INTRODUCTION
Hepatitis E virus (HEV) infection is a major cause of epidemic and acute sporadic hepatitis in many areas of Asia, Africa and Mexico [Reference Labrique1], where HEV is considered hyper-endemic. Traditionally, industrialized countries were considered non-endemic, with most HEV infections in these regions being sporadic and considered to be imported. However, in recent years, enhanced surveillance has detected an increasing number of non-travel-associated HEV infections, especially in Europe and Japan [Reference Okamoto, Takahashi and Nishizawa2]. In addition, unexpectedly high anti-HEV immunoglobulin G (IgG) seroprevalence among the general population has been reported from some industrialized countries, although the rates varied considerably between reports, ranging from 2·3 to 33% [Reference Boutrouille3–Reference Toyoda9].
HEV is a non-enveloped, ribonucleic acid (RNA) virus. Its genome is single-stranded, about 7·2 kb in length and contains three open reading frames. HEV is classified in the family Hepeviridae and consists of genotypes (gt) 1–4 that infect humans and other mammals, and a group of avian HEV strains that probably constitute a separate genus [Reference Meng10]. Of interest, the majority of HEV strains from human cases acquired in industrialized countries are different genetically to those isolated from cases in, or imported from, hyper-endemic countries. This geographical difference in genotype distribution contributed to the hypothesis that HEV gt1 and gt2 lead to hepatitis E with a different clinico-epidemiological entity compared to hepatitis E caused by gt3 and gt4 viruses [Reference Teo11, Reference Teo12] (Fig. 1). In contrast to hyper-endemic regions where HEV gt1 is predominately found during large waterborne outbreaks, autochthonous infections in Europe are usually sporadic and related to gt3. The higher standard of hygienic conditions in industrialized countries makes the waterborne route of HEV transmission unlikely in these regions and might explain the rare occurrence of gt1 infections among autochthonous cases. Thus, it is hypothesized that gt3 is linked to different sources and routes of infection than gt1.

Fig. 1. Summary of epidemiology and transmission of mammalian HEV.
As early as 1990, Balayan and co-workers postulated that HEV could be a zoonosis since a human HEV strain was found to be capable of infecting domestic pigs [Reference Balayan13]. In 1997, HEV gt3 strains were detected in swine in the USA [Reference Meng14]. HEV gt3 and gt4 have since been found to be endemic in pig herds worldwide [Reference Ha and Chae15, Reference Williams16]. Both genotypes, whether from pigs or humans, have been shown to be transmissible to non-human primates and a range of other animal species [Reference Meng17, Reference Zhang18]. Very close homology (>90%) has been found between HEV gt3 nucleotide sequences from pigs, wild boar and humans, suggestive of cross-species transmission [Reference Herremans6, Reference Borgen19]. As HEV gt3 spreads easily in pig herds but poorly in human communities it has been postulated that humans are not the main host or reservoir [Reference Purcell and Emerson20].
The diagnosis of a HEV infection is mainly dependent on serology. In general, a positive result for anti-HEV immunoglobulin M (IgM) antibodies, or a rise in anti-HEV IgG titre, is indicative of an acute infection. Recent studies have shown that the use of commercially available serological tests (which are mainly based on HEV gt1 antigens) in HEV gt3-endemic regions need further confirmation [Reference Herremans6, Reference Herremans21]. Moreover, a comparison of five serological tests showed that the agreement between the tests was moderate at best (kappa ∼0·5) [Reference Bouwknegt22]. A couple of IgG enzyme-linked immunosorbent assay (ELISA) commercial kits were withdrawn from the market or replaced by new tests by the manufacturer due to recognition of a high proportions of false-negative results [Reference Bouwknegt22, Reference Mast23]. There are also many reports on the detection of anti-HEV antibodies in serum samples from different animals [Reference Meng17] but these results are often obtained using tests that are developed and evaluated for detecting anti-HEV antibodies in human samples during the acute phase of the disease. The reliability of these results is therefore questionable.
Detection of a part of the virus, for example HEV RNA, in serum or faecal samples, instead of detection of an immune response against antigens of a virus, is more definite proof for HEV infection. Several reports state that the viraemic phase of HEV in humans is, in general, short and that the virus may disappear from peripheral blood at the height of the disease (reviewed by Jameel [Reference Jameel24]). Nevertheless, HEV RNA might be detectable in faecal samples for a longer period of time [Reference Takahashi25] and recent prolonged infection and shedding has been reported for some immunocompromised patients [Reference Haagsma26].
The clinical presentation of hepatitis E in humans is indistinguishable from hepatitis A. Commonly reported symptoms include jaundice, anorexia, abdominal pain and hepatomegaly accompanied by fever, nausea and vomiting [Reference Emerson and Purcell27]. Hepatitis E is usually self-limiting but very recently chronic infections have been described in organ transplant recipients in France [Reference Gerolami, Moal and Colson28, Reference Kamar29] and The Netherlands [Reference Haagsma26]. Fulminant and even fatal hepatitis E caused by gt3 have been reported in Japan [Reference Mizuo30] and in Europe [Reference Dalton31–Reference Peron35], often in older males with underlying chronic liver disease. In contrast, in hyper-endemic gt1 and gt2 areas, pregnant women are at higher risk for severe disease and death [Reference Boccia36, Reference Guthmann37]. This feature has not yet been reported for HEV gt3 infections.
In Europe, acute HEV infection is diagnosed in 5–15% of patients with acute hepatitis for whom hepatitis A, B and C has been ruled out [Reference Herremans6, Reference Dalton38–Reference Waar41]. Requests for HEV testing in patients without travel history continue to be infrequent and consequently the actual number of hepatitis E cases is underestimated.
In industrialized countries, little is known about possible sources and transmission routes for endemic human HEV infections. Research is hindered because outbreaks are rarely detected, many infections remain asymptomatic and there is a long and variable incubation period. Narrative, non-systematic reviews have commented on the zoonotic potential and other transmission routes of HEV [Reference Teo11, Reference Pavio42]. However, none have documented a systematic review process or specifically focused on Europe. Our objectives were to determine risk factors for HEV infection and disease and the likely routes of HEV transmission in Europe. We systematically reviewed the scientific literature to give an overview of the evidence available from the last ten years and make recommendations for future research, and prevention and control.
METHODS
Literature search and data extraction
A systematic review of the literature was performed to identify all primary reports and studies that have been published between January 1998 and September 2008, conducted on: (i) HEV infection or anti-HEV antibody prevalence in humans, or (ii) HEV or anti-HEV antibody prevalence in food, animals or the environment. The primary objective was to review and report on the current knowledge and evidence that exists on risk factors for hepatitis E and the most likely routes of HEV transmission in Europe. Thus, the review was limited to the occurrence of HEV infection and disease in Europe, and three report categories were included: (i) original studies published in peer-reviewed scientific journals, (ii) abstracts in conference proceedings, and (iii) correspondence with scientific experts.
For the first category, an electronic search of the United States National Library of Medicine and the National Institutes of Health Medical Database (Pubmed) from its inception was conducted in September 2008. Search words used were: (‘HEV’ or ‘hepatitis E’) and (‘zoonosis’ or ‘zoonotic’ or ‘epidemiology’ or ‘food’ or ‘environment’ or ‘animal’ or ‘pig’ or ‘meat’ or ‘rodent’ or ‘indigenous’ or ‘autochthonous’ or ‘industrialized’). Articles from 1998 onwards were picked out manually. To verify the search, reference lists of the five most recent literature reviews and five most cited primary articles investigating the zoonotic potential of hepatitis E were hand-searched and relevant citations were added to the database. For the second category, a hand-search included eight international conference proceedings held between 2006 and 2008: International Conference on Emerging Infectious Diseases (ICEID), Med-Vet-Net annual scientific meetings, International Meeting on Emerging Diseases and Surveillance (IMED) and International Congress on Infectious Diseases (ICID). Finally, when a published study could not be located, we contacted topic-related scientists of relevant conference abstracts to gather more detailed information.
Abstracts were examined to determine if the inclusion criteria were met. A relevance tool was then applied to the selected reports: (1) countries are contained entirely within continental Europe (i.e. excluding Turkey and the Russian Federation); (2) abstract is in English, French, German or Dutch; (3) diagnosis of hepatitis E or HEV infection was laboratory-confirmed using recognized tests; (4) report has relevance to transmission of, or risk factors for, autochthonous HEV infection (including reports with isolation of HEV gt3). If one of the four criteria was not met, the report was excluded.
For each report or study included, the following information was extracted (as applicable to study): type of study, location (country and setting), study population (number of cases, patients or risk groups, etc.), demographics (age and sex), laboratory tests utilized, positivity rates of humans and animals tested by serological tests and for RNA, HEV genotype, risk factors questioned about, and risk factors found.
Assessment of level of evidence and critical appraisal
Each identified study was categorized to an evidence level (EL) regarding the route of transmission of HEV in Europe, taking both study design and diagnostic methodology into account and using the risk assessment template developed by Palmer et al. [Reference Palmer, Brown and Morgan43] as follows:
Evidence level 1
• Single human case report confirmed by detection of anti-HEV IgM or HEV RNA, or,
• human hepatitis E case series diagnosed by detection of anti-HEV IgM or HEV RNA without specific exposures by subgroup, or,
• serosurveys carried out on acute non-A–C hepatitis patients (without comparison of risk groups) with a proportion of cases confirmed by detection of anti-HEV IgM or HEV RNA, or,
• animal or environmental studies finding HEV gt3 RNA in samples with <100% nucleotide sequence relatedness to human HEV.
Evidence level 2
• Enhanced surveillance with hepatitis E case series confirmed by detection of anti-HEV IgM or HEV RNA and cases with specific exposures by subgroup, or
• serosurvey data comparing risk groups vs. non-risk groups. The limitations of the diagnostic kits used in some of these studies were taken into account by limiting data interpretation to comparative data and consideration not being given to the absolute levels of seroprevalence found.
Evidence level 3
• Analytical epidemiological study (case-control study or cohort study), or
• single human case reports with 100% RNA sequence identity between source and human HEV.
RESULTS
Of 1884 de-duplicated citations published between 1998 and 2008, 110 reports or studies were relevant primary papers or conference proceedings that were suitable for inclusion (Table 1).
Table 1. Inclusion and exclusion criteria used for this review and number of reports identified

Identification of relevant studies by evidence level
Evidence level 1
For HEV in humans, 37 reports pertaining to HEV reservoir and transmission routes can be divided into single case reports (n=16) or case-series (n=18) with the remaining three studies adding molecular sequencing results (Table 2). Even though these reports provide only a weak level of evidence to evaluate risk factors for infection or disease, the majority do provide descriptive epidemiological information and description of the infecting HEV. In fact, in 23 of the 34 reports, information on the infecting genotype was given: of 75 HEV strains identified, 73 belonged to gt3. On most occasions, clinical reports included demographic information, details on symptoms and severity of disease and the questioning of patients on potential risk factors, including zoonotic ones. Nevertheless, it was not always clear from these case reports how thorough and structured the questioning was.
Table 2. Case reports and case series on autochtonhous hepatitis E infections in Europe, published 1998–2008 (Evidence level 1)
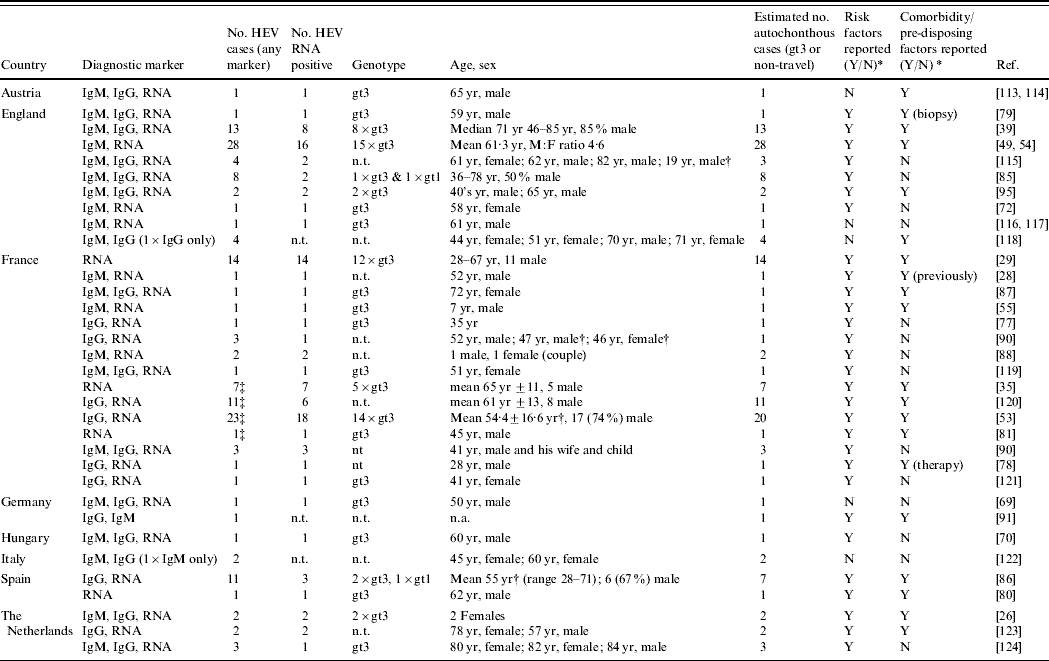
HEV, Hepatitis E virus; IgM/G, immunoglobulin M/G; n.t., not tested; n.a., not applicable as not covered by article; RNA, ribonucleic acid; yr, years.
* Reported risk factors and chronic comorbidity/pre-disposing factors are summarized in Appendix Table 1.
† Series included travel-related cases.
‡ Possible duplication of cases between reports.
We identified nine seroepidemiological surveys carried out on acute non-A–C hepatitis patients (Table 3). Within this group 4–39% were shown to have acute hepatitis E highlighting the probable under-detection of HEV since these patients are often classified as patients with ‘unknown hepatitis’. Most studies contained information on demographics, symptoms, and risk factor questioning. A proportion of the identified HEV cases were confirmed by reverse-transcriptase–polymerase chain reaction (RT–PCR), gt3 being the most frequent genotype (Table 3).
Table 3. Studies identifying autochthonous hepatitis E virus (HEV) cases among undiagnosed non A–C hepatitis patients (Evidence level 1)
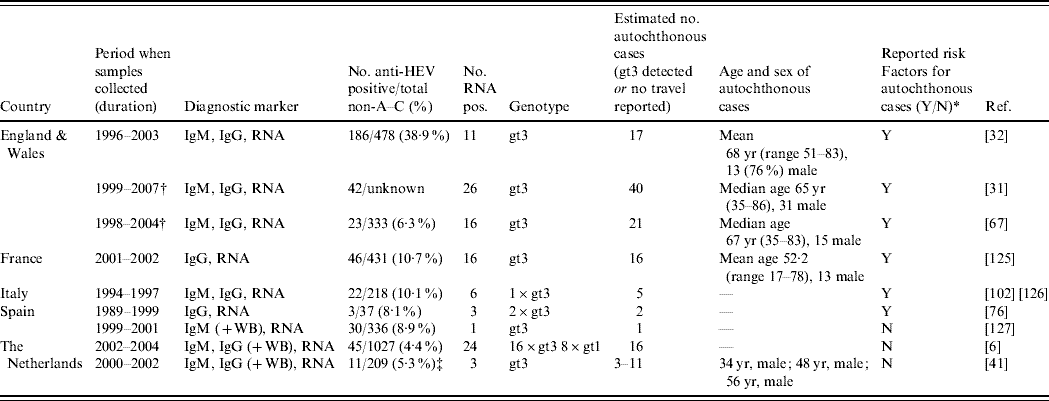
IgM/G, immunoglobulin M/G; RNA, ribonucleic acid; WB, Western blot; yr, years.
All IgG and IgM by enzyme immunoassay unless WB also carried out which means confirmation also done by WB, all RNA detected through RT–PCR.
* Reported risk factors are summarized in Appendix Table 2.
† Overlapping patients.
‡ Comparison of two IgG diagnostic tests.
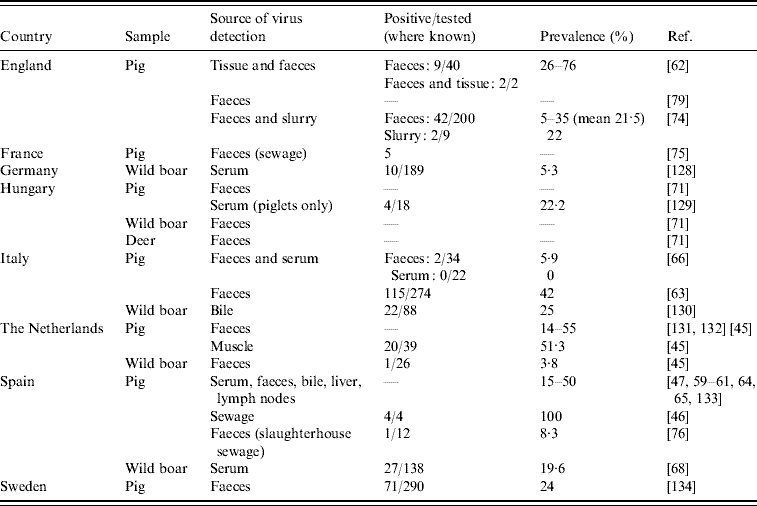
In animals, studies assessing HEV RNA prevalence and RNA sequence analysis are of interest. We identified 24 studies in eight European countries showing HEV RNA in animal samples, most often in domestic pigs (Table 4). The proportion of pigs excreting HEV in faeces ranged between 5% and 55%. A small number of the studies (n=7) have detected HEV RNA from food items (n=2) [Reference Bouwknegt44, Reference Bouwknegt, Rutjes and de Roda Husman45], sewage samples of human (n=4) [Reference Borgen19, 46–Reference Pina48] and animal (n=3) origin (Table 4), and from water (n=3) [Reference Bouwknegt, Rutjes and de Roda Husman45–Reference Clemente-Casares47].
Table 4. Studies on hepatitis E virus genotype 3 PCR-positivity in animal and animal sewage (Evidence level 1)
Evidence levels 2 and 3
A total of 31 seroepidemiological surveys in humans were identified as EL2. Two of these reports contained cases also reported under EL1 [Reference Dalton31, Reference Dalton49]. These studies were carried out in different groups of people (Tables 5 and 6) and compared groups considered at higher risk of acquiring HEV infection either to a group considered not be at risk or to a baseline – most often healthy blood donors. The seroprevalence rates in the different groups were compared, either prospectively or retrospectively, to see if those considered at higher risk have a higher seroprevalence. Care should be taken in comparing results between serosurveys as different methodologies and diagnostic tests were used, and sometimes different markers for infection.
Table 5. Comparative seroepidemiological studies assessing the risk of zoonotic hepatitis E virus (HEV) infection in occupational groups (Evidence level 2)

ELISA, Enzyme-linked immunosorbent assay; IgM/G, immunoglobulin M/G; RNA, ribonucleic acid; WB, Western blot; yr, years.
* Risk analysis done retrospectively in each group after administering questionnaire to those tested.
† Hunting found to be an independent predictor in multivariate analysis (P=0·038). Of those that did not travel, majority lived in rural areas (P=0·07).
‡ Bayesian stochastical model combined results of different assays therefore exact numbers cannot be given.
Table 6. Comparative seroepidemiological studies (IgG) assessing risk factors other than zoonotic exposure (Evidence level 2)
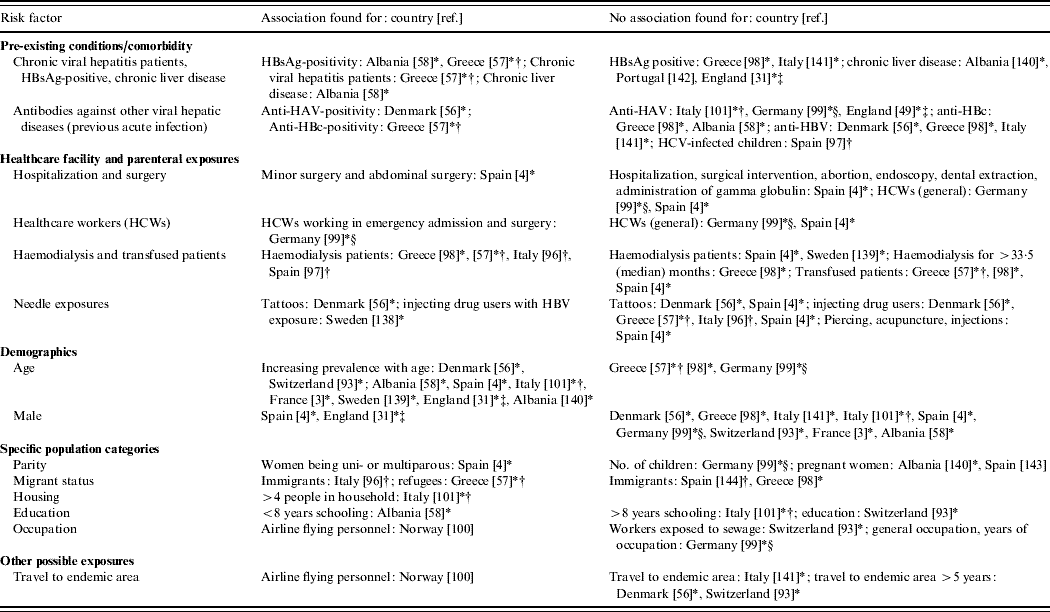
* Result is from retrospective study done in a certain population group (e.g. haemodialysis patients, blood donors, prisoners, healthcare workers) rather than comparing a previously identified risk group to a control group [either as this was (i) original study design, or (ii) in studies with risk and control groups that then looked at subgroups within these, (iii) studies which looked at risk factors within whole HEV positive population, i.e. risk and control group merged]. Study in Denmark [Reference Christensen56] included two different population groups: prisoners and drug-treatment-centre patients.
† Confirmation by immunoblot/Western blot.
‡ Report includes other study type of EL1 – [31] is also reported in Table 3 and [49] in Table 2.
§ Significant result from logistic regression.
It is only in recent years that nationwide epidemiological risk factor analyses and enhanced surveillance (Table 7) have been conducted. Three studies, in the UK [Reference Lewis50], The Netherlands [Reference Borgen19] and France [Reference Renou51] conducted enhanced surveillance and a standardized hypothesis-generating questionnaire was applied to cases of autochthonous hepatitis E so that risk-factor analysis could be undertaken. Through enhanced surveillance it was verified that a significant proportion of hepatitis E cases are indeed not travel-associated (Table 7). Currently, only one analytical epidemiological study, a case-control study in Germany, has been carried out on hepatitis E in Europe [Reference Wichmann52]. However, no European study has reported identical sequences between potential source and human HEV in a logical time order.
Table 7. Analytical studies (Evidence level 3) and case series with risk factor analysis (Evidence level 2) for hepatitis E virus (HEV) infection or hepatitis E
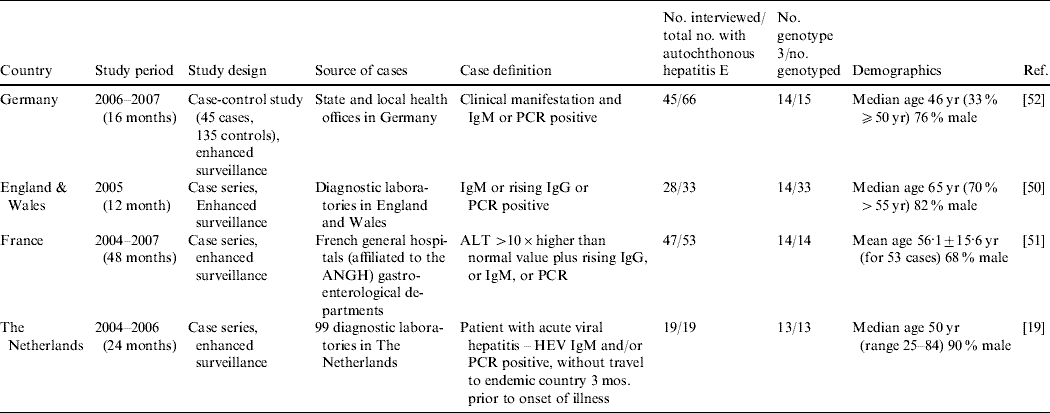
ALT, Alanine aminotransferase; ANGH, Association Nationale des Hépato-Gastroentérologues des Hôpitaux Généaux (a national network of 96 Hepato-gastroenterology centres in France); IgM/G, immunoglobulin M/G; PCR, polymerase chain reaction; RNA, ribonucleic acid; WB, Western blot.
Identification of risk factors and transmission routes
Age and sex as risk factors
There is a general trend for men and older people being reported with HEV gt3 infection as found in EL1 case series (Table 2), EL1 seroprevalence studies in non-A–C hepatitis patients (Table 3) and EL2 case series (Table 7). Acute hepatitis E patients are significantly more likely to be older than hepatitis A patients [Reference Peron53, Reference Dalton54]. Studies assigned to EL1–3 [Reference Ijaz32, Reference Lewis50, Reference Wichmann52] have found that autochthonous hepatitis E cases in Europe are significantly older than those who have travelled to hyper-endemic areas. At present, only one HEV gt3 case has been reported in a child aged <15 years, and this was an immunocompromised boy who received infected blood by transfusion [Reference Colson55]. A number of EL2 comparative seroprevalence studies (Table 6) have also found an association between increasing age and anti-HEV IgG prevalence. In contrast to the case series in which about 70% of HEV cases are male (Tables 2 and 7), being male is rarely associated with seropositivity for anti-HEV IgG. An explanation for the higher proportion of men and older people among acute hepatitis E cases is not provided by any study. However, the lack of an association in the seroprevalence studies conducted to date could suggest that the dominance of male cases is due to a higher risk for overt disease manifestations rather than for infection.
Comorbidity as a risk factor
Increasingly, cases of acute hepatitis E are being reported with chronic liver disease, liver cirrhosis, or history of high alcohol consumption (Table 2). Additionally, other comorbidities like diabetes mellitus, compromised immune status, hypertension, obesity, arthritis, ischaemic heart disease or previous HAV infection have been reported in acute hepatitis E cases. Even though some of these comorbidities were more likely associated with the comparatively older age of the patients, pre-existing diseases or behaviour affecting the liver do seem to have an impact on whether HEV infection results in clinical disease. Enhanced surveillance in France noted that 11/47 (23%) patients had high alcohol consumption [Reference Renou51], and the EL3 case-control study in Germany [Reference Wichmann52] found that a higher but non-significant percentage of cases than controls (6·7% vs. 1·5%) had chronic liver disease.
Although four EL2 seroepidemiological studies have also found that patients with comorbidities [Reference Christensen5, Reference Christensen56–Reference Kondili58] have a higher anti-HEV IgG positivity in comparison to the reference group a larger number of studies found no association (Table 6). This further supports the above-mentioned hypothesis that comorbidities affecting the liver are mainly associated with a higher risk of clinical disease but not infection.
Potential for zoonotic transmission by direct animal contact
The detection of widespread HEV gt3 RNA suggests that swine HEV is ubiquitous and epizootic in pigs in Europe (EL1) (Table 4). Infection in pigs can be symptomatic [Reference de Deus59–Reference de Deus61] but most often infections remain clinically undetected [Reference Clemente-Casares47, 62–Reference Fernandez-Barredo65]. Close relatedness has been found between HEV gt3 nucleotide sequences from pigs and wild boar and those from human cases [Reference Herremans6, Reference Ijaz32, Reference Waar41, Reference Renou51, Reference Martin60, Reference de Deus61, Reference Di Bartolo63, Reference Fernandez-Barredo64, Reference Caprioli66–Reference Reuter71]. HEV carried by a human case in the UK had 100% amino-acid sequence homology to a UK pig HEV sequence but no transmission link between them could be defined [Reference Banks72]. Such sequence comparison data suggest that in Europe, HEV carried by pigs and humans are genetically closely related and that cross-species transmission is occurring.
In a recent transmission study the R 0 (which defines the average total number of new infections caused by one typical infectious animal during its entire infectious period in a completely susceptible population) for transmission of HEV in pigs was estimated to be 8·8 under experimental conditions [Reference Bouwknegt73]. This indicates that HEV is able to spread efficiently between pigs and supports the hypothesis that pigs are a reservoir for HEV. During HEV infection, the virus is shed mostly via faeces. Consequently, HEV RNA has been found in the pig farm environment (Table 4) including in pig sewage [Reference Albinana-Gimenez46, Reference McCreary74, Reference Pavio, Boutrouille and Eloit75], slaughterhouse sewage [Reference Pina76], ditch manure [Reference Fernandez-Barredo65] and a pig water trough [Reference Fernandez-Barredo64].
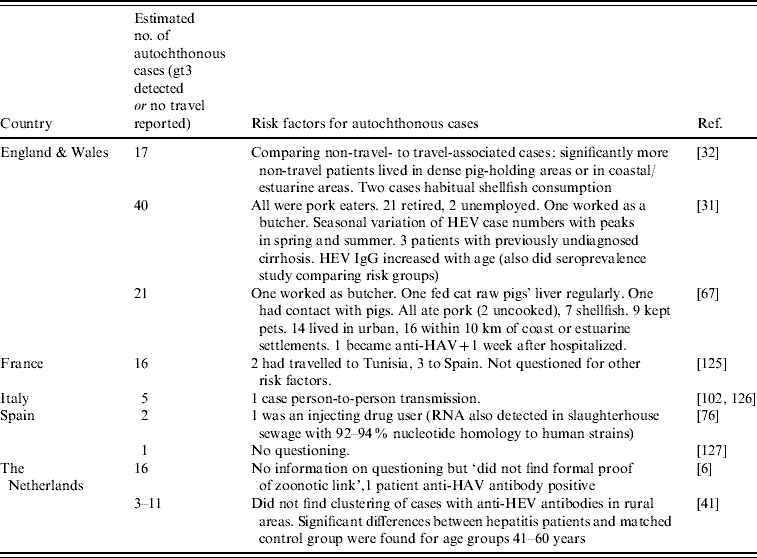
Several EL1 case reports (Table 2) and seroprevalance studies in non-A–C hepatitis patients (Table 3) have alluded to pig contact (i.e. from working with pigs to living in a rural area) as being potential risk factors for the acquisition of HEV infection [Reference Ijaz32, Reference Peron35, Reference De Silva39, Reference Peron53, Reference Dalton67, Reference Colson77–Reference Peron81]. Anti-HEV IgG seroprevalence studies have reported that those working with pigs are at increased risk of infection in some countries in Europe (Austria, Denmark, Moldova, Spain), but not in others (France, Italy, Sweden) (Table 5). In The Netherlands, an increased risk has been found for veterinary surgeons working with pigs when Bayesian statistics were applied to a set of five tests, but no increased risk was found when a validated diagnostic protocol was followed [Reference Bouwknegt22]. Apart from one patient in the case series in France being given a pet pig 8 weeks before onset of fatigue [Reference Renou51, Reference Renou82], remarkably, there seems to be no correlation between pig contact and being a hepatitis E case in the EL2 enhanced surveillance studies and the German EL3 case-control study [Reference Wichmann52]. Despite the ubiquity of HEV in a considerable proportion of pig herds in Europe, there have been no studies proving that contact with pigs is a risk factor for developing hepatitis E.
HEV gt3 RNA has been isolated from wild boar in a number of European countries (Table 4) and deer have also been found positive for HEV gt3 in Hungary [Reference Reuter71]. In The Netherlands, HEV RNA was not detected in faeces of a pet pig, horses, cows [Reference Borgen19], musk rats [Reference Bouwknegt, Rutjes and de Roda Husman45] or in liver tissues in dogs with hepatitis [Reference Boomkens83], but only a few samples were analysed. Having pets (dogs and cats) [Reference Borgen19, Reference Lewis50], contact with horses [Reference Christensen5, Reference Borgen19], hunting or living in rural areas (Table 5) was reported to be associated with HEV infection in some EL2 studies. The German EL3 case-control study did not find an association, indicating that further analytical studies are needed.
Potential for foodborne zoonotic transmission
Recent studies in Spain [Reference de Deus59, Reference Fernandez-Barredo64, Reference Fernandez-Barredo65] and Italy [Reference Di Bartolo63] have shown that RNA is present in faeces, serum and liver of pigs at the age of going to slaughter, which implies that infected meat and organs are potentially on the market. Two studies in Europe (EL1) have attempted to isolate HEV from pig livers. In The Netherlands, 4/62 (6·5%) pig livers on retail sale were found positive for HEV RNA and sequencing analysis found HEV gt3 to be closely related to HEV gt3 of pigs (97%) and humans (93%) in The Netherlands [Reference Bouwknegt44]. In contrast, no pig livers were found positive in a study conducted in 80 retail outlets in Cornwall, UK [Reference Banks84]. Moreover, in The Netherlands, 20/39 (51%) muscle samples from experimentally infected pigs, used as proxies for pork at retail, were found positive [Reference Bouwknegt, Rutjes and de Roda Husman45]. Positive livers contained infectious HEV as revealed by the virus extracted from them being used to successfully infect pigs. However, this success appears to be dose-dependent as it only occurred when the inoculum contained a high dose of virus [Reference Bouwknegt44].
EL1 case reports (Table 2) and seroprevalence studies in non-A–C hepatitis patients (Table 3) have suggested that the following food items are risk factors for the acquisition of HEV infection: pork pies [Reference Sadler85], liver paté [Reference De Silva39], wild boar [Reference Colson77, Reference Buti86], under-cooked or raw pork [Reference Dalton67, Reference Banks72, Reference Colson87, Reference Deest88], home-made sausages [Reference Reuter70], meat (in general) [Reference Dalton31, Reference Dalton67], unpasteurized milk [Reference Kamar78], shellfish [Reference Ijaz32, Reference De Silva39, Reference Dalton67, Reference Sadler85, Reference Ducancelle89, Reference Moucari90] and ethnic foods [Reference Hartmann, Frosner and Eichenlaub91]. Handling raw pig liver is also suggested [Reference Dalton67]. Unpasteurized milk was associated with HEV infection in an EL2 seroepidemiological study [Reference Drobeniuc92]. In a risk factor EL2 analysis using a structured questionnaire, dried sausages and uncooked shellfish (mussels, oysters, or both) were considered risk factors associated with cases in The Netherlands and France, respectively, but these studies did not use controls [Reference Borgen19, Reference Renou51]. In the EL3 case-control study in Germany, Wichmann et al. found that eating any offal or wild boar was independently associated with autochthonous HEV infection [Reference Wichmann52]. No significant association was found for other food products from other animals including shellfish.
Potential for transmission through human sewage
HEV RNA has been detected in raw human sewage in Barcelona, Spain [Reference Borgen19, Reference Albinana-Gimenez46–Reference Pina48] and in France [Reference Clemente-Casares47]. An EL2 study among sewage workers in the canton of Zurich did not find a higher seroprevalence in those exposed to waste water [Reference Jeggli93]. In the EL3 case-control study, one of the 45 autochthonous cases had contact with sewage, but there was no statistical difference compared to the control group [Reference Wichmann52].
Potential for water-borne transmission
A number of EL1 case reports have described water exposure as a possible risk factor for acquiring HEV infection: swimming in a lake [Reference Moucari90], through exposure to water from a high- pressure hose, and water sourced from a stream which ran adjacent to a free-range pig farm [Reference Lockwood79]. In The Netherlands 2/12 (17%) of monthly samples of river water used for drinking water production and recreation were found positive for HEV RNA in two separate years [Reference Bouwknegt, Rutjes and de Roda Husman45]. By contrast, river water samples in Spain were all found to be negative [Reference Albinana-Gimenez46].
During EL2 enhanced surveillance in The Netherlands, HEV RNA was found in a water sample taken from outside a patient's home and it had an identical sequence to that of the patient but it was thought more likely that the patient contaminated the water [Reference Borgen19]. An EL2 risk factor study conducted in France found that 15 cases (32%) drank from their own water supply (spring or well) [Reference Renou51] and 1/35 samples taken from the spring was positive for HEV RNA. An EL2 seroprevalence study found that untreated water consumption was a risk factor (OR 5·6, P=0·01) for anti-HEV IgG seroprevalence confirmed by immunoblot [Reference Galiana94]. Conversely, the EL3 case-control study found that drinking well water and contact with surface was not related to HEV infection [Reference Wichmann52]. No waterborne outbreaks have been reported in Europe to date.
Potential for parenteral transmission
As HEV does not usually produce a chronic carrier state, non-enteric (faecal–oral) sources were not considered as an important cause of HEV transmission. However, cases linked to blood transfusion have now been reported in England [Reference Boxall95] and France [Reference Colson55]. In The Netherlands, enhanced surveillance using a structured questionnaire detected two cases of hepatitis E who had previously received blood transfusions [Reference Borgen19]. The German EL3 case-control study did not identify blood transfusion as a risk factor, which shows that parenteral transmission does not play a significant role in Germany (none of 46 included autochthonous cases had received a recent blood transfusion) but does not exclude that parenteral transmission in single cases is possible [Reference Wichmann52]. In France, a student acquired HEV 7 weeks after practising surgical procedures on a pig [Reference Colson77].
The majority of EL2 seroepidemiological studies looking into risk factors involving needle exposure and many types of hospital exposure did not find a significant difference between groups tested (Table 6). However, haemodialysis patients in four different studies [Reference Dalekos57, Reference De Donno96–Reference Stefanidis98], those who had undergone minor surgery and abdominal surgery in Spain [Reference Buti4], healthcare workers in emergency admission and surgery in Germany [Reference Nubling, Hofmann and Tiller99] and male prisoners and drug treatment patients in Denmark [Reference Christensen56] were found to have a higher anti-HEV IgG prevalence than the reference group.
Potential for other transmission routes and risk factors
Some seroepidemiological studies have found other risk groups to have higher anti-HEV antibody prevalence in comparison to a reference group (Table 6). For immigrants [Reference De Donno96], refugees [Reference Dalekos57] and probably airline flying personnel [Reference Andenaes, Lie and Degre100], the HEV antibodies probably did not result from HEV gt3 infection but from gt1 infection when staying in an hyper-endemic country. In contrast, three seroepidemiological studies did not find an association with travel to endemic areas (Table 6) and in one case-control study no association was found with travel within Europe or close contact with returning travellers [Reference Wichmann52]. The finding of an association with a larger family size supports the possibility of person-to-person transmission [Reference Rapicetta101] as has been reported for gt1 and gt2 HEV infection and is further suggested in a case series from France [Reference Moucari90] and a case in Italy [Reference Zanetti102]. However, efficient person-to-person transmission is unlikely since very few reports mention outbreaks or family clusters of cases. In the EL2 enhanced surveillance conducted by Borgen et al. in The Netherlands it was specifically asked if any family members or close contacts had signs of hepatitis in the months preceding or following the disease period of the cases, but no positive responses were elicited and a limited contact investigation found no evidence of HEV infection [Reference Borgen19]. During the enhanced surveillance conducted for the case-control study in Germany, unstructured household investigations identified five additional IgM-positive HEV cases in three households of autochthonous cases [Reference Wichmann52], one of whom was PCR-positive. All were asymptomatic or complained only of mild abdominal pain or fatigue. Due to possibility of asymptomatic infection and the wide range of the incubation period, it was not possible to say if these were cases due to person-to-person transmission or if they were infected through the same source (in one household the whole family ate wild boar, in another they had all eaten offal).
In contrast to hyper-endemic countries [Reference Teo11], there have been few studies and no reports of sexual transmission or vertical transmission during pregnancy in Europe. Seroepidemiological studies have found no association with sexual contact [Reference Buti4] or pregnancy (Table 6). One HEV infection during pregnancy occurred in Germany but without complications [Reference Wichmann52].
DISCUSSION
This review found that there is no evidence for one main transmission route or risk factor for HEV infection or disease in Europe. Rather, multiple routes of transmission are likely to exist. Autochthonous HEV gt3-associated hepatitis E cases are on average older than the general population and predominantly male, which could be due to higher likelihood of pre-existing comorbidity, different dietary preferences and differences in other behaviours. Our results do indicate that zoonotic transmission of HEV, either by direct contact or food, is likely due to the following observations: (1) the virus is highly prevalent in domestic pigs, wild boar and to a lesser extent in deer; (2) animal stool and tissues may contain sufficient viral loads to be infectious; (3) high sequence relatedness has been found between animal and human HEV gt3 isolates; (4) several EL2–3 studies have identified zoonotic risk factors. It should be noted that not all autochthonous infections were attributable to these factors. Other routes of transmission reported in hyper-endemic countries do not appear to be applicable to gt3 HEV infection, and our results therefore provide further evidence for a unique clinical and epidemiological identity of disease conferred by this genotype [Reference Teo12]. We also found evidence suggesting that cases of hepatitis E with underlying diseases are at increased risk of developing clinical manifestations or severe disease.
Current epidemiology suggests that HEV gt3 is the predominant strain circulating in pigs in Europe and that pigs are the natural host [Reference Purcell and Emerson20, Reference Meng, Thomas, Lemon and Zuckerman103]. However, the virus can also infect a considerable proportion of the human population, but only a minority appear to develop symptoms [Reference Boutrouille3–Reference Olsen8]. The key to understanding HEV transmission in Europe may lie in determining how the virus that circulates among pigs is transmitted to humans in sufficiently high doses to cause infection or overt disease. Direct animal contact could lead to infection associated with low-dose exposure, thus one could argue that infection does not occur or that the resulting infection is more likely to be asymptomatic. Larger doses through, for example, the consumption of heavily contaminated and insufficiently cooked meat could lead to infections that are more likely to result in clinical symptoms. Occasional instances of HEV transmission through sewage or consumption of contaminated water seem possible, if a sufficiently high dose of HEV is ingested. The failure of the virus to generate an infecting dose when excreted by humans could be a plausible explanation for the low person-to-person transmission rates.
Evidence for foodborne zoonotic transmission of HEV in Europe is generally weak but this is mainly because of an insufficient number of good analytical epidemiological studies. The only case-control study conducted to date has suggested that, at least in Germany, foodborne zoonotic transmission is the main route [Reference Wichmann52]. Additional strong evidence for foodborne transmission is available from Japan where sequence identity between deer meat [Reference Takahashi104, Reference Tei105] and hepatitis E patients who consumed that meat, and HEV genetic similarity between boar meat and patient samples [Reference Li106], have been found. HEV has been shown to be viable and infectious in pigs' liver in the USA [Reference Feagins107] and resistant to temperatures up to 60°C [Reference Emerson, Arankalle and Purcell108]. Consequently, the role of foodborne transmission in European countries other than Germany should be assessed through further analytical studies. Interestingly, consumption of bivalve molluscs [Reference Ijaz32, Reference De Silva39, Reference Renou51, Reference Dalton67, Reference Sadler85, Reference Ducancelle89, Reference Moucari90] and contact with horses [Reference Christensen5, Reference Borgen19] are mentioned as possible sources of infection in studies we reviewed. Even though anti-HEV antibodies have been detected in many other animal species including rats, cats, monkeys, dogs, cattle, sheep, goats and mongooses [Reference Meng17, Reference Li, Miyamura and Takeda109, Reference Zhang110] it is not clear if these animals can be infected with the HEV strains known to date, and thus their role as possible reservoir is doubtful. Nonetheless, the possibility of another reservoir than swine transmitting HEV to swine and humans cannot be excluded.
It is of interest that questioning of HEV patients for enhanced surveillance studies and the case-control study (Table 7) often found evidence for food exposures whereas a number of seroepidemiological studies conducted in healthy populations (Table 5) found other risk factors such as animal contact. This could be due to confounding factors such as different dietary behaviour in rural areas where there is also more direct animal contact than in urban settings. Since case series and case-control studies involve interviewing patients with a known date of onset of hepatitis E symptoms it is also possible to enquire about the consumption of specific food items during the incubation period, but one should be aware of recall problems due to length and variation of incubation periods. This kind of assessment is not possible for seroepidemiological studies since the date of HEV infection cannot be estimated. Moreover, seroepidemiological studies assess risk factors for infection whereas case series and case-control studies assess risk factors for clinically overt disease. Risk factors for asymptomatic HEV infection might be different from risk factors for hepatitis E disease if, as suggested above, the ingested dose of HEV determines whether infection subsequently leads to overt clinical disease. A clear limitation especially of less recent seroepidemiological studies is the use of poor diagnostic kits and the results of these studies should be interpreted with caution.
When the opportunity presents, routes of transmission other than zoonotic ones may exist. The parenteral route of transmission has been documented in people who received HEV-contaminated blood transfusions [Reference Colson55, Reference Boxall95] and in a student practising surgical procedures on a pig [Reference Colson77]. It is plausible that patients develop disease in these instances due to the high infectious dose inoculated. Person-to-person spread of HEV is an uncommon event but this is not surprising if, as proposed earlier, infection and disease are dose-dependent [Reference Bouwknegt44] and require a higher infective dose than, for example, for HAV transmission.
This is the first systematic review attempting to define sources and transmission routes of HEV infection and disease. In conducting this review we found that studies of higher grade evidence are lacking and further case-control and cohort studies are required, preferably population-based and using sensitive diagnostic techniques such as Western blot and RT–PCR for HEV confirmation. Standardization of questionnaires and studies across a number of European countries would also assist evaluation and comparison of data. Where possible, water and food samples of HEV patients should be analysed and HEV strains should be genotyped to attempt to link their infection to a source [Reference Takahashi104, Reference Li106]. Further studies are also required to test for HEV RNA in potential commercial food sources in Europe (e.g. pork and pig offal, and wild boar meat) and in potential animal reservoirs other than pigs.
The lack of evidence for one main transmission route or risk factor, and the high possibility for multiple transmission routes, for HEV gt3 infection and disease in Europe limits the possibility to propose effective intervention strategies. However, proper cooking of all pork products and prevention of cross-contamination in the kitchen are generally accepted guidelines that should help to prevent HEV infections. Although the role of direct zoonotic or environmental transmission is still unclear, consideration should still be given to improve education of occupationally HEV-exposed people such as pig farmers and sewage workers. Although evidence for parenteral transmission is limited, it is recommended that a risk assessment, including determination of the frequency of HEV contamination, be conducted to determine whether general screening of organs for transplantation and blood products should be undertaken.
Several HEV vaccine candidates based on HEV gt1 recombinant capsid protein have been developed. To date, two have progressed to human clinical trials with promising preliminary results [Reference Shrestha111, Reference Zhang112]. Whether these vaccines provide long-term immunity to hepatitis E still needs to be established. Even though HEV gt1–4 are considered to belong to one serotype, whether they have similar efficacy to protect from HEV gt3 infections requires further study. If licensed, the main target of the vaccine would be those living in or travelling to HEV hyper-endemic countries but consideration should also be given to people living in industrialized countries with low HEV endemicity who are at risk of a severe outcome (e.g. patients with chronic liver disease or transplant organ recipients).
In summary, this first systematic review of HEV in Europe found that there is no evidence for one main transmission route or risk factor for HEV although evidence points towards zoonotic transmission. Multiple routes of transmission are likely to exist, and these need to be further defined so that specific and appropriate preventive measures can be implemented.
ACKNOWLEDGEMENTS
The authors thank Barbara Schimmer and Christian Winter for their comments on the manuscript.
DECLARATION OF INTEREST
None.
Appendix
Table A1. Risk factors in case reports and case series on autochthonous hepatitis E infections in Europe, published 1998–2008 (Evidence level 1)

CMV, Cytomegalovirus; EBV, Epstein–Barr virus; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HEV, hepatitis E virus; IgG, immunoglobulin G; RNA, ribonucleic acid.
Appendix
Table A2. Risk factors in studies identifying autochthonous hepatitis E virus (HEV) cases among undiagnosed non-A–C hepatitis patients (Evidence level 1)
HAV, Hepatitis A virus; IgG, immunoglobulinG; RNA, ribonucleic acid.












