Due to the high prevalence of obesity and the increased risk of morbidity and mortality associated with it, early-life prevention of childhood obesity has become a public health priority. The preconception and prenatal periods are critical developmental stages for later obesity and metabolic risk. In this context, many factors have been investigated, including maternal pre-existing medical conditions, lifestyles, possible gene–environment interactions and epigenetic mechanisms as underlying early life origins of metabolic risk(Reference Wang, Bartell and Wang1).
A growing body of evidence suggests strong links between maternal lifestyles before pregnancy and adverse effects on both pregnancy and child health outcomes(Reference Vézina-Im, Nicklas and Baranowski2–Reference Oostingh, Hall and Koster5). Among modifiable lifestyle factors, maternal pregestational BMI is a well-known risk factor for future obesity in the offspring from birth to adulthood. Maternal obesity is associated with an increased risk of gestational diabetes and gestational hypertensive disorders, and it is also a major determinant of offspring’s health during childhood and later adult life(Reference Godfrey, Reynolds and Prescott6,Reference Patro Golab, Santos and Voerman7) . A systematic review and meta-analysis conducted by Heslehurst et al. found a 264 % increase in the odds of childhood obesity for those children whose mothers had obesity before conception(Reference Heslehurst, Vieira and Akhter8).
On the other hand, cigarette smoke contains thousands of known toxic components and although pathways linking prenatal exposure to cigarette smoking and obesity are not well understood(Reference Rogers9,Reference McLean, Jun and Kozyrskyj10) , even very low doses of cigarette smoke exposure during pregnancy may increase the risk overweight and obesity(Reference Albers, Sobotzki and Kuß11,Reference Philips, Santos and Trasande12) in the offspring.
The present study aimed to assess the association between maternal pregestational BMI and offspring’s risk of overweight or obesity after accounting for the most important confounders, especially maternal smoking habit, among children of women participating in the Seguimiento Universidad de Navarra (SUN) cohort.
Materials and methods
Selection of the participants
The objectives and methods of the SUN prospective cohort have been detailed elsewhere(Reference Carlos, De and Fuente-Arrillaga13). Recruitment started in 1999, and it is permanently open. Currently, the SUN cohort includes more than 22 000 participants. Data on diet, lifestyle and clinical diagnoses are collected at baseline and every 2 years. The SUN project was conducted according to the guidelines laid down in the Declaration of Helsinki; its protocol was approved by the Institutional Review Board of the University of Navarra, and this cohort is registered at clinicaltrials.gov as NCT02669602. When obtaining informed consent, potential participants were of their right to refuse to participate in the SUN study or to withdraw their consent to participate at any time without reprisal.
In March 2018, we invited 3496 women who reported one or more pregnancies during their follow-up in the SUN cohort to complete a more detailed online supplemental questionnaire focused on their pregnancy history and the health of their offspring. Among them, 1527 (43·6 %) agreed to participate and reported information on 3013 children. We excluded children with BMI z-scores out of the predefined limits (percentile 1 and percentile 99, n 57) and those born after 2016 (n 165) due to the lack of International Obesity Task Force standards of reference under the age of 2 years. The final sample consisted of 2791 children from 2731 pregnancies in 1485 women (see online supplementary material, Supplemental Figure 1).
Data collection
Between March and June 2018, participants completed two brief questionnaires sent by email. The first questionnaire (Q1) consisted of seven items on pregnancy and delivery including questions about gestation, such as complications during pregnancy (gestational diabetes, pregnancy-induced hypertension and preeclampsia) and type of delivery (caesarean or vaginal). The second questionnaire (Q2) consisted of eight items on the health and conditions of the offspring, including retrospective questions about offspring’s date of birth, sex (male or female), birth weight (continuous), as well as questions about current weight (continuous) and height (continuous). Women completed one Q1 for each pregnancy during the follow-up in SUN and one Q2 for each child born after that pregnancy.
Exposure
Pre-pregnancy weight (continuous) and smoking habit (yes or no) were updated using information reported in the questionnaire immediately preceding the date of the delivery.
Outcome
Information about offspring’s current age, weight and height was obtained from the questionnaires that participants completed between March and June 2018. Children’s current age was calculated as the difference between the day in which the questionnaire was collected and their birth date. To analyse the BMI as a continuous variable, we calculated offspring’s sex- and age-specific BMI z-scores using the LMS parameters, which describe the skew (L for lambda), the median (M for mu) and the coefficient of variation (S for sigma) for a measurement(Reference Cole and Lostein14). To calculate the risk of overweight or obesity in the offspring, we first calculated offspring’s BMI as weight in kg divided by height in metres squared and then defined nutritional status using sex- and age-specific cut-off points based on the International Obesity Task Force standards of reference(Reference Cole and Lostein14).
Covariates
Information about maternal covariates was obtained from the baseline and follow-up questionnaires in the SUN cohort. Maternal age at delivery (continuous) was calculated as the difference between mothers’ birth date and the date of the delivery and categorised for the analyses. Information about covariates related to the pregnancy (or pregnancies) was obtained from the questionnaires that participants completed between March and June 2018.
Dietary information was obtained from a validated 136-item semi-quantitative FFQ collected when participants entered in the SUN cohort(Reference Martin-Moreno, Boyle and Gorgojo15). The FFQ had nine categories for intake frequency, from never to two or more servings/d. The nutritional content of each food was obtained from Spanish food composition guides(Reference Mataix Verdu16,Reference Moreiras, Carbajal and Cabrera17) and supplemented with information from food and supplement manufacturers when needed. The nutrient contribution of each food item was calculated by multiplying the frequency of food consumption by the nutrient composition of the specified portion size. Energy intake (kcal/d) for each food item was calculated by multiplying the frequency of each food item consumed by the energy content of its specified portion size. Total energy intake was calculated as the sum of energy provided by each food item. We also calculated the adherence to the Mediterranean dietary pattern based on the information from the FFQ using the classical Mediterranean Diet Score(Reference Trichopoulou, Costacou and Bamia18). We defined three categories of adherence to the Mediterranean Diet Score: low (from 0 to 2 points), medium (from 3 to 5 points) and high (from 6 to 9 points).
Physical activity was collected when participants entered in SUN cohort with a validated questionnaire that included seventeen activities and ten categories of response, from never to 11 or more h/week. We multiplied the metabolic equivalents of each activity by the weekly participation in that activity, weighted according to the number of months dedicated to each activity(Reference Ainsworth, Haskell and Whitt19) to obtain the metabolic equivalent-h/week for each activity. Total physical activity was quantified by summing the metabolic equivalent-h/week dedicated to all activities performed during leisure time.
Statistical analysis
We calculated the required sample size to reach a statistical power of 90 % using available data of estimates(Reference Heslehurst, Vieira and Akhter8) and considering the prevalence of offspring’s overweight in our sample (14·0 %). Therefore, assuming a 32·0 % prevalence for pregestational overweight, a risk ratio for offspring’s overweight associated with maternal pregestational overweight of 1·55 and a two-side alpha risk of 0·05, the minimum sample size resulted to be 1641 children, including 398 whose mother had overweight or obesity.
Main characteristics of mothers and their offspring were calculated by categories of maternal pregestational weight status. We used mean (sd) to describe quantitative variables and frequencies (%) for qualitative variables.
To analyse the association between maternal pregestational BMI and offspring’s z-score of the BMI, we used generalised mixed models with three hierarchical levels to account for the intra-cluster correlations between siblings from the same and different pregnancies. We used random intercept and the same single variance for all the random effects. To assess offspring’s risk of overweight or obesity, we used Poisson regression with mother-clustered variance–covariance matrix. For all analyses, we obtained crude- and multivariable-adjusted estimates. Non-linearity was assessed introducing a quadratic term for maternal BMI into the crude model. Confounding was evaluated in three progressively adjusted models. The first set of models was adjusted for offspring’s sex and current age (continuous). The second set of models was additionally adjusted for mother’s age (20–30, 30–35, ≥35 years old), maternal education (years of university), Mediterranean Diet Score (low, medium or high), total energy intake (quintiles of kcal/d) and physical activity (quintiles of metabolic equivalent-h/week). The third set of models was additionally adjusted for maternal smoking habit (yes or no).
To evaluate whether smoking habit modified the association between maternal pregestational BMI and offspring’s risk of overweight, we compared model 2 with and without the interaction term, using the likelihood ratio test (1 df). Subgroup analyses in smoker and non-smoker mothers were also performed.
In sensitivity analyses, we ran the same models using the WHO criteria to define overweight/obesity in children and adolescents and excluding participants with z-score of the BMI below –4 or above +4. Additionally, we assessed the association between maternal BMI and offspring’s z-score of the height.
Results
Our sample included 2791children born to 1485 mothers (528 singletons, 119 siblings from the same pregnancy – 116 twins and 3 triplets – and 2144 siblings from different pregnancies). Among women who reported information of more than one child, the mean number of children was 1·8 (range: 2–8 children). No major differences in baseline characteristics existed between women who did and women who did not agree to participate (see online supplementary material, Supplemental Table 1). Main characteristics of participants and their children are presented in Table 1. Four hundred fifty-nine women reported pregestational BMI equal to or above 25 kg/m2 (19·6 % of the pregnancies). Those women were slightly older at delivery and more likely to report complications during pregnancy, more specifically, hypertension and gestational diabetes. Pregestational overweight or obese women reported less years of university education, and lower both energy intake and physical activity. Children whose mother reported pregestational overweight or obesity were more likely born by caesarean delivery and had slightly higher birth weight. Overall, 392 out of the 2791 children were overweight or obese (14·0 %) when this study was set.
Table 1 Main characteristics of mothers and children by maternal pregestational weight status*

MET, metabolic equivalent.
* Adjusted for children factors: sex and current age; and maternal factors: age (20–30, 30–35 and ≥35 years), maternal education (years of university), adherence to Mediterranean diet (low/medium/high), total energy intake (quintiles), physical activity (quintiles) and smoking habit (yes/no).
† Normal weight and overweight/obesity were defined as BMI below and equal or over 25 kg/m2, respectively.
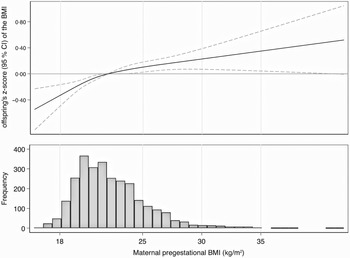
We found a linear association between maternal pregestational BMI and offspring’s z-score of the BMI (Fig. 1). Children whose mother reported pregestational BMI equal or above 25 kg/m2 showed significantly higher z-score of the BMI than those children whose mother reported pregestational BMI of 22 kg/m2 (reference category). Moreover, each 5 kg/m2 increase in maternal pregestational BMI was associated with a 0·22 (95 % CI 0·15, 0·29) higher offspring’s z-score of the BMI after adjusting for potential confounders (Table 2).

Fig. 1 Difference and 95 % CI in offspring’s z-score of the BMI associated with maternal pregestational BMI (kg/m2). Pregestational BMI of 22 kg/m2 was used as reference. The histogram represents the frequency distribution of maternal pregestational BMI (kg/m2)
Table 2 Change and 95 % CI in offspring’s z-score of the BMI associated with each additional 5 kg/m2 increase in maternal pregestational BMI

* Adjusted for children factor: sex and current age.
† Additionally adjusted for maternal factors: age (20–30, 30–35 and ≥35 years), maternal education (years of university), adherence to Mediterranean diet (low/medium/high), total energy intake (quintiles) and physical activity (quintiles).
‡ Additionally adjusted for maternal smoking habit (yes/no).
In further analyses, we observed that each 5 kg/m2 increase in maternal pregestational BMI was associated with a 1·61-fold (95 % CI 1·43, 1·82) higher risk of overweight or obesity in the offspring in the crude model (Table 3). The association was slightly attenuated but remained significant in the fully adjusted model (relative risk 1·57; 95 % CI 1·39, 1·77). Additional adjustment for breast-feeding (yes/no) did not change the estimates (data not shown).
Table 3 Risk ratio (RR) and 95 % CI for offspring’s overweight or obesity associated with each additional 5 kg/m2 increase in maternal pregestational BMI*,†

* Data: 334 overweight or obese children born to 2477 non-smoker mothers and 58 overweight or obese children born to 314 smoker mothers.
† Adjusted for children factor: sex and current age; and maternal factors: age (20–30, 30–35 and ≥35 years), maternal education (years of university), adherence to Mediterranean diet (low/medium/high), total energy intake (quintiles) and physical activity (quintiles).
‡ Adjusted for children factor: sex and actual age.
§ Additionally adjusted for maternal factors: age (20–30, 30–35 and ≥35 years), years of university, adherence to Mediterranean diet (low/medium/high), total energy intake (quintiles) and physical activity (quintiles).
|| Additionally adjusted for maternal smoking habit (yes/no).
A significant interaction was found between maternal smoking habit and pregestational BMI (P for interaction = 0·02; 1 df). Compared with the category of reference (z-score of children whose mother reported pregestational BMI of 22 kg/m2), the change in offspring’s z-score of the BMI associated with each 5 kg/m2 increase in maternal pregestational BMI was higher in those children whose mother reported smoking habit (difference 0·39; 95 % CI 0·21, 0·53) than in those who did not (difference 0·20; 95 % CI 0·13, 0·28) (see online supplementary material, Supplementary Figure 2). Similar results were found regarding offspring’s risk of overweight or obesity by maternal smoking habit; in non-smoker mothers, each 5 kg/m2 increase in pregestational BMI was associated with a 51 % (95 % CI 31 %, 73 %) relative increase in offspring’s risk of overweight or obesity, but that relative increase raised up to 91 % (95 % CI 48 %, 146 %) in smoker mothers (Fig. 2). Regarding the association between each additional 5 BMI units and risk of offspring overweight/obesity, the ratio of the relative risks in smoker and non-smoker mothers was 1·22 (95 % CI 1·00, 1·58).

Fig. 2 Risk ratio (RR) and 95 % CI for offspring’s overweight or obesity associated with each additional 5 kg/m2 increase in maternal pregestational BMI by maternal smoking habit (P for interaction = 0·02)
Similar results were found in sensitivity analyses using the WHO criteria to define overweight/obesity in children and adolescents and excluding participants with z-score of BMI below –4 or above +4 (see online supplementary material, Supplemental Table 2 and 3). In additional analyses, we found that maternal BMI was also directly associated with child’s z-score of height after adjusting for potential confounders (multivariable-adjusted difference = 0·20; 95 % CI 0·12, 0·28) (see online supplementary material, Supplemental Table 4).
Discussion
After assessing 2791 children born to 1485 mothers in the SUN study, we found that pregestational BMI was associated with higher offspring’s z-score of BMI and increased risk of overweight or obesity. More specifically, each 5 kg/m2 increase in pregestational BMI was associated with an increase of 0·22 (95 % CI 0·15, 0·29) in children’s z-score of BMI and a 57 % (95 % CI 39 %, 77 %) relative increase in offspring’s risk of overweight/obesity. Stronger estimates were observed for children born to smoker (relative risk 1·91; 95 % CI 1·48, 2·46) than to non-smoker mothers (relative risk 1·51 95 % CI 1·31, 1·73). Our results were robust in sensitivity analyses using the WHO criteria to define childhood overweight/obesity and excluding participants with z-score of BMI below −4 or above +4. The association that we found between maternal pregestational BMI and offspring’s z-score of height supports the hypothesis that maternal BMI is associated with child’s weight and that the strength of this association may be greater than the one observed for the BMI.
Taking into account that obesity is the most common problem in childbearing age women and according to the Developmental Origins of Health and Disease hypothesis, the periconceptional period has emerged as a critical stage that could lead offspring to have metabolic disruption and thus perpetuate the obesity epidemic across generations(Reference Santos Ferreira, Williams and Kangas20,Reference Mendez, Torrent and Ferrer21) .
It is well known that pregestational BMI is associated with offspring birth weight and future BMI(Reference Yu, Han and Zhu22), but it is still unclear whether that association is causally related to intrauterine factors, or if, on the contrary, it is explained by residual confounding (by genetic factors, environmental factors or both)(Reference Bond, Karhunen and Wielscher23). Although causality remains unclear, observational studies have reported epigenetic modifications in offspring of women with obesity(Reference Moholdt and Hawley24). Since obese women are more likely to have excessive gestational weight gain, they have an increased risk of preeclampsia and gestational diabetes, which leads to an increase in the insulin response, affecting early placental growth and gene expression(Reference Catalano and Shankar25).
Since parental modelling is associated with children’s energy balance-related behaviours(Reference Te Velde, ChinAPaw and De Bourdeaudhuij26), maternal overweight and smoking habit may increase offspring’s risk of overweight and obesity beyond the periconceptional period by facilitating children’s adherence to less healthy lifestyles. In line with our results, a recent meta-analysis of thirty-seven birth cohort studies concluded that higher maternal pre-pregnancy BMI was associated with an increased risk of childhood overweight/obesity, with the strongest estimates at later ages. Beyond the potential confounding by children’s lifestyle characteristics, the authors estimated that about 10·0–20·0 % of the cases of childhood overweight/obesity were attributable to maternal pre-pregnancy overweight or obesity(Reference Voerman, Santos and Golab27).
Interestingly, we found a positive interaction between maternal smoking habit and maternal pregestational BMI, meaning that offspring’s risk of overweight/obesity associated with maternal pregestational BMI was significantly higher in the subgroup of children whose mother was smoker (ratio of the relative risks 1·22; 95 % CI 1·00, 1·48). Evidence on joint effect in older children is scarce, and further research is need to fully elucidate the real magnitude of the association and the existence of a dose–response relationship(Reference Taylor, Wilding and Ziauddeen28).
Smoking before and during pregnancy is one of the most important modifiable risk factors for a wide range of adverse pregnancy outcomes(29,Reference Wang and Duan30) . Smoking in pregnancy remains an important public health issue, particularly in Europe and some regions of the USA that have the highest prevalence worldwide(Reference Campbell, Coleman-Haynes and Bowker31). In Spain, the INMA (INfancia y Medio Ambiente, Environment and Childhood) project, a multicentre prospective birth cohort study, assessed 2263 pregnant women and reported that 32·4 % were occasional or regular smokers when they became pregnant(Reference Aurrekoetxea, Murcia and Rebagliato32). The reported prevalence in INMA was similar to what we found in our sample.
A systematic review and meta-analysis concluded that, compared with children whose mother did not smoke during pregnancy, those born to mother who did had 37·0 and 55·0 % greater odds of overweight and obesity, respectively(Reference Rayfield and Plugge33). Furthermore, a recent systematic review concluded that maternal smoking inception and increasing the number of cigarettes were positively associated with adiposity in second- or higher-order children(Reference Taylor, Wilding and Ziauddeen28).
Plausible mechanisms for the association between maternal smoking and offspring’s overweight/obesity include hypoxia due to carbon-monoxide-nicotine-induced reductions in in utero placental blood flow and association with growth due to placental toxicity induced by chemicals in cigarette smoke(Reference Rogers9). More recently, tobacco exposure-induced changes in infant gut microbiota have also been hypothesised as a possible explanation for the association between maternal smoking and offspring’s overweight/obesity(Reference McLean, Jun and Kozyrskyj10).
Our study has several strengths including the large sample size and the use of updated information on pre-pregnancy weight and smoking habit of women for each pregnancy. Nevertheless, we acknowledge some limitations. First, we invited 3474 women, but only 44·0 % of women agreed to participate. However, no major differences in baseline characteristics were observed between responders and non-responder women. Second, our sample in this study was highly educated mothers, which could be not fully representative of general population. However, representativeness does not, in and of itself, deliver valid scientific inference and in many cases. Furthermore, since restricting the sample to highly educated participants raises the response rate and adds validity to their auto-referred data as well as reduce confounding by socio-economic covariates(Reference Rothman, Gallacher and Hatch34,Reference García Blanco, Ciriza Barea and Moreno-Galarraga35) , it may be considered a strength rather than a weakness of this study. Third, questionnaire on pregnancy history and offspring data was not specifically validated. However, previous validation studies on self-reported data in the SUN cohort including weight and BMI(Reference Bes-Rastrollo, Pérez Valdivieso and Sánchez-Villegas36) support the reliance of collected information as occurs in other cohorts based on highly educated participants(Reference Troy, Michels and Hunter37,Reference Moreno-Galarraga, Álvarez-Zallo and Oliver-Olid38) . Fourth, as we used mother-reported information, the possibility of potential measurement error must be acknowledged. Besides, the quality of the information regarding smoking habit is limited both by the dichotomisation of the responses, which prevents measuring a possible dose–response gradient, and by its retrospective nature, which does not completely remove a potential recall effect and consequent misclassification. However, the high motivation, commitment and educational level of participants in the SUN cohort may allay this possibility. Fifth, despite our significant findings, we must acknowledge that this study may be underpowered to detect some interactions. Finally, this investigation was mother-centred approach, since paternal factors and lifestyles were not available.
Conclusions
We found that each 5 kg/m2 increase in maternal pregestational BMI was associated with a 0·22 (95 % CI 0·15, 0·29) higher z-score of BMI and a 57 % relative increase in offspring’s risk of overweight or obesity. That risk was higher (relative risk 91 %; 95 % CI 48 %, 146 %) in children born to smoker mothers, suggesting that smoking might modify the association between maternal pregestational BMI and offspring’s nutritional status. Although more evidence is needed to clarify dose–response relationship and assess the association of other exposures, including paternal factors, these findings reinforce the importance of promoting healthy lifestyles in pregnant women in order to prevent childhood obesity.
Acknowledgements
Acknowledgements: We thank all the participants in the SUN study for their contribution to this work. Financial support: The SUN study is supported by the Spanish Government-Instituto de Salud Carlos III and the European Regional Development Fund (FEDER) with the grants RD06/0045, PI14/01764 and PI17/01795. Conflict of interest: The authors declare no conflicts of interest. Authorship: N.M.C. designed the data collection instruments, collected data and carried out the initial analyses. N.M.C. and S.S. drafted the initial manuscript. M.A.M., as the principal investigator of the SUN cohort, coordinated and supervised data collection. G.S. critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work. Ethics of human subject participation: The SUN project was conducted according to the guidelines laid down in the Declaration of Helsinki; its protocol was approved by the Institutional Review Board of the University of Navarra, and this cohort is registered at clinicaltrials.gov as NCT02669602. When obtaining informed consent, potential participants were of their right to refuse to participate in the SUN study or to withdraw their consent to participate at any time without reprisal.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980020005194