Introduction
For insects in temperate climates, thermoregulatory behaviours such as seeking suitable microhabitats for overwintering are critical to survival (May Reference May1979; Lombardero et al. Reference Lombardero, Ayres, Ayres and Reeve2000; Kausrud et al. Reference Kausrud, Økland, Skarpaas, Grégoire, Erbilgin and Stenseth2012; Woods et al. Reference Woods, Dillon and Pincebourde2015; Abram et al. Reference Abram, Boivin, Moiroux and Brodeur2017). Bark beetles (Coleoptera: Curculionidae: Scolytinae) are subcortical phloeomycetophagous insects with a range of developmental (Bentz et al. Reference Bentz, Régnière, Fettig, Hansen, Hayes and Hicke2010; Bentz and Jönsson Reference Bentz, Jönsson, Vega and Hofstetter2015) and behavioural adaptations for surviving winters (Lombardero et al. Reference Lombardero, Ayres, Ayres and Reeve2000; Sauvard Reference Sauvard2007; Kausrud et al. Reference Kausrud, Økland, Skarpaas, Grégoire, Erbilgin and Stenseth2012; Dworschak et al. Reference Dworschak, Meyer, Gruppe and Schopf2014). Overwintering microhabitats are characterised by temperature-buffering and insulating properties, such as those found beneath thick bark, litter, and snow. If overwintering microhabitats occur within vital tree tissues, occupation of these sites can negatively impact host tree fitness (Rexrode and Baumgras Reference Rexrode and Baumgras1984; Ye Reference Ye1991; Hanavan et al. Reference Hanavan, Adams and Allen2012; Kirkendall et al. Reference Kirkendall, Biedermann and Jordal2015).
The alder bark beetle, Alniphagus aspericollis (LeConte) (Coleoptera: Curculionidae: Scolytinae), infests the main stems and is associated with mortality of mature red alder trees, Alnus rubra (Bongard) (Betulaceae), throughout the range of its host (Chamberlin Reference Chamberlin1958; Borden Reference Borden1969). Red alder is the most prevalent hardwood tree in the Pacific Northwest of North America (Harrington et al. Reference Harrington, Zasada and Allen1994) and, as a nitrogen fixer (Torrey Reference Torrey1978), is an essential early successional species (Hu et al. Reference Hu, Finney and Brubaker2001; Hanley et al. Reference Hanley, Deal and Orlikowska2006; Deal et al. Reference Deal, Orlikowska, D’Amore and Hennon2017; Perakis and Pett-Ridge Reference Perakis and Pett-Ridge2019). Knowledge of the biotic agents that injure and kill red alder is thus critical for sustainable forest management.
Alder bark beetles have been documented overwintering as late-instar larvae, pupae, and callow adults within brood galleries in the main stems of their host trees and as emergent adults that construct shallow galleries in the bark of tree boles (Chamberlin Reference Chamberlin1958; Borden Reference Borden1969). More recently, adults have been observed overwintering in shallow galleries excavated in branch and bud nodes of 1- to 3-m-high saplings grown in plantations (J.H.B., unpublished data). We hypothesised that in the absence of saplings, adult alder bark beetles might overwinter in the branch and bud nodes of mature red alder crowns.
Methods
The crowns of mature red alders were surveyed for overwintering alder bark beetles; three windthrown trees (T1–T3, where T = tree) were assessed at two coastal British Columbia locations in the winters of 2003 and 2004, and three felled trees (T4–T6) at an additional site, also in coastal British Columbia, were evaluated in winter 2018–2019 (Table 1). The sampled trees were growing along access roads leading to sites where mature red alder had been harvested and replaced by plantations of red alder saplings (T1–T3) or along the margins of recently cleared land slated for development (T4–T6) and were either dominant or codominant in position while standing. Branches from each tree were examined for overwintering sites, which were dissected and classified as old or new (current year) based upon the presence or absence of callous tissue and, after further dissection, classed as occupied by live beetles or unoccupied (Fig. 1; Table 1). The numbers of live beetles in each occupied site were recorded. Windthrow of T1–T3 occurred after autumn leaf fall, and we concluded that occupied overwintering sites were entered or constructed while the trees were still standing.
Table 1. Summary of samples collected to assess alder bark beetle branch-overwintering sites within the crowns of six mature red alder trees: one windthrown tree (T1) on 21 January 2003 from Annis Bay, Nelson Island, British Columbia (N 49° 46′ 03.31″, W 124° 00′ 52.48″), two windthrown trees (T2, T3) on 20 January 2004 from Theodosia Arm, north of Powell River, British Columbia (N 50° 03′ 35.65″, W 124° 41′ 33.92″), and three trees (T4, T5, T6) felled in September 2018 and sampled from December 2018 to February 2019 on the University of British Columbia Endowment Lands, Vancouver, British Columbia (N 49° 15′ 47.00″, W 123° 14′ 01.41″). Branch groups (T4–T6) comprise all of the branch segments of each branch type (primary (oldest), secondary, or tertiary (youngest) growth) per branch. The numbers of occupied and unoccupied overwintering sites indicated per tree (T1–T3) or crown position (T4–T6) includes both old and new (current year) overwintering sites.
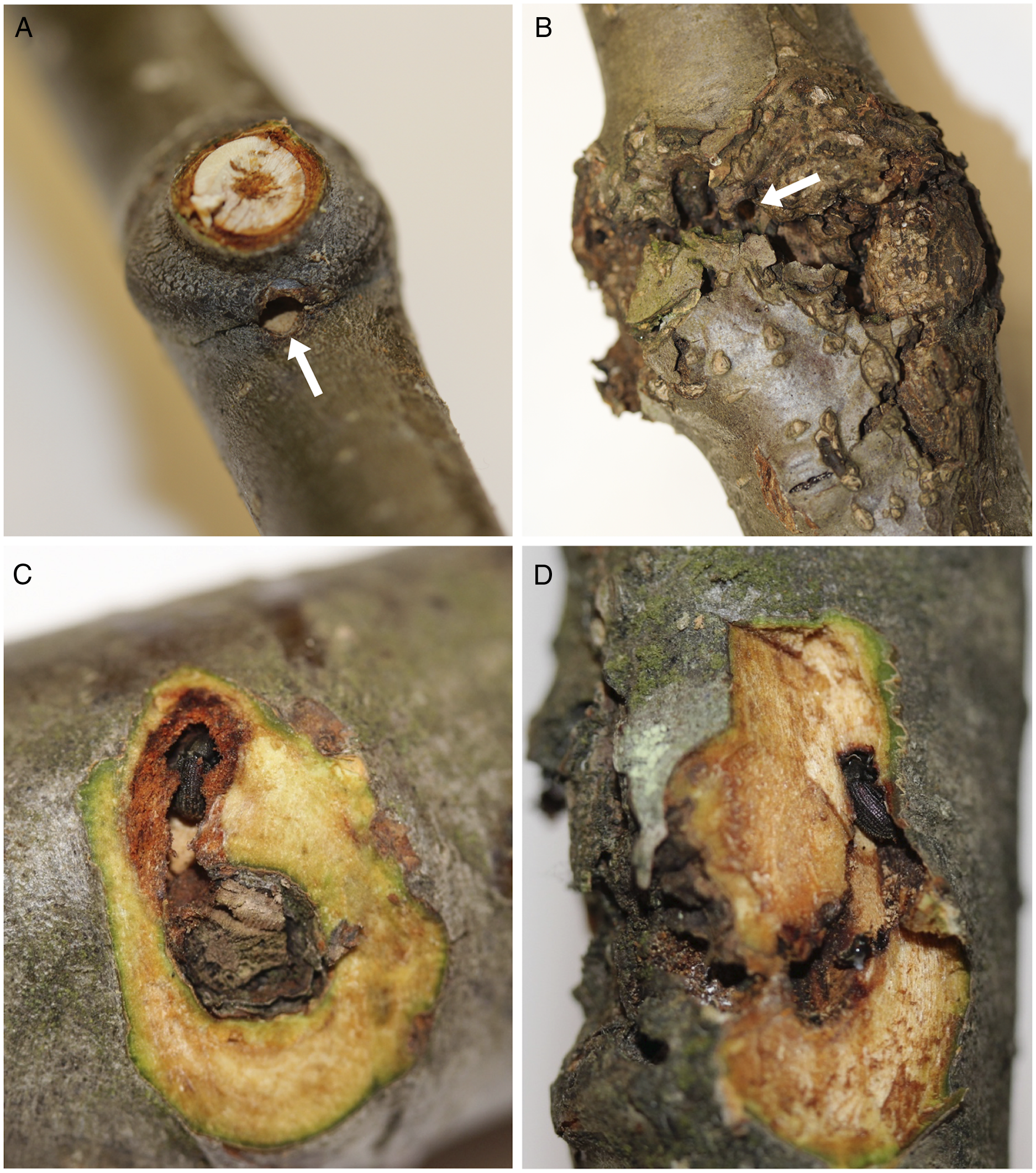
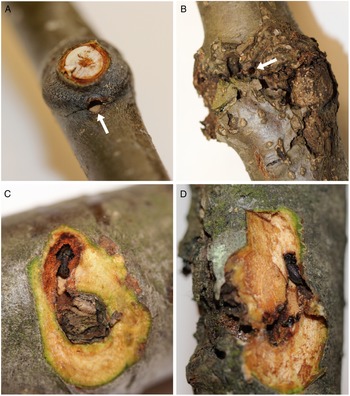
Fig. 1. A, Red alder branches showing a new (current year) overwintering site (arrow indicates an entrance hole through the branch node); B, an old, or re-occupied (from previous year(s)), overwintering site with characteristic callous tissue (arrow points to an entrance hole); C, a new (dissected) overwintering site adjacent to a branch scar with resident alder bark beetle; and D, an old overwintering site, dissected to reveal a beetle within.
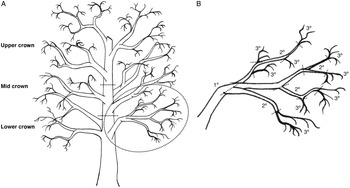
Three branches were removed from each of the lower, mid, and upper crowns of felled trees T4–T6 and returned to the laboratory, where they were classified by crown position and sectioned into segments based on age: tertiary (youngest; defined herein as 1–2 years old), secondary (defined as 2–3 years old), and primary (oldest; defined as 3+ years old) segments (Fig. 2). Branches were processed quickly at a cool room temperature and stored beneath tarps to limit beetle escape before dissection (branches from T1 to T3 were dissected onsite). Data on old, new, occupied, and unoccupied overwintering sites were summed and averaged across each branch group. Branch groups lacking overwintering sites were excluded from analysis.
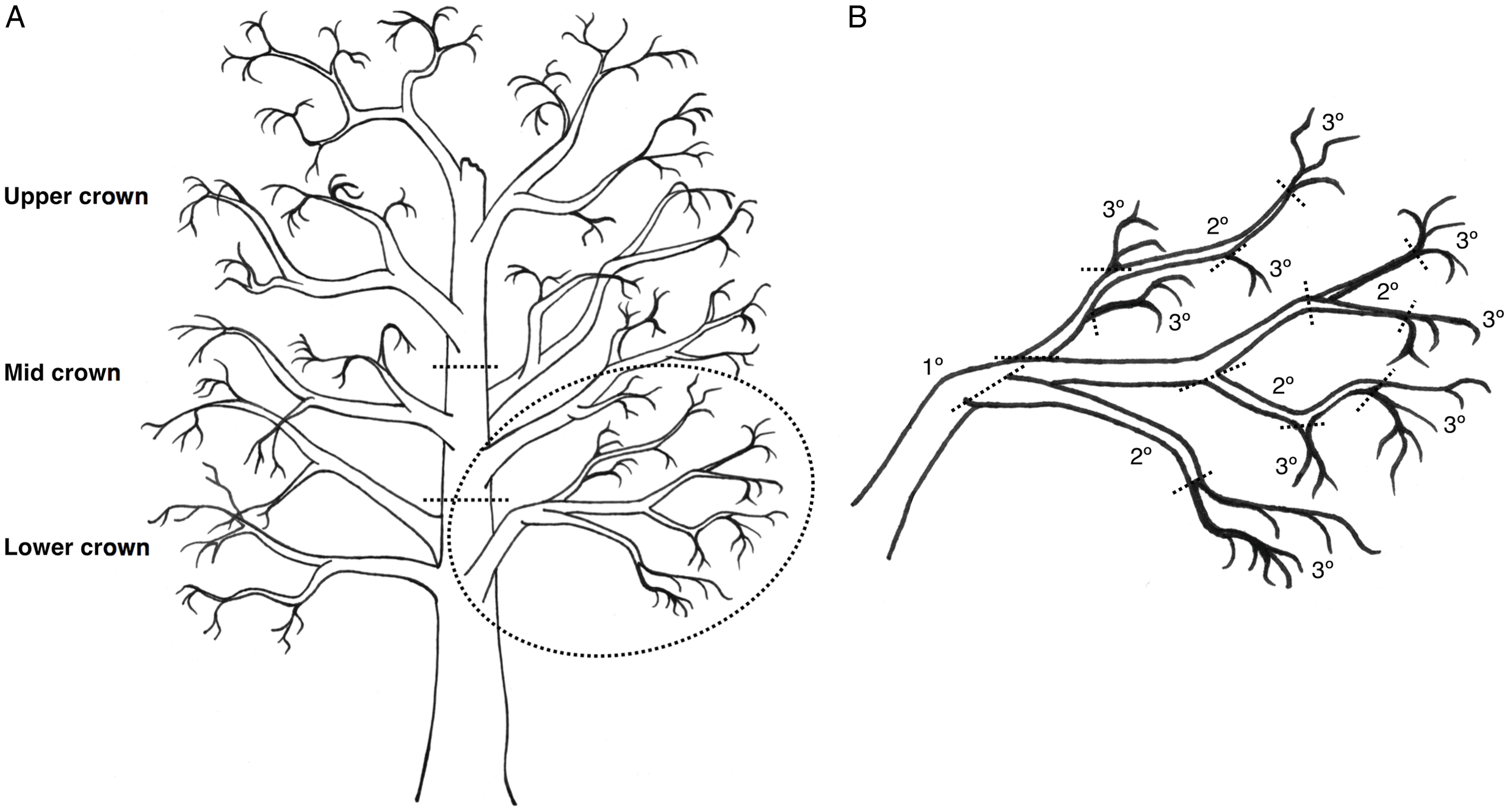
Fig. 2. Illustration of a hypothetical red alder tree, with A, the crown divided into lower, mid, and upper sections, with three branches per section; and B, the circled lower crown branch is shown in greater detail, with dashed lines showing where the branch would be cut into segments according to three branch types: tertiary (youngest, defined as 1–2 years old), secondary (defined as 2–3 years old), and primary (oldest, defined as 3+ years old) growth. From each branch sampled, there was one primary segment, while secondary and tertiary segments were pooled into groups for data analysis.
For T4–T6, the number of beetles per branch group was modelled against three discrete predictor variables (tree, crown position, and branch type) using negative binomial regression to accommodate overdispersion in the count data. Employing backwards stepwise selection, we produced a simple negative binomial model with two of the three factors (tree and branch type) and no interactions. A significant tree × crown position interaction was omitted because the measurement of zero beetles from the mid crown of T5 produced a computational error, and crown position and the two other interaction terms (crown position × branch type and tree × branch type) were excluded due to nonsignificance.
To assess the likelihood of overwintering site occupancy for T4–T6, the proportion of occupied to unoccupied overwintering sites was modelled against the same three predictor variables (tree, crown position, and branch type) using binomial logistic regression. The logistic model developed using backwards stepwise selection included all three factors and two two-way interactions (tree × crown position and crown position × branch type) and excluded the third two-way interaction (tree × branch type), which was nonsignificant.
To assess whether overwintering site age affected occupancy by alder bark beetles, we performed a Chi-squared analysis of a contingency table containing the summed totals of occupied old, unoccupied old, occupied new, and unoccupied new overwintering sites for all six trees (T1–T6). This analysis allowed us to assess the effect of overwintering site age on site occupancy independent of crown position and branch type, whereas two additional Chi-squared analyses were conducted to assess the effects of tree and survey site location on overwintering site occupancy.
All analyses were conducted in RStudio (R Core Team 2021; Rstudio Team 2021). The negative binomial regression was constructed using the MASS package (Venables and Ripley Reference Venables and Ripley2002), and associated significance levels for terms were obtained with the ANOVA function in car (Fox and Weisberg Reference Fox and Weisberg2019; test = “LR”). Count data dispersion was assessed with the AER package (Kleiber and Zeileis Reference Kleiber and Zeileis2008). The logistic model was visualised using the visreg package (Breheny and Burchett Reference Breheny and Burchett2017), and data figures were generated with ggplot2 (Wickham Reference Wickham2016).
Results
Alder bark beetles were found overwintering in branch and bud nodes within the crowns of all six mature trees at all three sites and in both sampling periods (Table 1). None of the trees contained brood galleries in the bole, so the beetles must have flown to mature trees after they emerged from brood hosts located elsewhere. Callous tissue formed around each branch-overwintering site during the year following initial occupation (Fig. 1B, D), and thus the presence or absence of callous tissue was a reliable indicator of previous years’ sites.
Overwintering site occupancy within crowns was highly variable among trees and branch types (Tables 2 and 3). A total of 324 adult alder bark beetles was recovered from 923 overwintering sites, of which 242 (26%) were occupied, across the six trees sampled. Most frequently, one beetle was found alone, but in several cases, five or more beetles were found in the same overwintering site (Fig. 3). Instances of multiple beetles occupying the same overwintering site were observed in primary (oldest) and secondary branches, whereas only single beetles were found in the tertiary (youngest) branches (T4–T6; Fig. 4).
Table 2. Mean ± standard error number of alder bark beetle overwintering sites, proportion of occupied sites, and proportion of newly excavated sites within the canopies of three felled mature red alder trees (T4–T6) by crown position and branch type (primary (oldest, defined as 3+ years old), secondary (2–3 years old), or tertiary (youngest, 1–2 years old)). A branch group consists of all of the branch segments of each branch type (primary, secondary, or tertiary growth), per branch.
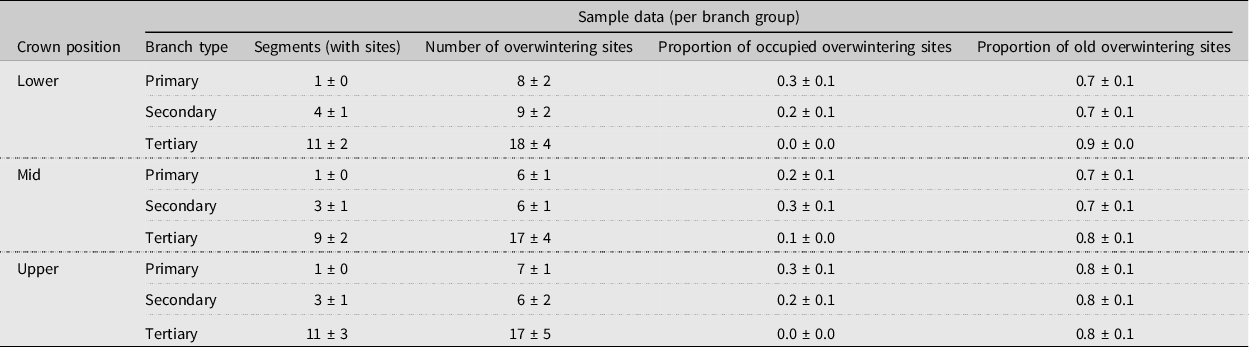
Table 3. Analysis of deviance table (test = Chi) for binomial logistic regression analysis of the effects of tree (T4–T6), crown position (lower, mid, and upper), and branch type (primary (oldest), secondary, and tertiary (youngest)), and two interactions (tree × crown position and crown position × branch type) on the proportion of occupied alder bark beetle overwintering sites within the crowns. The unit of replication in the model is branch group, which consists of all of the branch segments of each branch type, per tree branch.
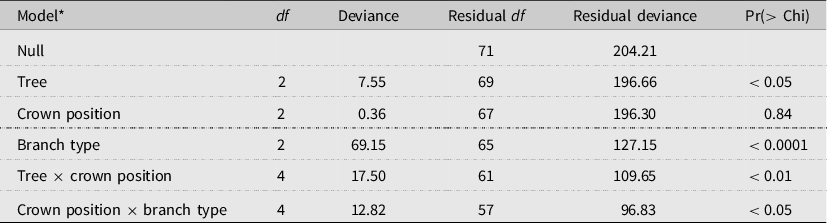
* Model deviance = 107.38 on 14 df, a 52.6% reduction in residual deviance.

Fig. 3. Frequency distribution of the number of alder bark beetles recovered from each occupied overwintering site for trees T1–T6 combined.
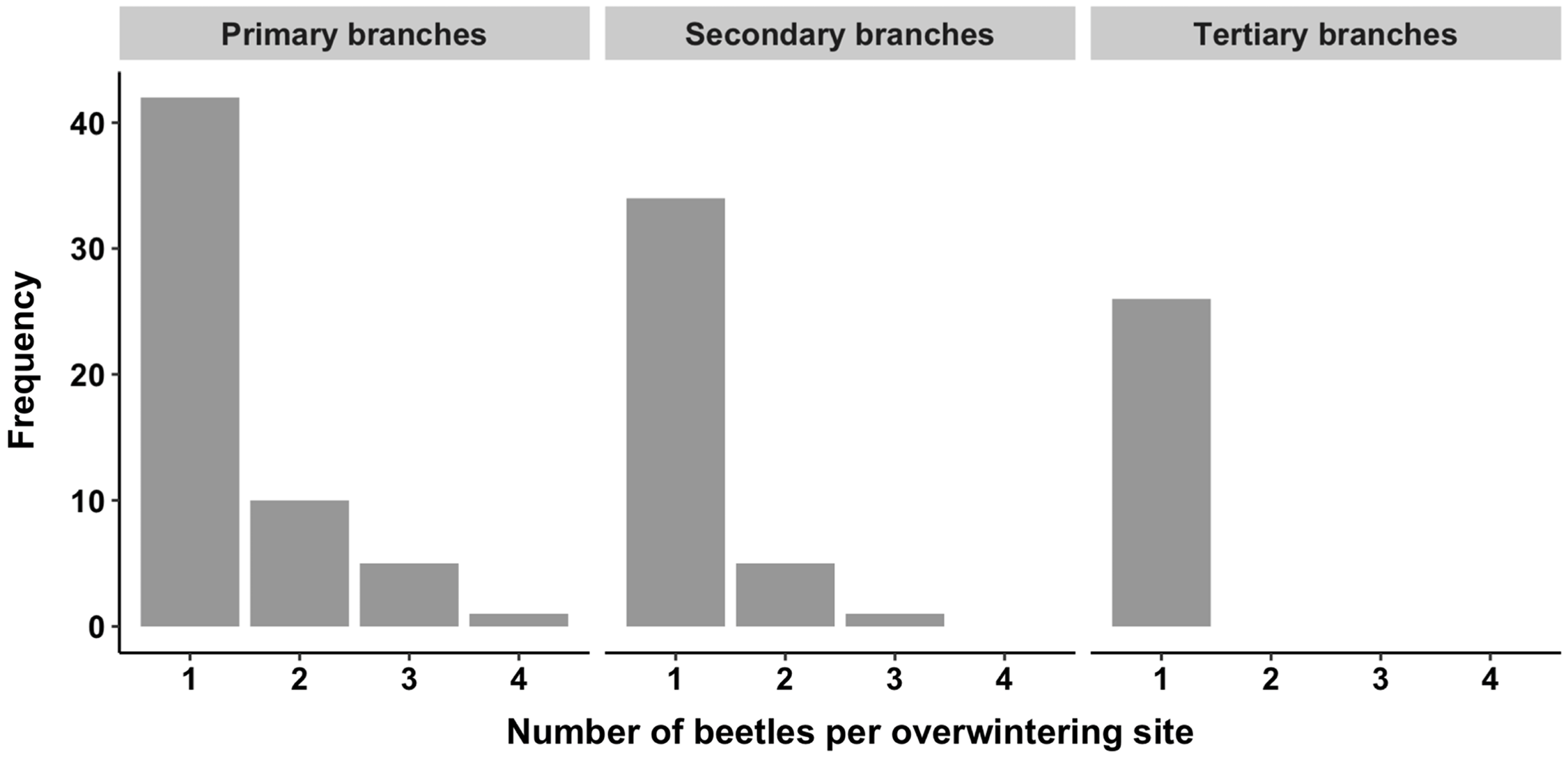
Fig. 4. Frequency distribution of the number of alder bark beetles recovered from individual occupied overwintering sites within the canopies of three mature red alder trees (T4–T6). Data are shown for primary (oldest), secondary, and tertiary (youngest) branches.
The absolute distribution of overwintering alder bark beetles within red alder trees (T4–T6) was best predicted by tree (P < 0.01; χ 2 2 = 9.23) and branch type (P < 0.05; χ 2 2 = 7.43) (model deviance = 15.20 on 4 df, a 17.5% reduction in residual deviance) (Fig. 5). By contrast, the likelihood of overwintering site occupancy within different canopy sections was best predicted by tree (P < 0.05), branch type (P < 0.0001), and two interactions (tree × crown position (P < 0.01) and crown position × branch type (P < 0.05)), indicating that, although crown position itself was not significant (P = 0.84), the effect of crown position was dependent upon tree and the effect of branch type was dependent upon crown position (Table 3). Across trees and crown positions, the number of overwintering beetles and the proportion of occupied overwintering sites were both highest in primary (oldest) branch types and lowest in tertiary (youngest) branch types (Table 2; Fig. 5).
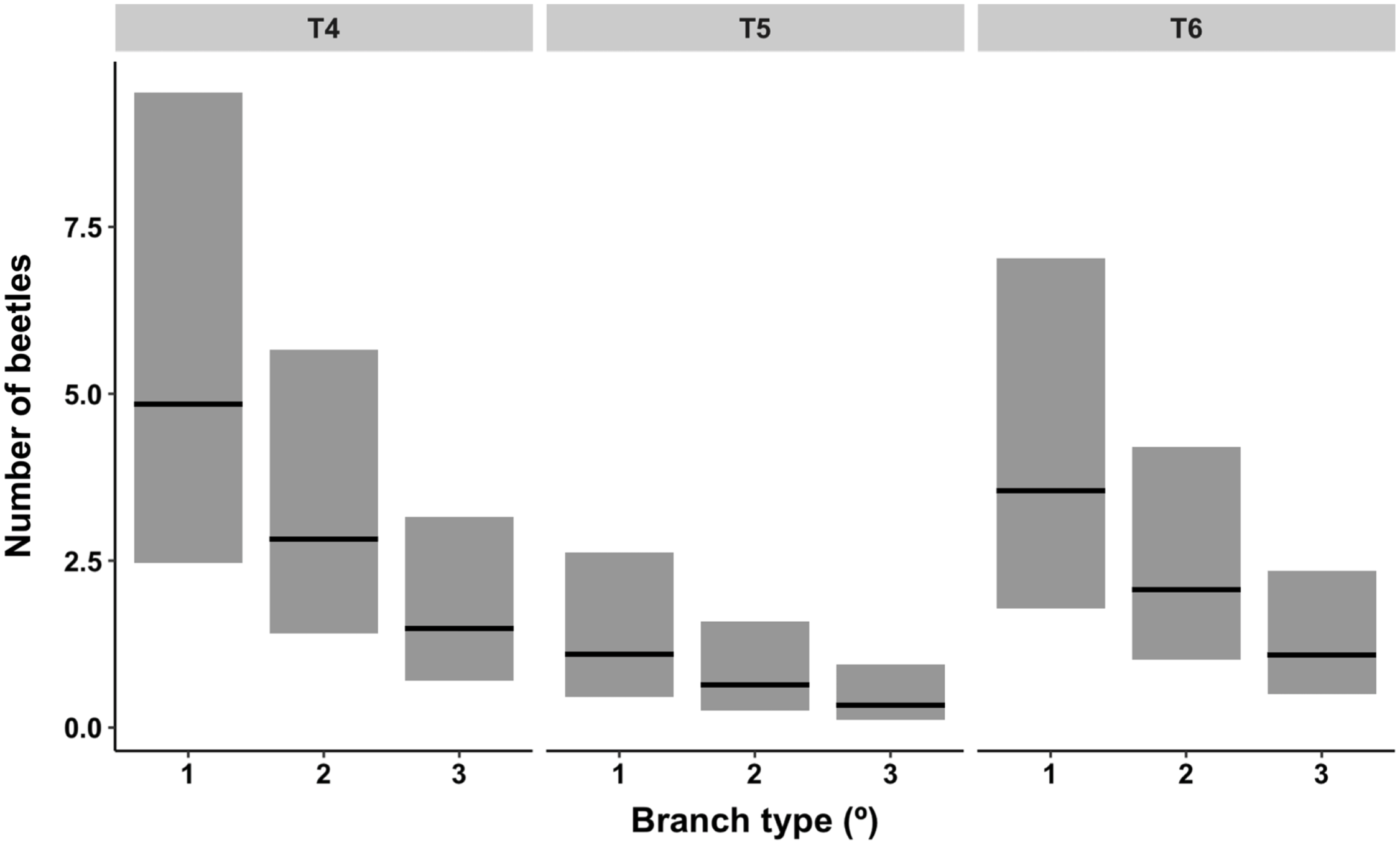
Fig. 5. Contribution of branch type (primary (1; oldest), secondary (2), and tertiary (3; youngest)) faceted by tree (T4–T6) to a negative binomial model describing the distribution of overwintering alder bark beetles. Lines across bars represent model estimates flanked by 95% confidence bands.
Across all six trees, alder bark beetles were found more frequently in new than in old overwintering sites than would be expected if the beetles were occupying sites of different ages evenly (P < 0.0001; χ 2 1 = 194.2), although survey site location (P < 0.0001; χ 2 2 = 170.8) and tree (P < 0.0001; χ 2 5 = 189.9) also significantly influenced patterns of overwintering site occupancy. Of 923 total overwintering sites, 86 occupied and 567 unoccupied old sites and 156 occupied and 114 unoccupied new sites were found. In each case, the distributions deviated significantly from an even distribution (observed versus expected occupied new (156 > 71), unoccupied old (567 > 482), occupied old (86 < 171), and unoccupied new (114 < 199) overwintering sites).
Discussion
We have discovered that adult alder bark beetles occupy branch and bud nodes within the crowns of mature host trees during the winter, representing a previously unknown overwintering strategy for this species. Bark beetles generally overwinter as larvae or immature adults within the phloem or outer bark of brood trees, or as emerged adults within bark near the root collar or on the forest floor in litter or soil (Chamberlin Reference Chamberlin1958; Lombardero et al. Reference Lombardero, Ayres, Ayres and Reeve2000; Sauvard Reference Sauvard2007; Kausrud et al. Reference Kausrud, Økland, Skarpaas, Grégoire, Erbilgin and Stenseth2012; Dworschak et al. Reference Dworschak, Meyer, Gruppe and Schopf2014). Overwintering as emerged adults inside branches and shoots on standing trees is documented in a few species, including the olive bark beetle, Phloeotribus scarabaeoides (Bernard) (González and Campos Reference González and Campos1996; Szauman-Szumski et al. Reference Szauman-Szumski, Peña, Kelly and Campos1998), the pistachio twig borer, Chaetoptelius vestitus (Mulsant and Rey) (Braham Reference Braham2016), and Tomicus Latreille spp. (Russo Reference Russo1946; Masutti Reference Masutti1969; Långström Reference Långström1983; Ye Reference Ye1991; Sauvard Reference Sauvard2007). We propose three hypotheses for the evolution of branch overwintering in the alder bark beetle: (1) branches and twigs are thermally protective overwintering sites for this species; (2) branches provide opportunities for maturation feeding during warm winter periods; and (3) branch overwintering enables beetles to acquire and disseminate symbiotic phytopathogens. It is also possible that branch overwintering decreases beetle susceptibility to woodpecker predation (Fayt et al. Reference Fayt, Machmer and Steeger2005), as these birds tend to focus on main stems for foraging (Hammond and Theimer Reference Hammond and Theimer2020).
Bark beetles that overwinter as adults employ behavioural strategies that vary along latitudinal, local, and microhabitat temperature gradients (Långström Reference Långström1983; Lombardero et al. Reference Lombardero, Ayres, Ayres and Reeve2000; Sauvard Reference Sauvard2007; Kausrud et al. Reference Kausrud, Økland, Skarpaas, Grégoire, Erbilgin and Stenseth2012; Dworschak et al. Reference Dworschak, Meyer, Gruppe and Schopf2014). We found overwintering alder bark beetles throughout the crowns of mature red alders, although trends of site occupancy varied among sites, trees, crown position, and branch type. In many temperate bark beetles, including northern populations of Tomicus piniperda (Linnaeus) (Salonen Reference Salonen1973; Långström Reference Långström1983), Ips grandicollis (Eichhoff), Ips pini (Say) (Orr Reference Orr1935; Lombardero et al. Reference Lombardero, Ayres, Ayres and Reeve2000), Ips typographus (Linnaeus) (Annila Reference Annila1969; Kausrud et al. Reference Kausrud, Økland, Skarpaas, Grégoire, Erbilgin and Stenseth2012), Dendroctonus rufipennis Kirby (Massey and Wygant Reference Massey and Wygant1954; Knight Reference Knight1961), and Hylesinus californicus (Swaine) (Langor and Hergert Reference Langor and Hergert1993), adults emerge from their hosts and navigate to alternative overwintering sites before the onset of winter. These microhabitats include galleries constructed by one or more beetles in the outer bark of the lower bole (Borden Reference Borden1969; Poland et al. Reference Poland, Haack and Petrice2002), where there is often thermal protection under a layer of snow (Chamberlin Reference Chamberlin1958; Salonen Reference Salonen1973). We found that large, old branches, which might offer superior thermal insulation (Vermunt et al. Reference Vermunt, Cuddington, Sobek-Swant, Crosthwaite, Lyons and Sinclair2012) to beetles in mild winter climates, had a higher likelihood of occupation and hosted more overwintering beetles per site than younger branches did. We suggest that alder bark beetles may abandon feeding sites in secondary and tertiary branches as temperatures drop in early fall and move to more thermally protective primary branches.
Microhabitat selection by overwintering alder bark beetles likely occurs using strategies similar to those used to select reproductive hosts – that is, by employing sensory integration of visual, tactile, olfactory, and gustatory cues (Campbell and Borden Reference Campbell and Borden2006; Raffa et al. Reference Raffa, Andersson and Schlyter2016). The effect of tree on site choice and occupancy (T4–T6) can be attributed to microhabitat selection within heterogenous stand and canopy structures, as well as to the proximity of brood trees, from which alder bark beetles emerge in late summer (Borden Reference Borden1969). Landscape disturbance caused by clearcutting (T1–T3) and opening of a road right of way (T4–T6) would have contributed to microhabitat differences among and within trees at the study sites.
Branch overwintering by the alder bark beetle could have evolved as a consequence of maturation feeding in nutrient-rich and moist new buds and twigs, as occurs in other bark beetles, including Tomicus spp. (Långström Reference Långström1983; Ye Reference Ye1991; Poland and Haack Reference Poland and Haack2000), Pseudohylesinus nebulosus (LeConte) (Stoszek and Rudinsky Reference Stoszek and Rudinsky1967), Pseudopityophthorus minutissimus (Zimmermann), P. pruinosus (Eichhoff) (Buchanan Reference Buchanan1958; Rexrode and Jones Reference Rexrode and Jones1970), Phloeosinus Chapuis spp. (Faccoli and Sidoti Reference Faccoli and Sidoti2013), Hylesinus californicus (Langor and Hergert Reference Langor and Hergert1993), Hypocryphalus mangiferae (Stebbing) (Masood et al. Reference Masood, Saeed, Erbilgin and Jung Kwon2010), and Hylurgopinus rufipes (Eichhoff), and Scolytus Geoffroy spp. (Webber and Brasier Reference Webber and Brasier1984; Anderson and Holliday Reference Anderson and Holliday2003; Santini and Faccoli Reference Santini and Faccoli2014). This hypothesis is supported by observations of beetles feeding in adventitious shoots on the lower boles of alders during mid-summer (D.L.W., unpublished data). In a mild maritime climate, moving from terminal shoots to sites in larger-diameter branches and stems as winter approaches, as occurs in Tomicus spp. in southern Europe (Masutti Reference Masutti1969; Salonen Reference Salonen1973; Långström Reference Långström1983) and southern China (Ye Reference Ye1991), in Phloeotribus scarabaeoides in the Mediterranean (González and Campos Reference González and Campos1996; Szauman-Szumski et al. Reference Szauman-Szumski, Peña, Kelly and Campos1998), and in Chaetoptelius vestitus in Tunisia (Braham Reference Braham2016), could enable beetles to avoid the coldest temperatures at the periphery of the crown while retaining the nutritious and competitor-free advantages of spending winter inside previously unaffected tissues (Reid Reference Reid1961; Reid and Robb Reference Reid and Robb1999; McNee et al. Reference McNee, Wood and Storer2000; Poland and Haack Reference Poland and Haack2000). This hypothesis is substantiated by our findings that beetles were most frequently found in newly excavated overwintering sites across trees and survey locations and that beetles recovered from old overwintering sites were usually mining in living tissue adjacent to callous tissue. We suggest that an affinity for previously occupied sites might be a consequence of callous tissue offering an ideal textured surface for gripping and excavating into the bark (Ferrenberg and Mitton Reference Ferrenberg and Mitton2014) and that old overwintering sites could harbour microorganisms that nutritionally benefit the beetles during winter feeding (Ayres et al. Reference Ayres, Wilkens, Ruel, Lombardero and Vallery2000; Bleiker and Six Reference Bleiker and Six2007). It is also possible that the beetles acquire symbiotic organisms from, or disseminate them to, these tissues before dispersal to reproductive hosts (Bleiker et al. Reference Bleiker, Potter, Lauzon and Six2009; Wingfield et al. Reference Wingfield, Garnas, Hajek, Hurley, de Beer and Taerum2016), as occurs for bark beetles that vector pathogens causing Dutch elm disease (Webber and Brasier Reference Webber and Brasier1984) and oak wilt (Rexrode and Jones Reference Rexrode and Jones1970) and for cerambycids that carry pine wilt nematode (Mamiya Reference Mamiya1983).
Acknowledgements
The authors thank J. Burleigh, A. Gustafsson, J. Mullan, Z. Melo, and K. Kitchens for assistance in the field and S. Pokorny for statistical and ecological insights. Support for this research was provided by Weyerhaeuser Canada Ltd., GFC Forest Management Ltd., and the Natural Sciences and Engineering Research Council of Canada.
Competing interests
The authors declare none.