Gum arabic is a dried exudate of the acacia tree (Acacia senegal or Acacia seyal), a tree commonly encountered in various tropical and subtropical parts of the world, especially in Africa. It is a heteropolysaccharide of high molecular weight (approximately 350–850 kDa) containing galactose, rhamnose, glucuronic acid and arabinose residues, but also minerals like calcium, potassium and magnesium(Reference Williams, Phillips, Phillips and Williams1). The total amount of protein is limited to less than 3 %. Gum arabic is highly soluble in water, concentrations up to 40 % are feasible without a major impact on viscosity(Reference Williams, Phillips, Phillips and Williams1), rendering it an attractive candidate compound for various applications, like beverages.
One of the most promising applications linked with gum arabic, due to its relative inaccessibility to the various enzymes within the small intestines, concerns its use as prebiotic, being defined as a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon without stimulating that of unwanted bacteria, and thus contributes to host well-being and health(Reference Gibson and Roberfroid2, Reference Roberfroid3). Various studies mention its potential as a prebiotic agent. Wyatt et al. (Reference Wyatt, Bayliss and Holcroft4) addressed the issue in one volunteer by applying 10 g gum arabic and noted an increase in the numbers of Bacteroides and Bifidobacterium. In healthy human volunteers Cherbut et al. (Reference Cherbut, Michel, Raison, Kravtchenko and Meance5) demonstrated that consumption of 10 and 15 g/d for 10 d yielded an increase in counts of both lactic acid-producing bacteria and Bifidobacteria as determined in faecal samples. Using in vitro techniques Michel et al. (Reference Michel, Kravtchenko, David, Gueneau, Kozlowski and Cherbut6) as well as May et al. (Reference May, Mackie, Fahey, Cremin and Garleb7) yielded conflicting results on the selective outgrowth of the unwanted Clostridium difficile. The latter might be explained by a difference of the host providing the faecal sample: man in the former and pigs in the latter. Moreover, differences in the production of SCFA upon incubation of various fibre sources were also encountered(Reference May, Mackie, Fahey, Cremin and Garleb7).
The effect on the composition of SCFA in vitro (Reference May, Mackie, Fahey, Cremin and Garleb7, Reference Annison, Trimble and Topping8) and enhancement of gut membrane function underline the potential functionality of gum arabic on gastrointestinal health as well. As demonstrated in experimental animal models, optimizing the host resistance in the colon(Reference Campbell, Fahey, Lichtensteiger, Demichele and Garleb9, Reference Rehman, Codipilly and Wapnir10) represents another biological function associated with the consumption of gum arabic. Although a possible effect on lowering of the glycaemic index is also suggested, scientific proof is lacking yet. All of these considerations render gum arabic a potentially interesting compound for gastrointestinal health functionality. Moreover, gum arabic is already used as a coating agent and it is known to enhance the survival of probiotica under physiological conditions in the gut(Reference Desmond, Ross, O'Callaghan, Fitzgerald and Stanton11, Reference Ross, Desmond, Fitzgerald and Stanton12).
Since most of the studies mentioned earlier concern in vitro and animal models, there is a lack in fundamental understanding in the use of gum arabic in man. Especially the impact of gum arabic consumption on the qualitative and quantitative composition of micro-organisms within the colon flora in healthy man has not been evaluated over a relatively long period of time. To assess the prebiotic potential during 4 weeks of consumption, gum arabic was compared with that of a well-established compound, inulin(Reference Kruse, Kleessen and Blant13), and with water as the negative control because the various doses of gum arabic were dissolved in water. Therefore the aim of the present study was to investigate a potential prebiotic functionality by gum arabic, and if any, establish a dose–effect relationship after a period of 4 weeks of daily consumption.
Methods
Material
Gum arabic (EmulGold®) was provided by Kerry Ingredients (Cam, UK) in a spray-dried form as a water-soluble free-flowing food-grade powder. EmulGold® consists of at least 80 % soluble fibre and 3 % protein. Gum arabic has GRAS status (E414, EU directive 95/2/EC); no recommended daily dose has been established so far.
Inulin (Fibruline® Instant), kindly provided by Cosucra (Warcoing, Belgium), is a fructo-oligosaccharide polymer derived from the chicory root. It was also obtained as a spray-dried powder and typically consists of at least 90 % non-digestible fibre and a maximum of 10 % free fructose, glucose and saccharose. The mean degree of polymerization is 9. All test products were of food-grade standard.
Subjects and study design
The present study was performed as a randomized, double-blinded, double-controlled trial with six parallel test groups. The study protocol was approved by the Medical Ethics Committee of the University of Maastricht, The Netherlands (MEC 05-148) and conducted in accordance with the World Medical Association Declaration of Helsinki. Healthy volunteers (n 54) were initially selected according to specific inclusion criteria, such as BMI between 19 and 27 kg/m2; normal blood cell counts and normal non-fasted blood glucose concentrations; absence of glucose and/or albumin in urine; no use of medication affecting gastrointestinal physiology, such as antimicrobial drugs; no use of specific supplements, functional foods or other products claiming specific pro- and/or prebiotic effects (yoghurts, etc.). Every volunteer was asked to sign the Informed Consent Form. In total eighty-one subjects were screened for the study of which fifty-one actually started. At the onset of the trial three subjects dropped out due to various personal reasons. Individuals were randomized over the various groups.
Experimental procedures
Every volunteer was randomly assigned to one of the six groups via Latin-square arrangement with date of entry as controlling factor. Subjects consumed either 5, 10, 20 or 40 g EmulGold®/d, water as a negative control or a dose of 10 g Fibruline/d for 4 weeks.
Every product was dissolved in 250 ml water and consumed at around 10.00 hours. The negative control consisted of 250 ml water without any addition. Volunteers were instructed to maintain their usual eating habits throughout the entire study period.
At the start of the study, as well as at 1, 2 and 4 weeks the volunteers were asked to collect two fresh faecal samples of approximately 10–15 ml in special tubes via a standardized method and store them directly in the freezer ( − 20°C) or in the fridge at 4°C if they did not have access to a freezer. They were asked to deliver the samples to the laboratory as quickly as possible.
At weeks 0, 1, 2 and 4 volunteers were requested to rank their well-being with respect to gastrointestinal drawbacks, such as abdominal pain, intestinal bloating, flatulence, nausea, borborygmi, diarrhoea and constipation. The respective ranks given were: 0 = none, 1 = mild, 2 = moderate, 3 = severe. Moreover, stool frequency and consistency were also monitored and ranked as follows: 1 = watery/diarrhoeal, 2 = soft, 3 = normal, 4 = hard.
Microbial determination of frozen stool samples
The frozen samples were regularly collected and sent to the appropriate microbiological laboratory (Laboratorium Pro Health BV, Weert, The Netherlands). Quantification of the various genera or species of bacteria, Bifidobacteria, Lactobacilli, Bacteroides, C. difficile and Enterococci, was achieved via real-time PCR with 16S ribosomal DNA-targeted genus-specific primers(Reference Rintilä, Kassinen, Malinen, Krogius and Palva14) (Table 1) using the LightCycler® System (Roche Diagnostics, Mannheim, Germany). The used primers showed a high PCR efficiency (96·5–100·1 %) and no cross-reactivity with other micro-organisms(Reference Rintilä, Kassinen, Malinen, Krogius and Palva14). Extraction of DNA from the respective faecal samples was done via QIamp® DNA stool mini kit (Qiagen, Hilden, Germany). The amount of PCR product within each amplification cycle was determined via SYBR Green I fluorescence intensity. For each bacterial group, or species as in the case of C. difficile, standard curves were made by plotting threshold cycles obtained after real-time PCR analyses of different culture dilutions of bacterial strains representative for the groups. This enabled direct comparison between the threshold cycle and the 10-logarithm of the number of bacteria, as established via plate counting(Reference Matsuki, Watanabe and Fujimoto15, Reference Gueimonde, Tölkkö, Korpimäki and Salminen16). From the precise amount of faeces obtained the results were calculated and given as log10 number of bacteria/g wet faeces.
Table 1 Primers used within the present study to quantify the various (groups of) bacteria by means of real-time PCR

Power analysis
Since to our knowledge no similar study has been conducted, data from Kruse et al. (Reference Kruse, Kleessen and Blant13) were used. The α has been set at 0·05 and the power (1 − β) at 0·8, for a one-sided test. The formula used for the power analysis(Reference Geller and Pocock17) was:
in which Z is the value to determine that a standard normal deviate has certain probability of exceeding a specified value; σ is the standard deviation of the outcome variable, assumed to be equal for both treatments; δ0 is the smallest treatment effect which has to be detected with probability of at least 1 − β.
Calculation revealed that in this parallel study a number of at least eight persons is needed, taking into account a drop-out rate of one person per group.
Statistical analyses
Bacterial numbers were expressed as log10 numbers/g wet faeces. The Δ values (log10 numbers at t x (x = 1, 2 or 4) minus log10 numbers at t 0) per individual were calculated and used for statistical evaluation. Since every group consisted of different individual subjects (parallel test group design) unpaired tests were applied for statistical analysis between groups and paired tests within groups.
Initially, t tests were used to detect differences between groups at various time intervals and between groups receiving various doses. Subsequently, via ANOVA and multiple regression(Reference Armitage18), the effect of the various doses of gum arabic on the change in log10 number of bacteria over 4 weeks was addressed. The formula used(Reference Armitage18) was:
in which y = Δ log10; X is the dose of gum arabic used; a 1–4 are coefficients.
Significance was obtained via t test and F test. The software used for the statistical analyses was STATA version 8 (Stata Corp., College Station, TX, USA).
With respect to the adverse effects as experienced by the volunteers the results were standardized between 1 (100 % increase in occurrence) and − 1 (100 % decrease in occurrence).
Throughout the study P values of 0·05 or lower were considered to be significant.
Results
Compliance of volunteers
Table 2 provides information on baseline characteristics of the various groups. There were no statistically significant differences in these characteristics between the various groups. Subjects' compliance to the protocol was assessed on the basis of the test product intake as recorded in the study diaries. In all test groups compliance was found to be very high: 97·0–99·6 %.
Table 2 Demographic baseline characteristics of the study population used for statistical analyses*
(Mean values and standard deviations)

* For details of subjects and procedures, see Methods.
Quantification of bacterial numbers over 4 weeks of intervention
The actual numbers of the various groups of bacteria at the start and at the end of the period are listed in Table 3. There were no significant differences observed in the initial number of the various bacteria between the different groups of volunteers. Analyses were done on the difference in the 10-logarithmic number at 4 weeks to that at the start. Only in the volunteers who consumed gum arabic was a significant change in these bacterial numbers observed. At a dose of 10 g a significant increase in Bifidobacteria, Lactobacilli and Bacteroides was noted. A decrease in numbers was found for Lactobacilli at a dose of 20 g and for Bacteroides at a dose of 40 g.
Table 3 The 10-logarithmic numbers of bacteria within the various groups at the start (week 0) and at the end (week 4) of the study†
(Mean values and standard deviations)

Mean values were significantly different from those of week 0 (paired t testing (one-sided)): *P < 0·05, **P < 0·01.
† For details of subjects and procedures, see Methods.
Bifidobacteria spp.
The change in numbers of Bifidobacteria within the faeces followed a dose-dependent effect (Table 4). The increase in the numbers of Bifidobacteria in subjects having consumed 10 g gum arabic/d was significantly (P < 0·01) higher than in those who consumed 0 g gum arabic (water, negative control): approximately 40 fold difference in outgrowth. Moreover, at a dose of 10 g this increase was also significantly (P < 0·05) higher for gum arabic than for inulin: an approximately 10-fold difference. Higher doses of gum arabic did not lead to an additional effect.
Table 4 The change in 10-logarithmic numbers of bacteria during 4 weeks of consumption of various doses of gum arabic, 10 g inulin or water (Mean values and standard deviations)

Mean values were significantly different from those of water (t testing (one-sided)): *P < 0·05, **P < 0·01.
† For details of subjects and procedures, see Methods.
Using the regression model the optimal dose with respect to the highest number of bacteria was found to be around 10 g gum arabic.
Lactobacilli spp.
After consuming 10 g gum arabic/d for 4 weeks the increase in the number of Lactobacilli was significantly (P < 0·05) higher than observed at 0 g gum arabic (water, negative control): an approximately 6-fold difference in numbers. Interestingly, consumption of 10 g gum arabic yielded a significantly (P < 0·03) higher increase in numbers than that observed for 10 g inulin: approximately 7-fold (Table 4).
An optimum dose of EmulGold® with respect to the numbers of Lactobacilli was established at about 5–10 g.
Bacteroides spp.
No differences in the Δ numbers of Bacteroides were detected between volunteers consuming gum arabic for 4 weeks and those consuming water, except at a dose of 10 g at which approximately a 2-fold increase was found. Interestingly, intake of 10 g gum arabic yielded a significantly (P < 0·01) higher increase in counts than that of 10 g inulin (approximately 2·5-fold; Table 4).
Relatively large changes in numbers of Bacteroides were found at doses of 5 and 10 g gum arabic. As was observed with the Lactobacilli the number of Bacteroides declined at higher doses, this difference being significant (P < 0·01).
Clostridium difficile
No differences in the numbers of C. difficile at the start and at 4 weeks were encountered between the various groups (Table 4). However, the variation in numbers per time-point was relatively large, especially after consumption of higher doses of gum arabic, but also at the start of the intervention. No significant differences were identified, not only between water and the various doses of gum arabic, but also between inulin and gum arabic. There was no dose-dependency in the changes of bacterial numbers after 4 weeks of intervention.
Enterococci spp.
In general, during the 4 weeks of intervention a non-significant reduction in bacterial numbers was encountered, especially in the water group (Table 4). Statistically significant differences in the numbers of bacteria between the various test groups were not encountered. A potential dose-dependency in the outcome for gum arabic was not seen.
Potential drawbacks associated with the intake of gum arabic for 4 weeks
Stool frequency and stool consistency were monitored throughout the study and various gastrointestinal symptoms were assessed via questionnaire: consistency of faeces, abdominal pain, nausea, borborygmi, colic cramps, bloating, flatulence, diarrhoea and constipation. Overall, all subjects tolerated the 4 weeks of consumption of gum arabic even at the highest dose of 40 g/d very well. Changes in the reported gastrointestinal symptoms were minimal and not significant during the intervention period as compared to subjects who consumed the negative or positive control. As an example of the discomfort the change in the intensity of diarrhoea is given in Fig. 1. The values below 0 represent those people who listed diarrhoea at the previous measurement. Evidently, the 4 weeks of consumption of all investigated compounds and doses did not result in a substantial diarrhoeal incidence.

Fig. 1 Mean change in intensity (in arbitrary units (AU)) of diarrhoea in the various test groups during the intervention as reported via questionnaires. Positive values correspond with improving stool consistency whereas negative values correspond with worsening, leading to less consistency. –♦–, Water; ■, 10 g inulin; ▲, 5 g EmulGold®; × , 10 g EmulGold®; *, 20 g EmulGold®; - -♦- -, 40 g EmulGold®.
Discussion
The results of the present study clearly demonstrate that consumption of gum arabic (EmulGold®) at a dose of 10 g/d for 4 weeks is associated with higher numbers of Bifidobacteria and Lactobacilli as compared with that of water (negative control). When compared with the intake of 10 g inulin (positive control) the numbers of Bifidobacteria, Lactobacilli and Bacteroides were found to be significantly higher after gum arabic intake. The effect on numbers of other bacteria was not significantly different from that of water or inulin. It is concluded that gum arabic, by yielding higher numbers of beneficial bacteria without stimulation of unwanted bacteria, can be considered a prebiotic fibre with functionality at least as good as inulin.
An important issue within the prebiotic topic is the balance in the floral composition. The issue of beneficial to the host (or not) is associated with numbers of bacteria in the colon being out of balance. Species such as Bifidobacteria and Lactobacilli are well recognized for their health-improving characteristics (e.g. increase in the colonization resistance or production of SCFA(Reference Jenkins, Kendall and Vuksan19, Reference Duggan, Gannon and Walker20)) and are therefore widely used as probiotics. Moreover, groups of bacteria in the colon consist of various species potentially beneficial or non-beneficial. One example is the Bacteroides group. An interesting finding within this study was the increase in log10 numbers of Bacteroides by gum arabic. As with any group of bacteria, Bacteroides consists of beneficial and non-beneficial members. Recently manuscripts have been published stating the beneficial impact of members of this group on gut health(Reference Turnbaugh, Ley, Mahowald, Magrini, Mardis and Gordon21, Reference Ley, Turnbaugh, Klein and Gordon22). Therefore, research into the contribution of the individual members within bacterial groups might reveal the really health-improving properties.
With respect to the bacterial development, within 4 weeks the present study demonstrated that the most optimal dose of gum arabic to achieve prebiotic efficacy lies around 5–10 g. Interestingly, at higher doses the numbers generally decreased. For Bifidobacteria the numbers remain at the same level as obtained at 10 g, reaching a plateau level. The present finding suggests that Bifidobacteria are able to utilize gum arabic up to doses of 40 g whereas the other bacteria have more difficulty in metabolizing gum arabic at higher doses. This might be explained by competition for substrate(Reference Guiot23): at high doses other bacterial strains have easier access to the substrate and subsequently, less becomes available for the ones determined within the present study. The enzyme systems within Bifidobacteria might be more adapted to degrade gum arabic than those of other micro-organisms. Another option is the composition within the various layers of microflora present in the gastrointestinal tract and subsequent utilization of substrate able to degrade the prebiotic at higher doses: surface-associated v. mucosa-associated flora(Reference Langlands, Hopkins, Coleman and Cummings24, Reference Kleessen, Hartmann and Blaut25). It might be speculated that at higher doses, bacteria present in the mucosa layer are able to degrade the compounds more substantially than at lower doses, simply because they have easier access. To what extent release of compounds by specific gut bacteria as a result of gum arabic fermentation has an impact on the outgrowth of others remains to be seen.
In the present study a real-time PCR assay was used to quantify the number of relevant bacteria in the faecal slurry representing the bacteria in the lumen of the gut. There are various limiting factors in this approach: not only the correlation in the number and composition of bacteria between a faecal slurry sample and the gut lumen, but also the validity of the primers used: how reliable is the binding of the used amino acid array to specific DNA in the stool. As reported(Reference Rintilä, Kassinen, Malinen, Krogius and Palva14), the correlation between DNA and threshold cycles as well as the correlation between bacterially spiked faecal samples and signal were significant. Moreover, in the present study internal validation using multiple analyses within a sample as well as spiking faecal samples with a known inoculum have been performed and showed statistically reliable results (data not shown).
An important characteristic of the present study was the huge variation in bacterial numbers, not only during the study but also at the start. Especially in the group of volunteers consuming 10 g gum arabic the individual variation within the numbers of C. difficile was extreme: more than five log numbers. A clear relation between the initial number of bacteria in the stool and the net increase during intervention has been recognized before(Reference Roberfroid, Van Loo and Gibson26, Reference Rao27) and was obvious from the present study as well (Fig. 2). Fig. 2 reveals a significantly (P < 0·01) negative correlation between the log10 number of C. difficile at the start and the rate of change in numbers after 4 weeks of gum arabic consumption. At low numbers of bacteria at the start the room for a decrease is limited and numbers can only increase, whereas this situation is reversed for high numbers at the start. At the intake of the study no evaluation of the numbers of bacteria in the stool of the volunteers was performed. However, by checking the diet per volunteer, those people who were expected to have a high number of lactic acid bacteria due to yoghurt consumption were excluded from the trial. Room for change in the number of bacteria over time should be achievable by the intervention. Apparently the inclusion criteria should be optimized in the sense that volunteers with either very low or very high numbers at the start should be taken out to minimize bias.
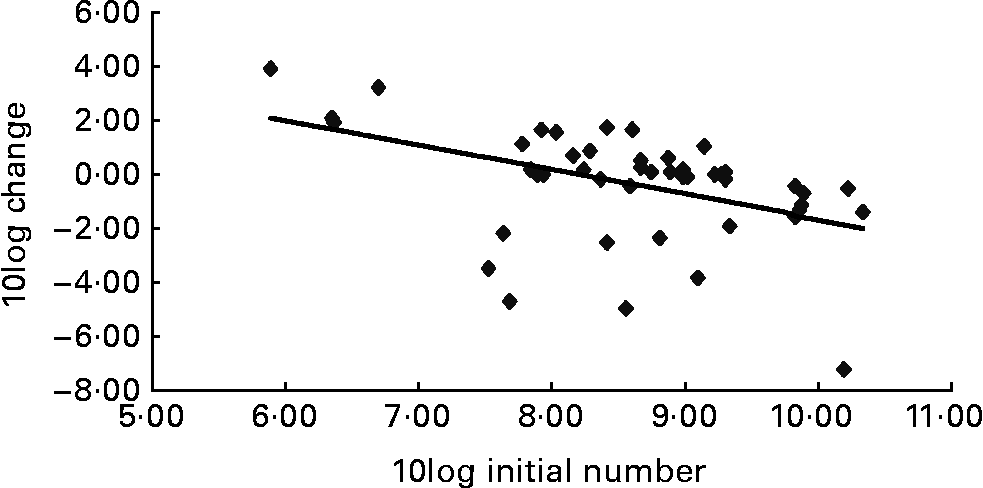
Fig. 2 Relationship between the number of Clostridium difficile at the start of the intervention and the change in numbers 4 weeks later. Every point is one individual, all data pooled. Equation of the regression line: y = − 0·916x+7·50, P < 0·01.
At present there are only a few studies documenting relatively long-lasting prebiotic effects in man by gums in general. With respect to the prebiotic behaviour of gum arabic, other gums demonstrate similar physiological effects upon consumption. Partially hydrolysed guar gum in combination with fructo-oligosaccharides (6·6 and 3·4 g/d respectively) over 21 d yielded a significant increase in Bifidobacteria, but not in Lactobacilli, Bacteroides, Clostridia and Enterococci(Reference Tuohy, Kolida, Lustenberger and Gibson28). Moreover, partially hydrolysed guar seems to play a role in the reduction of inflammatory bowel syndrome as well(Reference Giannini, Mansi, Dulbecco and Savarino29). In vitro research using bacteria isolated from the human colon demonstrated that gum arabic is rapidly utilized by these micro-organisms, more than any other tested compound(Reference Bourquin, Titgemeyer, Fahey and Garleb30). Not only by increasing the numbers of bacteria is the application of fibres well known, but also by stimulating the physiology of bacteria and thereby affecting the release of specific compounds, such as SCFA(Reference Cummings, Macfarlane and Englyst31), and the reduction of compounds with known carcinogenic properties(Reference Saito, Takano and Rowland32).
The results of the present study demonstrate that gum arabic (EmulGold®) bears prebiotic efficacy within a dose range similar to or lower than inulin, as established via the quantitative development of bacteria in stool samples. Taking into account its functional properties within food matrices this compound will be an attractive ingredient for the functional food segment. To what extent it will also exhibit other physiologically relevant properties remains open for further studies.
Acknowledgements
The technical support of Dr Inge Verkooijen (NutriScience, Maastricht, The Netherlands) and Dr Neil Bourke (Kerry Bioscience, Almere, The Netherlands) is gratefully acknowledged. W. C. was the principal investigator for this study and responsible for the design, statistics, evaluation of the data and writing of the various drafts of the manuscript. A. R. W. contributed significantly to the design of the study, the carrying out of the experiment, interpretation and critical reading of the manuscript. C. V. and C. F. were responsible for the delivery of the various products to be tested in the volunteers and contributed in critical reviewing of the manuscript. A. D. S. was involved in the design of the study, theoretical contributions and critical reading of the manuscript. All authors approved the publishing of the final version of the manuscript. None of the authors had a financial or personal conflict of interest in relation to this study.








