Dyspnea is a common non-motor symptom linked to Parkinson’s disease (PD) with nearly 40% prevalence in patients.Reference Baille, Chenivesse and Perez1 It tends to occur in progressed PD patients who also experience anxiety.Reference Baille, Chenivesse and Perez1 Causes of this symptom can vary, including upper airway obstruction, restrictive respiratory change, levodopa-induced dyskinesia, and hyperventilation, making its management difficult. Here, we report a PD patient who experienced dyspnea after oral intake of levodopa. We hypothesized that dopamine-induced peripheral stimulation of the respiratory center triggered hyperventilation, which manifested as dyspnea in this patient.
A 76-year-old male was hospitalized with complaints of frequent dyspnea. At age 65, REM sleep behavior disorder (RBD) and left-hand tremor appeared. At age 73, the patient was diagnosed with PD based on the presence of bradykinesia, mask-like face, and salivation. While magnetic resonance imaging (MRI) of his brain appeared normal, the accumulation of 6-[18F]-fluoro-meta-tyrosine (FMT) was reduced in the basal ganglia, suggesting dysfunction of dopaminergic pathways. The administration of 300 mg/day of levodopa/carbidopa in addition to 4.5 mg/day of rotigotine resulted in a partial improvement of his motor symptoms. However, non-motor symptoms such as hallucinations, nausea, and overeating became more apparent as the disease progressed. Soon after his diagnosis, he began experiencing shortness of breath.
The patient presented normal consciousness and left-dominant parkinsonism. During wakefulness, his breathing rate was 15 breaths/min, but was also unstable and would spontaneously increase to 30 breaths/min for a time before returning to the normal range. Conversely during sleep, his breathing was calm and regular. His breathing would also increase in response to changes in body position from lying down to standing up. These clinical findings led to the diagnosis of hyperventilation, but results from his arterial blood gas test were normal at baseline.
The results of laryngoscopic examination, respiratory function test, and chest computed tomography (CT) were deemed normal, excluding the possibility of organic diseases including upper airway obstruction and restrictive respiratory change as potential causes of his hyperventilation. When the dose of levodopa/carbidopa was increased to 900 mg/day, his hyperventilation worsened and seemed to appear every time after taking his medication, indicating that levodopa administration could trigger the symptom. While intravenous injection of 100 mg of levodopa led up to 20% improvement in his parkinsonism according to the UPDRS part III score, hyperventilation deteriorated. His breathing frequency increased to 33 breaths/min, and primary respiratory alkalosis became apparent from follow-up arterial blood gas tests (Figure 1). Interestingly, his breathing would normalize during sleep and only became rapid during wakefulness. Dyskinesia was not observed before or after levodopa treatment, and amantadine, an anti-dyskinesia agent, was not effective.
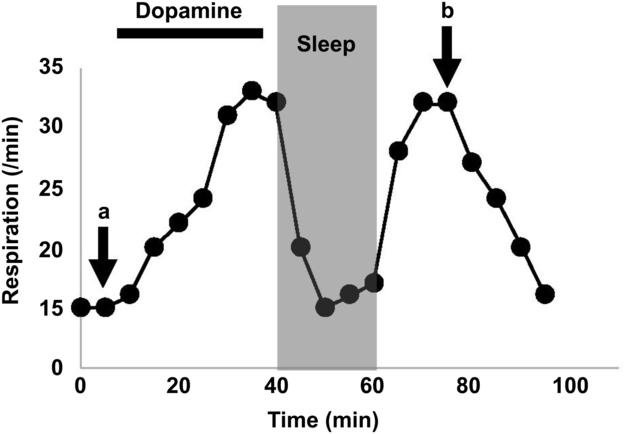
Figure 1: The respiratory alternation during the dopa challenge test. While intravenous dopamine administration induced hyperventilation, sleeping attenuated it. The arterial pH, CO2, O2, and HCO3 were 7.444, 38.6 mmHg, 90.5 mmHg, and 25.9 mmol/L at a and 7.485, 33.1 mmHg, 106.1 mmHg, and 24.4 mmol/L at b, respectively.
These results indicate that his hyperventilation was induced by levodopa administration; therefore we reduced the dosage of levodopa/benserazide to 300 mg/day and prescribed diazepam, which is known to decrease breathing. Combination treatment was effective in alleviating his symptoms.
Recent clinical studies revealed that dyspnea is a common symptom associated with PD.Reference Baille, Chenivesse and Perez1 It often appears during “off” times and is associated with reduced ventilatory function and dysphagia,Reference Baille, Chenivesse and Perez1 all of which were not observed in the current case. Respiratory dyskinesia has been reported to be the main cause of levodopa-induced respiratory disturbances.Reference Rice, Antic and Thompson2 While respiratory dyskinesia often presents concomitantly with facial or orobuccal dyskinesia and is not accompanied with the alteration of arterial blood gas,Reference Jankovic and Nour3 our patient did not show any dyskinetic movement including in the respiratory muscles and had marked respiratory alkalosis. In addition, oral intake of amantadine, an anti-dyskinesia agent, was not effective. Taken together, we hypothesized that levodopa-induced hyperventilation, rather than focal dyskinesia, caused his respiratory disturbance.
The mechanism behind levodopa-induced hyperventilation remains elusive. This may be in part due to the highly complex respiratory central system, which contains multiple interacting groups of neurons in the brain stem, making it difficult to pinpoint the pathological location associated with levodopa-induced hyperventilation.Reference De Keyser and Vincken4 However, experimental studies have shown that stimulation of either peripheral chemoreceptors or the central respiratory center in the brain stem can lead to hyperventilation.Reference Bongianni, Mutolo, Carfi and Pantaleo5,Reference Iturriaga, Alcayaga and Gonzalez6
Hyperventilation often occurs in patients experiencing psychological or physiological stress, but other forms, such as central neurogenic hyperventilation (CNH), can be triggered organically.Reference Takahashi, Tsunemi, Miyayosi and Mizusawa7 CNH is usually observed in patients with brain stem lesions and can result in dysregulated activation of the central respiration center. Therefore, we hypothesize that a mild form of CNH can also be induced in PD patients with basal ganglia abnormalities. While CNH is clinically characterized by sustained hyperventilation during both wakefulness and sleep, our patient exhibited normal breathing while asleep, suggesting that the central respiratory system was not functionally damaged (Figure 1). It is possible that his respiration was stimulated by increased peripheral dopamine following levodopa challenge. Experimental studies in rabbits have demonstrated that the area postrema, which lacks a blood brain barrier and functions as a vomiting center, consists of highly dense dopamine receptors that can positively influence respiration upon a sudden increase in peripheral dopamine.Reference Bongianni, Mutolo, Carfi and Pantaleo5 Peripheral dopamine might additionally stimulate chemoreceptors in the carotid body, which can sense the arterial PO2, PCO2, and pH, controlling cardiorespiratory system.Reference Iturriaga, Alcayaga and Gonzalez6 The carotid body can also be stimulated through orthostasis.Reference Malmberg, Tamminen and Sovijarvi8 Our patient consistently experienced hyperventilation induced by orthostatic change, specifically after standing from a spine position, supporting this hypothesis. Considering that levodopa has been the gold standard treatment for millions of PD patients, levodopa-induced hyperventilation is admittedly quite rare. Patients experiencing levodopa-induced hyperventilation may possess a unique respiratory center that is highly sensitized to peripheral stimuli. In support of this hypothesis, our patient was highly anxious and exhibited unstable breathing prior to receiving medications, indicating a low stimulus threshold to manifest hyperventilation.
In summary, we treated a PD patient with dyspnea resulting from levodopa-induced hyperventilation. Decreased dosage of levodopa and addition of a minor tranquilizer, diazepam, relieved the dyspnea without significantly deteriorating his parkinsonism, leading to improvements in quality of life. Levodopa-induced hyperventilation is rare but manageable; therefore clinicians should be mindful especially when treating dysprenic PD patients who have symptoms of anxiety.
Acknowledgements
We thank Maria Nguyen for proofreading and comments that improved the manuscript.
Disclosures
Drs. AH, TT, TS, EN, and NH have no conflicts of interest to declare.
Statement of Authorship
AH reviewed literature and drafted the manuscript. TS and EN revised the manuscript. TT and HN finalized the manuscript.



