PUFA resulted from significant reductions in the concentrations of 18 : 2, 18 : 3, 20 : 4 and 22 : 5, while that in n-3 PUFA resulted from significant reductions in 18 : 3, 20 : 5 and 22 : 6. Concomitant supplementation of FSO with CLA increased the wt% of n-6 PUFA by 33 % and that of n-3 PUFA by 342 % as compared with the corresponding values in the CLA group. The increase in n-6 PUFA resulted from a 26 % increase in the wt% concentration of 18 : 2n-6 (NS) and a 70 % increase in that of 20 : 4n-6; increases in n-3 PUFA resulted from 550, 300 and 323 % increases in the wt% concentrations of 18 : 3n-3, 20 : 5n-3 and 22 : 6n-3, respectively. FSO also decreased the n-6:n-3 ratio by 70 % when compared with that found in the CLA group. Thus, CLA supplementation increased the wt% concentration of total MUFA and n-6:n-3 ratio and decreased the concentrations of both n-6 and n-3 PUFA in liver lipids; FSO supplementation partially or completely reversed the changes in fatty acid composition induced by CLA.
Table 3 Fatty acid composition of livers of mice fed experimental diets
(Mean values (n 5) with their standard errors for each fatty acid measured)

CLA, trans-10, cis-12-conjugated linoleic acid; FSO, flaxseed oil.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05; ANOVA; a < b < c).
* Fatty acids with concentrations less than 0·5 g/100 g fatty acids are grouped as ‘minor’, unless their concentrations were significantly different between the three groups.
Effects of dietary fatty acids on plasma lipids, glucose, hormones and cytokines
CLA supplementation did not alter the plasma concentrations of total cholesterol, HDL-cholesterol, LDL-cholesterol and NEFA when compared with the corresponding concentrations in the control group; however, it reduced the plasma TAG concentrations by 38 % (Table 4). Concomitant supplementation of FSO and CLA reduced total cholesterol, HDL-cholesterol and LDL-cholesterol by 30–38 % and TAG by 57 % when compared with those found in the CLA group.
Table 4 Effect of experimental diets on plasma lipids, glucose, hormones and cytokines in mice
(Mean values (n 10) with their standard errors for each variable measured)

CLA, trans-10, cis-12-conjugated linoleic acid; FSO, flaxseed oil; HOMA1-IR, homeostatic model assessment 1 for insulin resistance; HOMA1-% B, homeostatic model assessment 1 for β cell function.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P ≤ 0·05; ANOVA; a < b < c).
CLA supplementation did not alter the fasting plasma glucose, but increased the concentration of circulating insulin, HOMA1-IR and HOMA1 for β cell function by 636, 982 and 968 %, respectively as compared with the corresponding values in the control group (Table 4). It also lowered concentrations of circulating leptin and adiponectin by 87 and 85 % respectively. Concomitant supplementation of FSO and CLA decreased the concentration of fasting plasma glucose by 20 % (P ≤ 0·05) when compared with that found in the CLA group. FSO supplementation along with CLA decreased the elevated circulating insulin, HOMA1-IR and HOMA1 for β cell function to levels found in the control group (Table 4). However, it had no effect on the circulating concentrations of leptin and adiponectin. Neither of the fatty acid treatments altered the circulating concentrations of the inflammatory cytokines TNFα and IL-6.
Discussion
We tested the hypothesis of whether CLA-induced fatty liver and IR are aggravated by an inadequate intake of n-3 PUFA. The present results demonstrate that the small amount of ALA (0·3 g/100 g diet) from FSO completely prevented the CLA-induced IR and significantly decreased the fasting plasma glucose concentration (Table 4). It also significantly reduced the development of fatty liver; however, it was not a complete prevention. The disparity of the effects of ALA on fatty liver and IR may suggest the involvement of different mechanism(s) or that a higher concentration of ALA may be needed to completely prevent the CLA-induced fatty liver than that required to prevent IR. Prevention of CLA-induced IR by ALA was associated with reversal of the changes in liver fatty acid composition induced by CLA. Dietary FSO increased the hepatic concentration of n-6 PUFA (linoleic acid, arachidonic acid and docosapentaenoic acid) and n-3 PUFA (ALA, EPA and DHA) and it decreased the n-6:n-3 PUFA ratio by 71 %. The most dramatic increases were in the concentrations of ALA and DHA, which most probably was the basis for improved insulin sensitivity. Therefore, we believe that the prevention of IR and NAFLD in the present study resulted from the increase in liver concentration of n-3 and not n-6 PUFA. Whether it was a direct effect of ALA or its elongation product (DHA) cannot be determined from the present results. Although addition of FSO to the diet created minor changes in other dietary fatty acids, the major change was an increase in the intake of ALA with a concomitant decrease in the n-6:n-3 ratio; hence we attribute the prevention of IR and fatty liver by FSO to the increase in the intake of ALA.
The present results showing the development of IR and fatty liver by CLA are consistent with those of previous animal studies(Reference Clement, Poirier, Niot, Bocher, Guerre-Millo, Krief, Staels and Besnard20, Reference Hargrave, Azain, Kachman and Miner21, Reference Poirier, Rouault, Clement, Niot, Monnot, Guerre-Millo and Besnard24, Reference Kelley, Bartolini, Warren, Simon, Mackey and Erickson26, Reference Ohashi, Matsushita, Kimura, Miyashita and Saito27, Reference Purushotham, Wendel, Liu and Belury29). Effects of ALA on the CLA-induced fatty liver and IR in the present study are consistent with results from studies with fish oils and purified DHA(Reference Vemuri, Kelley, Mackey, Rasooly and Bartolini7, Reference Ide35, Reference Winzell, Pacini and Ahren36) and with those of ALA on the fatty liver induced by high-fat or high-sucrose diets(Reference Murase, Aoki and Tokimitsu37–Reference Ghafoorunissa, Ibrahim, Rajkumar and Acharya39).
A review of the published studies in which trans-10, cis-12-CLA was used to induce IR and NAFLD in normal mice shows that many of these diets contained high concentrations of linoleic acid and were inadequate in ALA. Fat sources used in these studies included maize, sunflower-seed, safflower-seed and palm oils, which have insignificant amounts of ALA(Reference Clement, Poirier, Niot, Bocher, Guerre-Millo, Krief, Staels and Besnard20, Reference Kelley, Bartolini, Warren, Simon, Mackey and Erickson26, Reference Kelley, Bartolini, Newman, Vemuri and Mackey30, Reference Winzell, Pacini and Ahren36, Reference Javadi, Beynen, Hovenier, Lankhorst, Lemmens, Terpstra and Geelen46–Reference Yanagita, Wang, Nagao, Ujino and Inoue50). Other studies supplemented sunflower-seed oil with 0·1 % linseed oil(Reference Degrace, Demizieux, Gresti, Chardigny, Sebedio and Clouet51, Reference Degrace, Demizieux, Gresti, Chardigny, Sebedio and Clouet52), which failed to prevent the development of fatty liver. The concentration of linseed oil used in these studies may not be adequate, because it was only 20 % of that used in our present study. Other investigators used soyabean oil (6·5 %), which has a linoleic acid:ALA ratio of 7:1, and still observed fatty liver and IR(Reference Purushotham, Wendel, Liu and Belury29); the development of IR and fatty liver in this study was most probably due to a relatively high concentration of CLA (1·5 % as a mixture of isomers) compared with the low concentration of ALA. In general, the results of these studies support our hypothesis that fatty liver and IR induced by CLA may result from n-3 fatty acid deficiency.
The concentration of ALA (0·3 g/100 g diet) used in the present study was smaller than the concentrations of DHA (1·5 g/100 g diet) and fish oils (6 g/100 g diet) previously reported to prevent CLA-induced fatty liver and IR(Reference Vemuri, Kelley, Mackey, Rasooly and Bartolini7, Reference Ide35). A DHA concentration of 0·5 g/100 g diet only partially prevented the increase in liver weight, and it failed to prevent IR in an earlier study in mice where NAFLD and IR were induced by feeding a mixture of CLA isomers (2 g/100 g diet)(Reference Yanagita, Wang, Nagao, Ujino and Inoue50). The results of the present and those of previously published studies(Reference Vemuri, Kelley, Mackey, Rasooly and Bartolini7, Reference Ide35, Reference Yanagita, Wang, Nagao, Ujino and Inoue50) suggest that ALA may have a direct effect and may be more effective than DHA or fish oils in preventing IR. It is also possible that the prevention of IR and NAFLD in the present study resulted from an increase in liver DHA, because the liver DHA concentration in the FSO group was comparable with DHA concentration in the livers from the control group (Table 3). Further studies with increasing concentrations of DHA and ALA are needed to determine the minimum dose of the n-3 PUFA needed to prevent the NAFLD and IR induced by a known concentration of trans-10, cis-12-CLA. Body weights in both the CLA and CLA+FSO groups were significantly lower than that in the control group, and it did not differ between the CLA and CLA+FSO groups (Fig. 1). Further studies are also needed to determine the specific ratios between dietary CLA and ALA that may reduce body and adipose tissue weights without causing IR, NAFLD, or other adverse effects of CLA.
Studies in mice have reported that the hyperinsulinaemia seen after CLA supplementation was preceded by decreases in plasma leptin and adiponectin(Reference Vemuri, Kelley, Mackey, Rasooly and Bartolini7, Reference Poirier, Rouault, Clement, Niot, Monnot, Guerre-Millo and Besnard24, Reference Ohashi, Matsushita, Kimura, Miyashita and Saito27, Reference Ide35). It has also been reported that maintaining leptin or adiponectin in the CLA-fed mice attenuated CLA-induced IR(Reference Purushotham, Wendel, Liu and Belury29, Reference Toyoshima, Gavrilova, Yakar, Jou, Pack, Asghar, Wheeler and LeRoith53). In the present study, concentrations of both circulating leptin and adiponectin were reduced by 85 and 87 % by CLA supplementation (Table 4); concomitant supplementation of CLA and FSO failed to prevent the CLA-induced decline in circulating concentrations of both leptin and adiponectin, yet it maintained the circulating insulin concentrations to levels found in the control group. These findings suggest that the effects of ALA on the prevention of fatty liver and IR may be independent of both leptin and adiponectin. These effects of ALA on circulating concentrations of leptin and adiponectin differ from those of DHA which partially restored the concentrations of both leptin and adiponectin in mice fed CLA-containing diets(Reference Vemuri, Kelley, Mackey, Rasooly and Bartolini7). The present results that showed no change in the concentrations of circulating TNFα and IL-6 by the feeding of trans-10, cis-12-CLA are at variance with those of others who reported increased expression of these cytokines in liver and adipose tissue(Reference Poirier, Shapiro, Kim and Lazar54, Reference Cai, Yuan, Frantz, Melendez, Hansen, Lee and Shoelson55). This discrepancy may be due to site-specific effects of CLA.
We are not sure about the underlying mechanism(s) by which CLA increased and ALA reduced the plasma insulin concentrations. CLA feeding in mice has been reported to cause pancreatic β cell hyperplasia with a concomitant increase in insulin secretion in vivo (Reference Poirier, Niot, Clement, Guerre-Millo and Besnard28). β Cells isolated from mice fed CLA-containing diets and then cultured ex vivo demonstrated an increased secretion of insulin as compared with that observed in cells isolated from animals fed a diet without CLA(Reference Winzell, Pacini and Ahren36). Hence, changes in concentrations of circulating insulin caused by CLA and ALA in the present study may reflect the changes in insulin secretion. The reduction in fasting glucose caused by ALA may be due to increased insulin sensitivity and/or decreased glucose output by the liver. In rats fed fish oil-containing diets, DHA was the major n-3 PUFA that accumulated in liver and it suppressed hepatocyte levels of sterol regulatory element binding protein-1 (SREBP-1) nuclear abundance, SREBP-1c mRNA and Insig-2 mRNA(Reference Botolin, Wang, Christian and Jump56). Thus, the reduction in liver fat caused by DHA formed from ALA in the present study may be due to a decreased expression of SREBP-1, which controls hepatic lipogenesis.
In summary, the results of the present study show that a relatively small amount of ALA (0·3 g/100 g diet v. CLA at 0·5 g/100 g diet) completely prevented the CLA-induced IR and partially prevented the CLA-induced fatty liver. ALA increased the n-3 and n-6 PUFA and markedly reduced the n-6:n-3 PUFA ratio in liver lipids. These effects of ALA were independent of the changes in the concentrations of circulating leptin and adiponectin. Future studies are needed to determine the response of fatty liver and IR to increasing concentrations of ALA and DHA to distinguish between the direct effects of ALA and its elongation products (EPA and DHA), to determine the clinical relevance of ALA in the management of IR and fatty liver and to understand the mechanisms involved.
Acknowledgements
The funds for the present study were provided by the US Department of Agriculture. The authors have no conflicts of interest to disclose. D. S. K., M. V. and B. E. M. designed and planned the study; M. V., Y. A., S. S. G. and D. F. conducted the study and performed the sample analysis; D. S. K., M. V., Y. A., B. E. M. and D. F. analysed the data and prepared the manuscript.
References to a company or product name do not imply approval or recommendation of the product by the US Department of Agriculture to the exclusion of others that may be suitable.