Frailty is a multidimensional concept characterized by reduced resistance to stressors due to depleted physiological reserve. Its consequences are diverse and deleterious, and can include undernutrition and weight loss, as well as reduced strength and balance (Bergman et al., Reference Bergman, Ferrucci, Guralnik, Hogan, Hummel, Karunananthan and Wolfson2007). These, in turn, lead to a myriad of adverse sequelae such as falls, worsening mobility, hospitalization, and death. Frailty is increasingly recognized as an important geriatric condition whose identification and management are key to comprehensive geriatric care (Cesari et al., Reference Cesari, Marzetti, Thiem, Perez-Zepeda, Abellan Van Kan, Landi and Bernabei2016; Gladman et al., Reference Gladman, Conroy, Ranhoff and Gordon2016). A number of studies have identified genetic loci involved in the development of the frailty phenotype (Almeida et al., Reference Almeida, Norman, Van Bockxmeer, Hankey and Flicker2012; van den Beld et al., Reference Van Den Beld, Huhtaniemi, Pettersson, Pols, Grobbee, De Jong and Lamberts1999; Walston et al., Reference Walston, Arking, Fallin, Li, Beamer, Xue, Ferrucci and Chakravarti2005), as well as environmental determinants including socioeconomic status (SES) and educational attainment (Alvarado et al., Reference Alvarado, Zunzunegui, Beland and Bamvita2008; Andrew et al., Reference Andrew, Mitnitski and Rockwood2008; Cramm & Nieboer, Reference Cramm and Nieboer2013; Hoogendijk et al., Reference Hoogendijk, van Hout, Heymans, van der Horst, Frijters, Broese van Groenou and Huisman2014; Lang et al., Reference Lang, Hubbard, Andrew, Llewllyn, Melzer and Rockwood2009; Marshall et al., Reference Marshall, Nazroo, Tampubolon and Vanhoutte2015; Moreira & Lourenco, Reference Moreira and Lourenco2013; Peek et al., Reference Peek, Howrey, Ternent, Ray and Ottenbacher2012; Romero-Ortuno, Reference Romero-Ortuno2014; Szanton et al., Reference Szanton, Seplaki, Thorpe, Allen and Fried2010; Woo et al., Reference Woo, Goggins, Sham and Ho2005; Woods et al., Reference Woods, LaCroix, Gray, Aragaki, Cochrane, Brunner, Masaki and Newman2005). However, to our knowledge, no study has yet examined both the genetic and environmental determinants of frailty in the same individuals. Thus, our study aims to extend our understanding of the determinants of frailty by examining its heritability and the impact of various behavioral and environmental factors in a population of UK twins.
The two principal instruments for the measurement of frailty are the Fried frailty phenotype (Fried & Walston, Reference Fried, Walston, Hazzard, Blass, Halter and Ouslander2003) and Rockwood's Frailty Index (FI). A FI is based on the number, rather than the type of one's health problems (Rockwood et al., Reference Rockwood, Fox, Stolee, Robertson and Beattie1994) and is calculated as the proportion of an array of possible deficits (Searle et al., Reference Searle, Mitnitski, Gahbauer, Gill and Rockwood2008). Deficits occur when damage at the subcellular level is unrepaired or unremoved. Eventually, in the absence of repair mechanisms to compensate for the damage, or an inability of intrinsic repair mechanisms to cope with requirements for repair, subcellular damage scales up to become macroscopically visible health impairment. In this deficit accumulation model of frailty, the more health deficits (whether signs, symptoms, diseases or disabilities) that a person has, the more likely they are to experience other adverse outcomes and to die (Rockwood et al., Reference Rockwood, Mitnitski and Howlett2015). Thus, the FI predicts adverse health states, such as disability, hospitalization, dependency, institutionalization, and mortality (Clegg et al., Reference Clegg, Young, Iliffe, Rikkert and Rockwood2013), and is also related to age (Theou et al., Reference Theou, Brothers, Pena, Mitnitski and Rockwood2014).
Using data from a population of volunteer twins enrolled in the UK Adult Twin Registry (Moayyeri et al., Reference Moayyeri, Hammond, Hart and Spector2013a; Spector & Williams, Reference Spector and Williams2006), we applied the classical twin model (Boomsma et al., Reference Boomsma, Busjahn and Peltonen2002) to investigate the heritability of frailty as well as its association with the twins’ unique and shared environment. The classical twin model derives its ability to separate genetic etiology of variance in a trait from environmental etiologies (both shared and unique) from the fact that monozygotic (MZ) twins share almost 100% of variable hereditary information encoded in DNA, whereas dizygotic (DZ) twins, on average, share only half (as for any non-twin siblings; Boomsma et al., Reference Boomsma, Busjahn and Peltonen2002). Further, once the genetic effect on variance in a trait is known, the additional difference in variance between MZ and DZ twins allows the effects of their shared versus unique environment to be discerned. Although previous studies in twins have examined genetic variance in frailty, they have all used the Fried frailty phenotype (Dato et al., Reference Dato, Montesanto, Lagani, Jeune, Christensen and Passarino2012; Miles, Reference Miles1997), whereas we used Rockwood's FI in order to better understand the determinants of frailty when conceptualized as an ‘accumulation of deficits’ (Mitnitski et al., Reference Mitnitski, Song and Rockwood2004).
In the second analysis reported here, the association between frailty and a specific element of shared environment — that of childhood SES, as embodied by father's occupational status — was selected for further examination. The aim of this additional analysis was to extend our understanding of the environmental determinants of frailty with a life-course approach. There is an extensive literature documenting how an array of childhood conditions, including SES, educational attainment and living arrangements, continue to have an impact on mortality and health into adulthood and old age (Hayward & Gorman, Reference Hayward and Gorman2004). If frailty in older age is shaped by environmental factors, it is of interest to understand if these persist across the life course. Hence, in this analysis, multivariate logistic regression analysis was used to determine the association between father's occupational status and frailty. Age, age at end of education, marital status, and the health behaviors of smoking, alcohol consumption, and level of physical exercise were included as covariates, as these have also been shown to be associated with the development of frailty (Etman et al., Reference Etman, Kamphuis, van der Cammen, Burdorf and van Lenthe2015; Hoogendijk et al., Reference Hoogendijk, van Hout, Heymans, van der Horst, Frijters, Broese van Groenou and Huisman2014; Hubbard et al., Reference Hubbard, Searle, Mitnitski and Rockwood2009; Leigh & Fries, Reference Leigh and Fries2002; Ortola et al., Reference Ortola, Garcia-Esquinas, Leon-Munoz, Guallar-Castillon, Valencia-Martin, Galan and Rodriguez-Artalejo2016; Strawbridge et al., Reference Strawbridge, Shema, Balfour, Higby and Kaplan1998; Trevisan et al., Reference Trevisan, Veronese, Maggi, Baggio, De Rui, Bolzetta and Sergi2016). Birth weight (self-reported) was also included as a covariate as there is some evidence that it is a robust indicator of health across the life course (United Nations Children's Emergency Fund [UNICEF] and World Health Organization [WHO], 2004). We believe this to be the first study to have applied the classical twin model (Boomsma et al., Reference Boomsma, Busjahn and Peltonen2002) and standard epidemiological modeling in the same sample of adult twins.
Methods
Subjects
Study participants were volunteer adult twins from across the United Kingdom recruited into the St. Thomas’ UK Adult Twin Registry since 1992 using national media campaigns. At this time, the registry consisted of approximately 12,000 volunteer MZ and DZ twins aged 18–103 years, as well as some parents and siblings (Moayyeri et al., Reference Moayyeri, Hammond, Hart and Spector2013a; Reference Moayyeri, Hammond, Valdes and Spector2013b). Zygosity of subjects has been determined using a validated questionnaire (Peeters et al., Reference Peeters, Van Gestel, Vlietinck, Derom and Derom1998). Participants have previously been shown to be comparable to age-matched population singletons in a wide range of characteristics, including bone mineral density, osteoarthritis, blood pressure, hypertensive drug use, height, history of hysterectomy and ovariectomy, menopausal status, and alcohol and tobacco consumption (Andrew et al., Reference Andrew, Hart, Snieder, de Lange, Spector and MacGregor2001).
Measurements
Multivariate logistic regression analysis used to construct a model describing the association between FI and father's occupational classification employed data from four self-report questionnaires — Q2, Q10, Q11A, and Q17D (the latter also known as the Healthy Ageing Twin Study [HATS]) — all collected between 1999 and 2010 by the TwinsUK registry (Moayyeri et al., Reference Moayyeri, Hammond, Hart and Spector2013a; Reference Moayyeri, Hammond, Valdes and Spector2013b; Spector & Williams, Reference Spector and Williams2006) and covering a wide range of health and lifestyle issues (questionnaires can be found in full on the TwinsUK website at http://www.twinsuk.ac.uk/). See supplementary material, Table 1, for questionnaire items used in the current analysis.
TABLE 1 Mean (SD) FI by Category of Variables and p Value for Test of Trend with FI

†% of total number of cases (n = 3,375).
‡statistical test with age as a continuous variable; FI = Frailty Index.
Derivation of the Rockwood Frailty Index
Thirty-nine items (see supplementary material, Table 2) in the domains of comorbid conditions, physical measures, biochemical measures, mental health, self-reported general health, disability, social functioning, polypharmacy, and pain that were collected via a questionnaire administered by a nurse practitioner and supplemented by physiological data and blood tests collected in HATS were used to derive the FI (Mitnitski et al., Reference Mitnitski, Song and Rockwood2004) as described by Searle et al. (Reference Searle, Mitnitski, Gahbauer, Gill and Rockwood2008). Subjects who completed fewer than 20 domains were excluded from the analysis. The FI was a continuous variable with a value between 0 and 1.
TABLE 2 Results of Hierarchical Multiple Regression Showing Unstandardized Beta Coefficients (Reference Groups are Indicated)
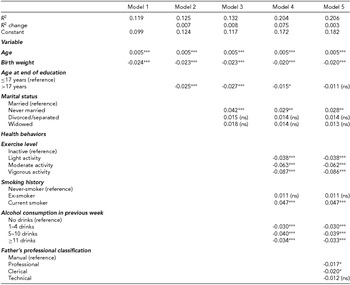
ns = not significant.
***p ≤ .001.
**p ≤ .005.
*p ≤ .05.
Operationalization of Variables for Multivariate Logistic Regression
Father's occupational classification
The independent (predictor) variable of FI in multivariate logistic regression was childhood SES, measured using father's occupation as recalled by the respondent and categorized using the National Statistics Socioeconomic Classification (Office for National Statistics, 2005). The variable consisted of the following four categories: (1) modern and traditional professions and senior managers/administrators (referred to as ‘professional’); (2) clerical and intermediate professions and middle and junior managers (‘clerical’); (3) technical and craft professions (‘technical’); and (4) semi-routine and routine manual professions (‘manual’). A further binary variable was created consisting of technical and craft, semi-routine and routine manual categories (referred to as ‘manual’) and modern and traditional professions, senior managers/administrators, clerical and intermediate professions plus middle and junior managers (referred to as ‘non-manual’).
Covariates
A binary categorical variable for age at end of education of ≤17 years or >17 years was constructed using items from two questionnaires asking ‘At what age did you finish full time education?’ Where unavailable, this information was supplemented with responses as to which of 12 different educational qualifications a respondent had received (none, clerical, 1–4 O-levels, low vocational, 5+ O-levels, middle vocational, A-levels, nursing, teaching, higher vocational, university and other). Marital status was categorized as married, divorced/separated, never married or widowed. Using questionnaire responses on hours spent on various types of physical exercise and activity undertaken during leisure time in the previous week, a variable was constructed to describe a respondent's total physical activity level for the previous week as inactive, light, moderate, or vigorous. A categorical smoking variable was created with the categories never smoked; ex-smoker; and current smoker (see supplementary material, Table 1). Likewise, a variable with four possible values was constructed to describe alcohol consumption as none, 1–4, 5–10, or ≥11 alcoholic drinks in the previous week. Age and birth weight were used as continuous variables. As the core sample was all female and 98% white, neither sex nor ethnic origin was retained as covariates in this analysis.
Statistical Analysis
Transformation of the Rockwood frailty index
A square root (sqrt) transformation was applied to the FI to provide a distribution that was close to normal (skewness statistic with standard error [SE] = 0.370 [0.042]; kurtosis statistic [SE] = 0.185 [0.084]). This transformed variable is denoted as sqrt[FI].
Standard structural equation modeling: the classical twin model
For our first analysis, standard structural equation modeling, as used in the classical twin model (Boomsma et al., Reference Boomsma, Busjahn and Peltonen2002), was used to estimate the relative contribution of genetics and of shared and unique environment to the variance in FI adjusted for age, using MX open source software (Neale et al., Reference Neale, Walters, Eaves, Maes and Kendler1994).
Multivariate logistic regression analysis: father's occupational classification
In our second analysis, standard hierarchical multiple linear regression techniques were used to explain variance in sqrt[FI] as a function of father's occupational classification — adjusting for age and other covariates. Tests for outliers and multicollinearity were conducted prior to analysis. All statistical tests were one-sided and associations were deemed statistically significant with p < .05. All statistics were conducted using IBM SPSS® Version 22 software.
Additional path analysis was performed to examine any moderating and mediating influences of covariates on the proposed effect of father's occupational classification on FI. Mediation analysis was conducted using the PROCESS plug-in tool for SPSS (http://afhayes.com/spss-sas-and-mplus-macros-and-code.html) (Preacher & Hayes, Reference Preacher and Hayes2004).
Results
Study Participants
The core dataset comprised 3,375 participants (all female and 98% white) with FI data. Participants were 1,644 twin pairs and 87 respondents with no twin in the dataset. Of the 1,644 twin pairs, 841 were MZ, 802 were DZ, and one was of unknown zygosity. Table 1 shows the key sample characteristics and the mean FI for each of the variables considered. The age of respondents ranged from 40.0 to 84.5 years with a mean (standard deviation [SD]) age of 59.12 (9.3) years. Self-reported birth weight ranged from 0.793 to 4.129 kg with a mean (SD) of 2.382 (0.578) kg (n = 2,804). The distribution of respondents by father's occupational class ranged from 17.7% for those with clerical fathers to 33.7% for those with professional fathers.
Frailty: Descriptive Statistics and Heritability
In this sample, the FI followed the expected gamma distribution (Figure 1), with a maximum value of 0.67 consistent with previous studies (Searle et al., Reference Searle, Mitnitski, Gahbauer, Gill and Rockwood2008). The mean (SD) FI of the total sample was 0.132 (0.105) with 25th, 50th, and 75th quartiles of 0.059, 0.105, and 0.178, respectively. There was a significant relationship between FI and age (standardized beta of 0.035; p < .001) (Figure 2).

FIGURE 1 Distribution of frailty indices.

FIGURE 2 Frailty index by chronological age.
The mean correlation in FI scores between the twins of the MZ twin pairs was 0.47, compared to 0.27 for the twins of the DZ twin pairs, indicating greater similarity in the frailty scores of the MZ than DZ twins. Consistent with this, modeling revealed that 45% (95% confidence intervals [CIs] of 30–53%) of the inter-individual variation in FI was attributable to additive genetic effects and 52% (95% CIs 47–57%) to the respondents’ unique environment. In this analysis, environmental factors that were shared by twins had a non-significant effect on FI (3%; 95% CIs 0–16%).
Association Between Frailty and Father's Occupational Classification
In the second analysis, standard hierarchical multiple linear regression was conducted to construct a model to describe the relationship between a specific element of shared environment — father's occupational classification — and FI. Covariates were tested in blocks in a logical order, following a life-course conceptualization as follows: (1) the intrinsic characteristics of birth weight and age; then the mid-life characteristics of: (2) age at end of education and (3) marital status; (4) concurrent health behaviors of physical exercise, smoking and alcohol consumption; and (5) father's occupational classification. As father's occupational classification was the key predictor variable, this was included last in the model.
Overall, the regression model comprising a number of specific unique and shared environmental characteristics explained 20.6% of the observed variance in sqrt[FI] (Table 2). Father's occupational classification was seen to make a small, though significant contribution to the observed variance in sqrt[FI] in the final regression model. Thus, a professional or clerical occupational status for one's father (as opposed to the reference manual status) resulted in a small (R 2 change of 0.3%) but statistically significant (p < .05) reduction in mean sqrt[FI]. The largest contributors to variance in FI in the regression model were age and birth weight (Model 1; 11.9%) followed by the health behaviors (Model 4; 7.5%) of exercise, smoking, and alcohol consumption, which were statistically significant in the final model (with the exception that being an ex-smoker rather than having never smoked had no effect). The only marital status that was significant in the final regression model was the never-married status: people who had never married had a mean sqrt[FI] which was 0.028 higher than that of married people. As health behaviors and marital status are aspects of one's unique environment, these results are consistent with those of the standard structural equation modeling described above. In contrast, the fact that father's occupational classification, part of one's shared environmental characteristics, was significant in the logistic regression analysis, appears to run counter to the results of standard structural equation modeling described above, which did not find a significant effect of shared environment in its totality.
Testing for Moderation and Mediation Relationships
To further understand the pathway linking father's occupational classification and sqrt[FI], moderation and mediation analyses were conducted. Marital status, physical exercise, smoking history, and alcohol consumption had no statistically significant moderating effect on the observed effect of father's occupational class on frailty and can therefore be said to exert a direct effect on FI.
However, mediation analysis revealed that father's occupational classification exerts its effect on sqrt[FI] via birth weight and (separately) via age at end of education. The total effect of father's occupational classification (now considered as a binary, categorical variable — either manual or non-manual) on sqrt[FI] was -0.0329 (p < .0001); the direct (unmediated) effect was -0.0310 (p < .0001), leaving a small but statistically significant indirect effect of -0.0019 (95% CIs of -0.0037, -0.0006) that is the part of the effect of father's occupational classification on frailty that is mediated by birth weight. Similarly, in mediation analysis evaluating the role of age at end of education (used as a continuous rather than a binary variable), the total effect of father's occupational classification on sqrt[FI] was -0.0291 (p < .0001); the direct (unmediated) effect was -0.0234 (p = .0001), leaving a statistically significant indirect effect of -0.0057 (95% CIs of -0.0098, -0.0030) that is the part of the effect of father's occupational classification on frailty that is mediated by an individual's own educational attainment.
Discussion
To our knowledge, this is the first study to have applied the classical twin model (Boomsma et al., Reference Boomsma, Busjahn and Peltonen2002) and standard epidemiological modeling in the same sample of adult twins. It is also the first to examine the heritability of frailty using Rockwood's FI. Genetic variance in frailty has been evaluated in previous studies in twins but using the Fried frailty phenotype (Dato et al., Reference Dato, Montesanto, Lagani, Jeune, Christensen and Passarino2012; Miles, Reference Miles1997). In those studies, heritability was found to explain less than half of the variance in frailty, consistent with the value of 45% found here (by comparison, longevity is just 25% inherited; Christensen et al., Reference Christensen, Johnson and Vaupel2006). Hence, genetic factors (of which there are likely to be many) contribute significantly to frailty. Moreover, the similarity between our finding and those of the previous studies suggests that Rockwood's FI and the Fried frailty phenotype capture similar measures of frailty. That there is a large heritable component to frailty is supported by the fact that the regression model, incorporating a number of selected, shared (viz. father's occupational classification) and unique (viz. education, marital status, and health behaviors) environmental factors (plus age and birth weight), accounted for just 20.6% of the variance in sqrt[FI].
Structural equation modeling found that 52% of the variance in frailty was the result of factors in the respondent’s unique environment, that is, environmental factors not shared with the respondent's twin. Multivariate logistic regression also found that specific aspects of the unique environment — namely, education, marital status, and health behaviors — had a significant association with frailty. However, structural equation modeling also found that shared environment, in its totality, had a non-significant impact on frailty, while multivariate logistic regression analysis found a statistically significant, inverse association between father's occupational classification in childhood and frailty.
There are a number of possible explanations for this apparent contradiction. First, the totality of ‘shared environment’ encompasses many other factors besides father's occupational classification and, although it was significant, the association of father's occupational classification with later life frailty was small. Indeed, it was smaller than the associations between frailty and all the other unique environmental effects incorporated into the model; thus, a person whose father had a professional occupation had a mean sqrt[FI] that was just 0.017 units less than that of someone whose father had been a manual worker. Also, twins may experience the same early life SES factors differently, in which case they are not shared but instead contribute to the unique environmental component.
Another important consideration is that the analyses conducted did not account for potential gene × environment interactions, but rather treated genetic and environmental factors as separate and distinct. However, in so-called epigenetic processes, environmental factors can cause chemical modification of the DNA, most notably changes in levels of DNA methylation, that in turn, alter gene expression. Importantly, these modifications do not involve an alteration in DNA sequence but do confer an additional layer of information within the genome (Bagot & Meaney, Reference Bagot and Meaney2010), that is itself heritable (Burdge et al., Reference Burdge, Slater-Jefferies, Torrens, Phillips, Hanson and Lillycrop2007). DNA methylation is characteristic of the aging process — so much so that it acts as an ‘epigenetic clock’ that generally correlates with chronological age across the life course. Thus, aging has an impact on the processes of DNA methylation, but, importantly, changes in DNA methylation can also affect the aging process. Indeed, studies have shown that low childhood and adult SES (Needham et al., Reference Needham, Smith, Zhao, Wang, Mukherjee, Kardia and Diez Roux2015) and adverse environmental factors and lifestyle (Fraga et al., Reference Fraga, Ballestar, Paz, Ropero, Setien, Ballestar and Esteller2005) are associated with changes in levels of DNA methylation, thus accelerating the epigenetic clock and one's biological age.
In turn, the epigenetic clock has been shown to be associated with measures of health, including markers of mental and physical fitness, in a population of older people (Marioni et al., Reference Marioni, Shah, McRae, Ritchie, Muniz-Terrera, Harris and Deary2015). Associations have also been seen between frailty and both global (Bellizzi et al., Reference Bellizzi, D'Aquila, Montesanto, Corsonello, Mari, Mazzei and Passarino2012) and locus-specific (Collerton et al., Reference Collerton, Gautrey, van Otterdijk, Davies, Martin-Ruiz, von Zglinicki and Strathdee2014) methylation levels (Breitling et al., Reference Breitling, Saum, Perna, Schöttker, Holleczek and Brenner2016).
Hence, it is possible that SES, in the form of father's occupational status, exerts its effects on frailty via an epigenetic interaction. Thus, in the current study, father's occupational status had a statistically significant association with frailty as shown by the results of multiple regression, but it may be that no effect for shared environment could be discerned in structural equation modeling because the analysis did not account for those epigenetic interactions.
Taken together, these results suggest that variance in frailty is predominantly genetically determined and highly age-dependent, but that it is also shaped by environmental factors, especially one's own health behaviors and characteristics such as marital status and education. Moreover, there is a small effect of childhood SES, as measured by father's occupational classification, which acts across the life course. This is in accordance with a large body of literature demonstrating that the effects of childhood disadvantage persist into adulthood (Adler & Stewart, Reference Adler and Stewart2010; Bengtsson & Lindstrom, Reference Bengtsson and Lindstrom2000; Crimmins & Cambois, Reference Crimmins, Cambois, Robine, Jagger, Mathers, Crimmins and Suzman2003) and lend support to a life-course approach to health. However, the effect seen is mediated through educational attainment, highlighting the importance of education as a pathway to countering familial disadvantage. These results are consistent with theories of frailty expounded by Rockwood and coworkers in which a high deficit count does not necessarily signify a high absolute risk to an individual, but rather a relative increase in risk, as risk can be exacerbated by factors such as social vulnerability (Rockwood et al., Reference Rockwood, Mitnitski and Howlett2015) or mitigated by protective factors including education, exercise, economic status, not smoking, social engagement, and urban living (Wang et al., Reference Wang, Song, Mitnitski, Fang, Tang, Yu and Rockwood2014).
This study had a number of limitations. First, the study sample was comprised of volunteers. This is a necessary aspect of the TwinsUK initiative as twins can only be enrolled voluntarily in the United Kingdom, but it may result in non-representativeness, as higher SES groups are known to be more likely to participate in voluntary studies than lower SES groups (Freudenstein et al., Reference Freudenstein, Arthur, Matthews and Jagger2001; Goldberg et al., Reference Goldberg, Chastang, Leclerc, Zins, Bonenfant, Bugel and Imbernon2001; Hoeymans et al., Reference Hoeymans, Feskens, Van Den Bos and Kromhout1998; Purdie et al., Reference Purdie, Dunne, Boyle, Cook and Najman2002). In addition, the final core sample, comprising respondents for whom there was an FI measurement, was entirely female and almost entirely white. Of note too, although about one third of twins in the United Kingdom are MZ and the rest DZ, this sample was made up of approximately 50% MZ and 50% DZ twins. Although participants have previously been shown to be comparable to age-matched population singletons across a wide range of physiological characteristics, as well as alcohol and tobacco consumption (Andrew et al., Reference Andrew, Hart, Snieder, de Lange, Spector and MacGregor2001), caution is needed in extrapolating the results of this study to the population as a whole.
There are also a number of well-known limitations to the classical twin model. First, recent evidence shows that MZ twins may not share 100% of their variable DNA as has been assumed (Bruder et al., Reference Bruder, Piotrowski, Gijsbers, Andersson, Erickson, Diaz de Stahl and Dumanski2008) which would lead to underestimates of heritability. Second, the classical twin model assumes an equivalent shared environment for MZ and DZ twins. Although numerous studies have broadly upheld this assumption (Derks et al., Reference Derks, Dolan and Boomsma2006; Kendler et al., Reference Kendler, Neale, Kessler, Heath and Eaves1993; Scarr, Reference Scarr1968), some have challenged it, though only for subjective traits, for which MZ twins may rate themselves more similarly than DZ twins (Richardson & Norgate, Reference Richardson and Norgate2005; Tishler & Carey, Reference Tishler and Carey2007). Notably, if MZ twins shared environment more than DZ twins, heritability estimates would be inflated. Third, the classical twin model assumes no assortive mating on the traits of interest, an assumption that may not hold in considerations of SES. However, assortive mating on SES would likely lead to an underestimate of heritability of frailty and an overestimate of shared environment, as DZ twins would share more than half of their variable hereditary information, an observation that was clearly not seen. In addition, the classical twin model assumes that there are no non-additive genetic effects. In practice, such effects are present only when dominance at a key locus exists, or when two loci interact. As frailty is likely to be a highly polygenetic trait, violation of this assumption appears unlikely. A further limitation expounded upon above is that the analyses did not account for possible epigenetic pathways.
One must also note the study's reliance on subject's recall of both birth weight and father's occupation. Recall bias is a common and significant challenge in social science research. One study has reported that agreement between social class of father recalled in adulthood and that measured in early life is ‘moderate’ (Batty et al., Reference Batty, Lawlor, Macintyre, Clark and Leon2005). The fact that the current study is comprised of twins provided a method of checking consistency of responses. In fact, 76% of twins gave identical responses to the question about their father's occupation, similar to the proportions seen in a previous study (Krieger et al., Reference Krieger, Okamoto and Selby1998). In general, however, it seems likely that associations between childhood social class based on adult recall of parental occupation and health outcomes will underestimate real effects.
Whether childhood SES has a significant effect on frailty in this twin sample because they are relatively young and whether the effects of childhood SES on frailty will dissipate as the population gets older and childhood events become more distant remains unresolved. The TwinsUK study will continue to evaluate this sample as they age so it will be possible to see whether the effects of father's occupational classification on frailty dissipate as respondents become older and whether conditions in adulthood become more important for frailty. If so, this would indicate that more distant experiences and events may become secondary to more proximal ones across the life course, as has been found by others (Herr et al., Reference Herr, Robine, Aegerter, Arvieu and Ankri2015).
Conclusions
Variance in frailty in a sample of twins, aged from 40.0 to 84.5 years, enrolled in the St. Thomas' UK Adult Twin Registry, was seen to be approximately equally attributable to heritable factors and those inherent in one's unique environment. Age was significantly and directly associated with frailty, while being married, never smoking, drinking alcohol, and engaging in physical exercise were inversely associated. In addition, a small but significant association was seen between frailty and father's occupational classification, mediated by birth weight and an individual's own educational attainment.
The advent of frailty in an older person has serious health implications, but if those most likely to develop frailty can be identified it may be possible to devise strategies to prevent progression into frailty. With aging populations seen as a global challenge, such strategies may enable the extension of healthy life, reduce dependence, improve quality of life, and reduce burden on governments. Our study demonstrates that prevention and management of this complex, multidimensional condition calls for a multifaceted approach that includes addressing deleterious environmental factors, some of which may act across the life course.
Financial Support
This work was supported by the Wellcome Trust (T.S., grant number 081878/Z/06/Z).
Conflict of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All respondents provided informed consent that was approved by St Thomas’ Hospital Research Ethics Committee.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/thg.2016.72.






