n-3 Fatty acids: dietary sources and metabolism
α-Linolenic acid (ALA) is an n-3 fatty acid found in plant-based foods such as green leafy vegetables, nuts and cooking oils. It is important to note that commonly used cooking oils vary a great deal in their ALA content, with oils such as flaxseed oil being particularly rich sources of ALA, with up to 50 g ALA/100 g oil, while sunflower oil contains almost no ALA(Reference Foster, Williamson and Lunn1).
The longer-chain n-3 fatty acids EPA, docosapentaenoic acid (DPA) and DHA are found in a range of animal products, including eggs, meat and milk, but the richest dietary source of long-chain n-3 is fish. White fish such as cod contain about 300 mg EPA + DHA per portion, while oily fish such as salmon can contain over 2 g EPA + DHA per portion. Current UK dietary advice recommends consuming two portions of sustainably sourced fish per week, of which one should be oily fish(2). The most recent National Diet and Nutrition Data(3) reveals that fat from fish and fish dishes accounts for about 4 % of adult total fat intake, with an average total fish consumption of 10–20 g daily for adults, which equates to approximately one serving of white fish every 2 weeks, falling far short of recommendations (Fig. 1). If oily fish is considered separately from total fish intake, current data indicate a median intake of 0 g daily across all age ranges and in both sexes(3), indicating that the vast majority of the UK population never consume oily fish. However, there is a bimodal population distribution of intakes, with adults on the 97·5th percentile of intakes consuming up to 67 g daily, equivalent to consumption of approximately 2–4 portions of oily fish per week.

Fig. 1. The biosynthesis of long-chain PUFA from essential fatty acids in human subjects. Adapted from figure presented in Childs et al.(Reference Childs, Romeu-Nadal and Burdge15).
Of the n-3 fatty acids, only ALA is considered an essential dietary fatty acid. This is because ALA can be converted by a series of desaturation and elongation reactions into the longer-chain n-3 PUFA including EPA, DPA and DHA (Fig. 2). The same series of enzymes is responsible for conversion of the essential n-6 fatty acid linoleic acid into its longer-chain n-6 PUFA including arachidonic acid and n-6 DPA. The proportion of n-6 to n-3 fatty acids consumed in the diet will therefore impact upon which branch of this shared pathway is likely to predominate. Across Europe, recommendations are to consume about 4 % of daily energy from linoleic acid, and 0·5 % of daily energy from ALA, and while these recommendations are not met in up to half of countries assessed(Reference Sioen, van Lieshout and Eilander4), adherence to recommendations will result in consumption of n-6 fatty acids exceeding that of n-3 fatty acids.
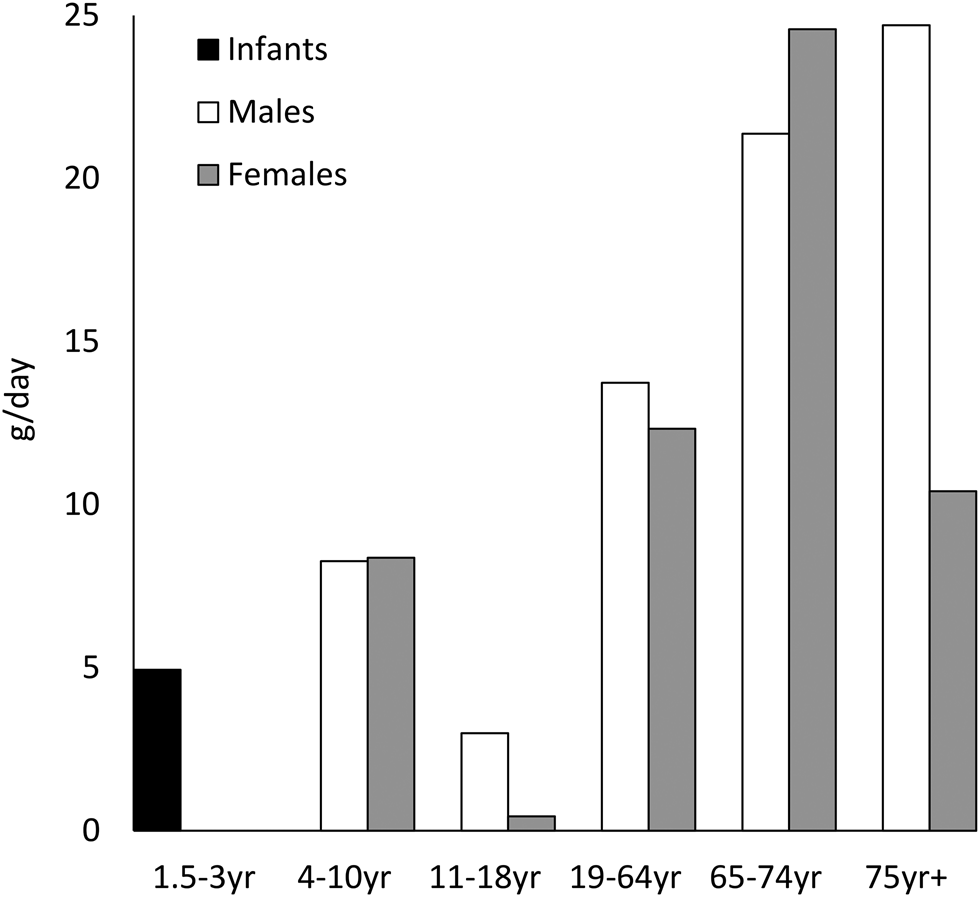
Fig. 2. Figures for median total fish consumption (g/d) reported in the National Diet and Nutrition Survey 2014/15–2015/16(3).
The role of sex in n-3 fatty acid status
A number of observational studies which assess the blood n-3 status in healthy adults have observed sex differences in circulating EPA, DPA and DHA concentrations, which remain after controlling for dietary intakes. Females have been found to have higher serum/plasma DHA content, in both total lipids and within lipid fractions including cholesterol esters, TAG, phospholipids and NEFA(Reference Crowe, Skeaff and Green5–Reference Nikkari, Luukkainen and Pietinen8). These sex differences are also reported in tissue samples, with DHA content in erythrocytes(Reference Metherel, Armstrong and Patterson9) platelets(Reference Geppert, Min and Neville10) and mononuclear cells, buccal cells and adipose tissue(Reference Walker, Browning and Mander11) higher in females. Meta-analyses of such studies have confirmed these sex differences in the DHA status in plasma lipids, phospholipids and erythrocytes(Reference Lohner, Fekete and Marosvolgyi12).
These sex differences are consistent when examining data across ethnicities, with both Caucasian and East Asian females having higher plasma DHA concentrations compared with males(Reference Abdelmagid, Clarke and Roke13). A potential interaction of sex differences with cardiovascular fitness is also indicated in fatty acid metabolism, as males with high levels of cardiovascular fitness have the lowest levels of longer-chain n-6 fatty acids, arachidonic acid and docosatetraenoic acid(Reference Tsou and Wu14).
The likely role of sex hormones in driving the higher DHA status seen in females is indicated by human study data demonstrating higher DHA concentrations among those using hormone therapies such as oral contraceptives and hormone replacement therapy(Reference Childs, Romeu-Nadal and Burdge15). Animal models are frequently used to enable investigation of the mechanisms behind such sex hormone effects. Such studies have revealed both sex differences and the effects of sex hormone administration upon mRNA expression of key enzymes involved in the endogenous synthesis of longer-chain fatty acids. Female rats have been found to have significantly higher mRNA expression of Δ5 and Δ6 desaturases(Reference Extier, Langelier and Perruchot16) and subcutaneous injection of oestrogen has been demonstrated to result in higher expression of Δ6 desaturase and elongase enzymes(Reference Kim, Choi and Park17).
In human studies, biopsies of organs such as the liver and brain are typically only available from those undergoing clinical interventions such as planned surgery, or post-mortem, as it is too invasive and dangerous to permit ethical collection of such tissues from healthy volunteers. Animal model systems therefore provide a valuable opportunity to access tissues from healthy animals undergoing dietary interventions, allowing exploration of the effect of dietary interventions upon tissue fatty acid composition, and of mechanisms which underpin such effects. Animal models also facilitate experimental assessment of the impact of dietary interventions during pregnancy upon the developing offspring. However, care must be taken when interpreting the translational value of data from animal models, as diets provided at times use fatty acid intakes which are not representative of those observed or achievable within human intervention studies. For example, a mouse study to explore the effects of dietary fat intakes provided mice with diets containing 8·3–80 % fat content(Reference Hu, Wang and Yang18). This is in contrast to median UK daily fat intakes of 70 g daily, providing approximately 35 % of daily energy, and even those on the 97·5th percentile consume about 130 g fat daily, providing approximately 60 % of recommended daily energy(3). High-fat diets used in animal research are also often rich in SFA, or are poorly defined, with little description of the relative contribution of saturated, MUFA and PUFA(Reference Engber19).
As is observed in human subjects, female animals including the rat have higher plasma(Reference Lin, Brown and DiMartino20) and erythrocyte(Reference McNamara, Able and Jandacek21) DHA status compared with males. Animal model systems have also identified significant positive correlations between liver DHA content and circulating progesterone, suggestive of an adaptation to ensure adequate DHA availability for the developing fetus during pregnancy. This positive correlation between progesterone and liver DHA content is apparent within both virgin(Reference Childs, Romeu-Nadal and Burdge15) and pregnant female rats(Reference Childs, Hoile and Burdge22). One mechanism which may mediate this effect of sex hormones is via changes to expression of Δ6 desaturase, a key enzyme in the metabolism of n-3 and n-6 fatty acids. Protein and mRNA expression of this enzyme is higher in the liver of female rats compared with males(Reference Kitson, Smith and Marks23), and is increased during pregnancy and positively correlated with circulating progesterone concentrations(Reference Childs, Hoile and Burdge22). This role of progesterone in meditating sex differences in long-chain n-3 status is further supported by in vitro studies using hepatocyte cell lines and primary hepatocyte cultures which identify that progesterone induces dose-dependent increases in Δ6 desaturase mRNA expression(Reference Sibbons, Brenna and Lawrence24).
Sex and α-linolenic acid dietary intervention studies
Given that the vast majority of the UK population never consumes oily fish, it is pragmatic to consider whether dietary ALA might have a role in optimising longer-chain n-3 fatty acid status, particularly for females and during pregnancy. Reviews are available which describe the limited ability of both infants and adults supplemented with ALA to convert this essential fatty acid into longer-chain n-3 fatty acids(Reference Brenna, Salem and Sinclair25,Reference Brenna26) . These reviews identify that increasing dietary ALA is effective at increasing blood EPA status, but does not tend to result in any significant changes to circulating DHA, suggestive of a limitation in the ability of human subjects to convert this essential fatty acid into its long-chain n-3 derivative. However, such studies of ALA supplementation frequently use all male cohorts, with only a minority of studies including female participants. Data from one study of ALA supplementation which included both male and female participants was re-examined to investigate potential sex differences in the response to increasing dietary ALA. This analysis confirmed that females provided with 9·5 g ALA daily had a greater increase in EPA compared with males, but did not demonstrate any significant increases in DPA or DHA status(Reference Childs, Kew and Finnegan27) (Fig. 3).

Fig. 3. Human plasma phospholipid n-3 fatty acid content after consuming a control or α-linolenic acid (ALA)-rich diet for 6 months. Adapted from data presented in Childs et al.(Reference Childs, Kew and Finnegan27). Data are mean values, n 10–13. *Significantly different from males in same dietary group (P < 0·05); †significant effect of diet within same sex (P < 0·05).
Given that sex hormones and pregnancy increase key enzymes involved in DHA synthesis within the liver, it is of interest to investigate the effects of an ALA-rich diet upon liver composition, and whether diet can also alter the expression of desaturase or elongase enzymes involved in n-3 fatty acid metabolism. Studies in rats reveal that there are tissue and lipid fraction specific effects upon longer-chain n-3 fatty acid status with enriched ALA diets. Diets providing 5 % w/w ALA significantly increased plasma phospholipid EPA content, but did not alter plasma DHA content (Fig. 4A)(Reference Childs, Romeu-Nadal and Burdge28), comparable with the effects observed in human dietary intervention studies. Examination of rat liver TAG, however, reveals that an ALA-rich diet significantly increases both EPA and DHA contents (Fig. 4B). Significantly higher liver Δ6 desaturase mRNA expression is observed in rats provided with an ALA-rich diet(Reference Childs, Romeu-Nadal and Burdge28), with inverse effects when a linoleic acid-rich diet is provided(Reference Kim, Choi and Park17). It is therefore possible that human studies are underestimating endogenous synthesis in response to ALA due to the limitations in human tissue accessibility.

Fig. 4. The effect of sex and dietary α-linolenic acid (ALA) upon longer-chain n-3 fatty acid status in rats. Adapted from data presented in Childs et al.(Reference Childs, Romeu-Nadal and Burdge28). Data are mean percentage fatty acid composition values, n 5–6. (A) Plasma phospholipids and (B) liver TAG. Significant effects of diet and sex are observed for all longer-chain n-3 fatty acids in both plasma phospholipids and liver TAG (P < 0·05). †Significant sex × diet interactions are observed for plasma phospholipid DHA (P = 0·002), liver TAG EPA (P = 0·029) and liver TAG DHA (P = 0·026).
Pregnancy and α-linolenic acid dietary intervention studies
Given that pregnancy induces increased Δ6 desaturase expression, most likely under the influence of increasing circulating progesterone, it would be anticipated that providing increased dietary ALA during pregnancy could enhance maternal endogenous DHA synthesis, and thereby increase DHA availability to the fetus. Indeed, animal studies indicate that an ALA-rich diet can achieve brain DHA and immune organ EPA concentrations which are comparable with those obtained with provision of a salmon-oil-rich diet(Reference Childs, Romijn and Enke29). This is suggestive of either specific transport of DHA, or fetal endogenous synthesis of DHA from ALA. The role of the placenta in metabolism and transport of longer-chain n-3 fatty acids requires further investigation, with the potential for fatty acids to act as both key signalling molecules in ensuring appropriate nutrient supply to the developing fetus and in the initiation of parturition(Reference Lewis, Childs and Calder30).
If an ALA-rich diet can be demonstrated to have comparable effects in human trials, it may be appropriate to revise dietary advice given to women during pregnancy to encourage increasing consumption of ALA. However, human studies will of course be limited in their ability to detect such effects if they are apparent only within inaccessible tissues such as the fetal brain and immune organs, and not detectable within readily available biosamples such as maternal plasma, cord blood or placenta. To date, human intervention studies have demonstrated that providing additional dietary ALA during pregnancy and lactation can significantly increase the erythrocyte EPA status of mothers and increase the DHA content of breast milk in early infancy(Reference Valenzuela, Bascunan and Chamorro31) and plasma EPA and umbilical vein and artery DPA concentrations status of neonates(Reference de Groot, Hornstra and van Houwelingen32).
Implications for personalised dietary advice
Women in the UK are advised to eat up to two portions of fish weekly during pregnancy, but not to eat more than two portions of oily fish weekly due to the risk of toxins from environmental pollution(33). Most women interpret this as advice to avoid fish during pregnancy, although the risks arising from avoidance have been identified as exceeding those risks likely to arise from excessive consumption(Reference Bloomingdale, Guthrie and Price34,Reference Hibbeln, Davis and Steer35) . Indeed, studies of communities which are high consumers of fish such as mothers in the Republic of Seychelles who consume on average over 500 g fish per week, indicate that there is a complex interrelationship between the risks of high methylmercury exposure and the protective benefits of a diet with a high DHA content(Reference Lynch, Huang and Cox36).
ALA could therefore prove an important and acceptable dietary alternative to fish during pregnancy, yet women in the UK are currently advised to only eat ‘small amounts’ of vegetable oils during pregnancy(33). Given that the majority of the UK population never consumes oily fish, it may be time to take a pragmatic approach to dietary recommendations for the general population and messaging targeted at pregnant women. Either fish oil capsules or provision of ALA may therefore prove to be acceptable alternatives to oily fish.
It is a challenge for the nutrition science community to consider how we can overcome the challenges of sample availability from human research, which may have resulted in underestimation of the potential impacts of dietary ALA, particularly for pregnant females. Future research will also need to balance the potential effects of dietary ALA with likely interactions with age, genetic polymorphisms in FADS genes(Reference Abdelmagid, Clarke and Nielsen37), BMI and smoking(Reference de Groot, Emmett and Meyer38) and use of hormone therapies such as the contraceptive pill and hormone-replacement therapy when considering other variables which may enhance or reduce the efficacy of targeted nutritional advice.
Acknowledgements
C. E. C. wishes to acknowledge the organising committee for the invitation to speak at the Nutrition Society Spring Meeting on ‘Inter-individual differences in the nutrition response: from research to recommendations’ in April 2019, Dundee.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors. C. E. C. receives research funding from HOST Therabiomics and honoraria to speak at an event organised by Yakult.
Conflict of Interest
None.
Authorship
The author had sole responsibility for all aspects of preparation of this paper.






