To the Editor—The contamination of the environment and the hands of health professionals, transfer of patients, and movements of health professionals between hospitals are all possible routes for the dissemination of Acinetobacter baumannii. Reference Harding, Hennon and Feldman 1 – Reference Weber and Rutala 3 In our region during 2004–2008, an endemic carbapenem-resistant A. baumannii (CRAb) was detected.Reference dos Santos Saalfeld, Viana, Dias Siqueira, Cardoso, Garcia and Tognim 4 Later, it was verified (2011–2014), with a change in the dissemination mode of this microorganism (ie, the endemic situation to polyclonal dissemination).Reference Viana, Zago, Menegucci, Zarpellon, Nishiyama, Cardoso and Tognim 5 However, the routes of spread of A. baumannii have not yet been established.
In this study, we analyzed the effect of constructing a new ICU in a Brazilian hospital on the dissemination of A. baumannii. In the first 6 months, 22 clinical isolates were collected from an old ICU (12 beds), and in the next 6 months, 26 clinical isolates were collected from a newly installed ICU (24 beds).
In the new ICU, the presence of A. baumannii in the environment was investigated for a period of up to 15 days before and 15 days after patient admission to the unit. The samples were collected from bedside table, antiseptic dispenser, cardiac monitor, infusion pump, and bedrail, using sterile swabs moistened with sterile saline solution. Each swab was then used to inoculate a MacConkey agar plate.
The identification and antimicrobial susceptibility of bacterial isolates were assessed using a BD Phoenix system (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). The minimum inhibitory concentrations of imipenem, meropenem, and polymyxin B were confirmed using the agar-dilution method. 6
A multiplex PCR assay was performed to detect the presence of MBL genes (bla IMP, bla VIM, bla GIM, bla SPM, and bla SIM) and oxacillinase genes (bla OXA23, bla OXA24, bla OXA51, and bla OXA58).Reference Ellington, Kistler, Livermore and Woodford 7 , Reference Woodford, Ellington and Coelho 8
Molecular typing was performed with enterobacterial repetitive intergenic consensus-polymerase chain reaction (ERIC-PCR) assays. Computer-assisted analysis was performed with BioNumerics version 6.5 software (Applied Maths, Sint-Martens-Latem, Belgium) with Dice correlation coefficient≥0.93.Reference Silbert, Pfaller, Hollis, Barth and Sader 9
In total, 48 A. baumannii were isolated. The clinical and colonization isolates were obtained from tracheal aspirates (n=26), urine (n=6), blood (n=5), cerebrospinal fluid (n=1), wound secretion (n=1), and nasal swabs (n=4), oral swabs (n=4), and axillar swabs (n=1). During the study period, no patient infected or colonized with A. baumannii was transferred from the old to the new ICU, and no A. baumannii isolates were detected in the environment of the new ICU.
Of the 48 isolates, 65% and 50% were resistant to imipenem and meropenem, respectively. The most effective of the antibiotics tested was polymyxin B (100% sensitivity), followed by tetracycline (73%) and tobramycin (52%). Comparing the isolates of A. baumannii from the 2 ICUs revealed an increase in resistance to imipenem in the isolates from the new unit (from 50% to 69%).
All isolates carried bla OXA51, and 29 (60%) also carried bla OXA23 (14 isolates from the old ICU and 15 isolates from the new unit). No strain was identified as a producer of MBL, OXA-58 or OXA-24.
The findings that 60% of the A. baumannii isolates showed the bla OXA23 gene and that 11 (38%) were susceptible to carbapenems can be explained. The expression of resistance depends not only on the presence of the bla OXA23 gene but also on its association with an insertion sequence, such as ISAba1, which enhances the expression of the bla OXA23 gene. These findings are worrisome because this insertion sequence may be inserted into a plasmid that has a high capacity for mobilization and dissemination.Reference Turton, Ward and Woodford 10
Molecular typing by ERIC-PCR of the 48 A. baumannii isolates detected 17 different clusters (Fig. 1, A–Q). Two clusters were detected in both ICUs (I and J). Cluster J was detected in a patient admitted to the old ICU (negative for bla OXA23) and in another patient admitted to the new ICU (positive for bla OXA23). Cluster I was detected in 10 patients (5 in the old and 5 in the new ICU). This cluster was identical to the endemic CRAb detected in our region.Reference Viana, Zago, Menegucci, Zarpellon, Nishiyama, Cardoso and Tognim 5
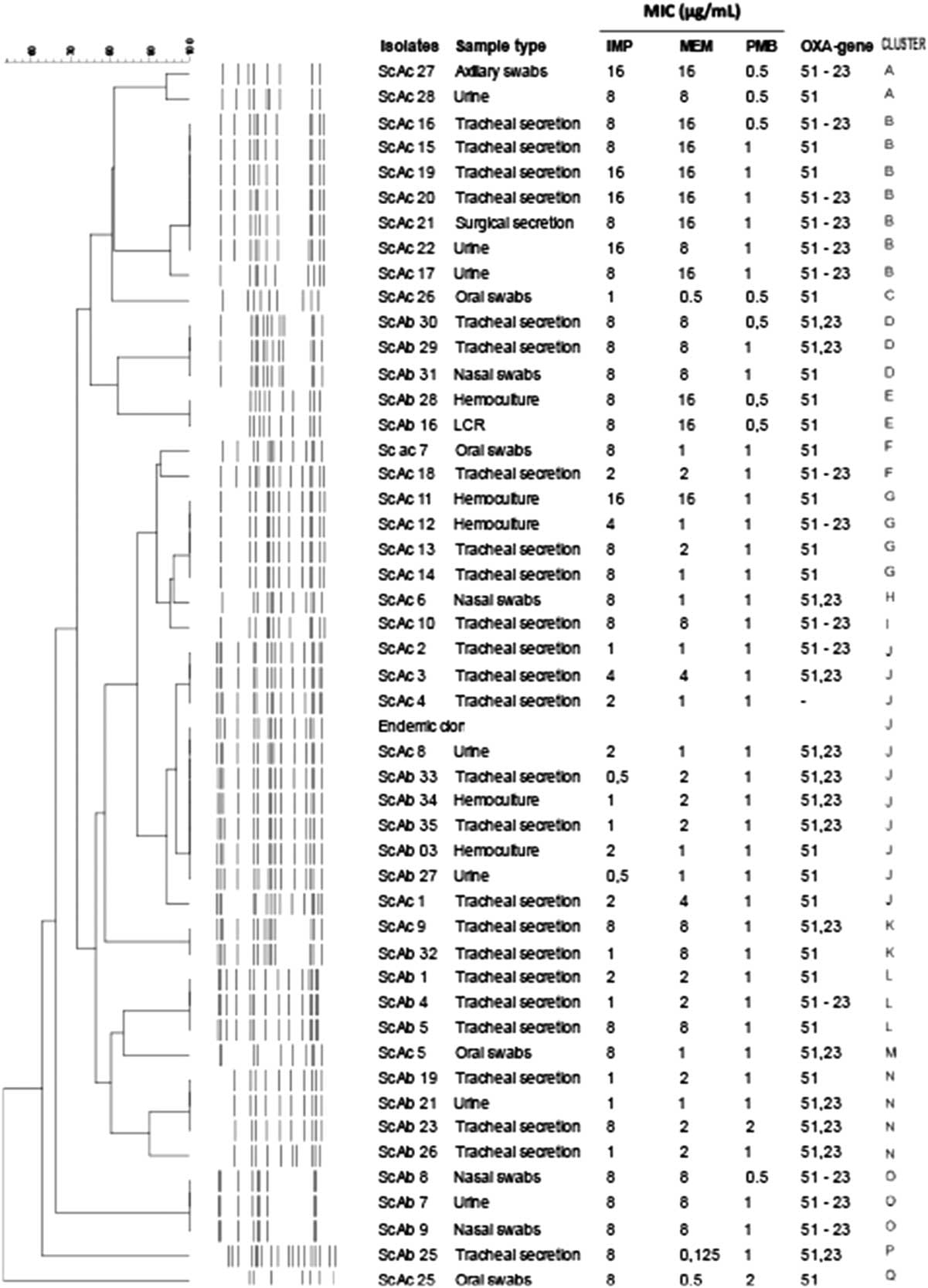
Fig. 1 Dendrogram, minimum inhibitory concentration (MIC) for imipenem (IMP), meropenem (MEM), and polymyxin B (PMB) of A. baumannii isolates from 2ICUs of Brazilian hospitals. Isolates from the old ICU are designated “ScAb,” and isolates from the new ICU are designated “ScAc.”
The old ICU showed no distinct clusters, and the new ICU had 10 clusters. The most common clusters were B, I, and H, which comprised ~50% (23 of 48) of all A. baumannii isolates. Clusters B and H were isolated only in the new ICU, while cluster I was detected in both units. All clusters showed at least 1 isolate carrying bla OXA23.
Molecular typing revealed a certain degree of clonal diversity, and despite the construction of the new ICU, several A. baumannii–producing OXA-23 coexist, making control more difficult. Moreover, the same endemic cluster persisted in both ICUs, that is, it was transferred to a completely new ICU.
Although many studies have demonstrated the importance of environmental contamination in the dissemination of pathogens,Reference Harding, Hennon and Feldman 1 – Reference Weber and Rutala 3 our study showed that an environment free of contamination was not sufficient to control either the dissemination of an endemic carbapenem-resistant or the emergence of new clusters of A. baumannii.
The presence of cluster I in both ICUs, identical to the endemic cluster described by Saalffeld et al,Reference dos Santos Saalfeld, Viana, Dias Siqueira, Cardoso, Garcia and Tognim 4 demonstrates the importance of interhospital dissemination. Although the environment was not the main cause of dissemination, we believe that the contamination of the hands of healthcare professionals may have contributed to the dissemination of A. baumannii isolates, and the failure to verify this dissemination route was a limiting factor in our study.
Our study showed that after the ICU was re-established in a new building (ie, a new ICU), the dissemination of endemic clone–producing OXA-23 was maintained even though the new ICU environment was not contaminated. This occurrence demonstrates that additional measures are required to control the dissemination of this important hospital pathogen.
Acknowledgments
We thank Dr Janet W. Reid (JWR Associates) for editing the English text.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.



