Introduction
Cogongrass [Imperata cylindrica (L.) Beauv.] is a C4 grass from Asia that was accidentally introduced to Mobile, AL, from Japan around 1912 (Dickens Reference Dickens1974). Intentional introductions as a potential forage from material collected in the Philippines occurred in McNeil, MS, in the 1920s and later in Florida in the 1930s and 1940s (Hubbard et al. Reference Hubbard, Gray, Brown and Whyte1944). Subsequent anthropogenic movements and natural wind-based dispersal have resulted in a widespread distribution across nine southern states. However, the majority of infestations occur in Mississippi, Alabama, and Florida. Impacts in forestry and natural areas have been well documented and continue to be a significant and growing threat (Brewer Reference Brewer2008; Daneshgar et al. Reference Daneshgar, Jose, Collins and Ramsey2008; Estrada and Flory Reference Estrada and Flory2015).
Box 1 Management Implications

Management of I. cylindrica in forestry and natural areas has been largely herbicide based, as no other effective control strategies have been developed for nonarable lands. Only two herbicides are widely used for I. cylindrica management, glyphosate and imazapyr (MacDonald Reference MacDonald2004; Minogue et al. Reference Minogue, Miller and Lauer2012). Both can be very effective with repeated applications (Aulakh et al. Reference Aulakh, Enloe, Loewenstein, Price, Wehtje and Miller2014). However, both may cause significant damage to desirable vegetation (Harmoney et al. Reference Harmoney, Stahlman and Hickman2007) from foliar treatments. Imazapyr also exhibits extensive soil residual activity (Czarnota and Derr Reference Czarnota and Derr2007; Westerman et al. Reference Westerman, Murray and Castner1993), and many land managers cannot use imazapyr due to non-target injury concerns for many woody and herbaceous plant species (Klaus and Klaus Reference Klaus and Klaus2011; Kochenderfer et al. Reference Kochenderfer, Zedaker, Johnson, Smith and Miller2001; Lewis and McCarthy Reference Lewis and McCarthy2008; Masters et al. Reference Masters, Stougaard and Nissen1994).
This leaves a single herbicide, glyphosate, which must be used in an aggressive, repeated manner for effective control. Weed resistance to glyphosate was rare before its widespread use in genetically modified crops (Dyer Reference Dyer1994), but at least 37 species are now glyphosate resistant (Heap Reference Heap2017). Although there are no published studies that have found evidence of I. cylindrica tolerance or resistance to glyphosate, sole reliance on glyphosate could lead to resistance selection in I. cylindrica with potentially catastrophic consequences. This is especially true for land managers who will not tank mix or rotate to imazapyr due to non-target concerns. Coincidentally, land managers have reported variation in glyphosate performance on I. cylindrica. The reasons for this are unclear and warrant further investigation. However, multiple studies have established a high degree of genetic diversity (Burrell et al. Reference Burrell, Pepper, Hodnett, Goolsby, Overholt, Racelis, Diaz and Klein2015; Lucardi et al. Reference Lucardi, Wallace and Ervin2014) among I. cylindrica populations across the southeastern United States.
Aminocyclopyrachlor, a relatively new herbicide labeled for use in non-crop settings, controls a wide range of weeds and invasive plants (Enloe et al. Reference Enloe, Loewenstein, Streett and Lauer2015; Minogue et al. Reference Minogue, Enloe, Osiecka and Lauer2011). It primarily has activity on eudicots, but some grasses have demonstrated sensitivity to it (Flessner et al. Reference Flessner, McElroy and Wehtje2011c; Wirt and Lym Reference Wirt and Lym2016). Aminocyclopyrachlor functions as an auxin mimic similar to pyridine carboxylic acids such as aminopyralid. Previous research on I. cylindrica control with aminocyclopyrachlor has yielded mixed results. Greis (Reference Greis2012) and Enloe et al. (Reference Enloe, Belcher, Lowenstein, Aulakh and van Santen2012) in separate studies in Florida and Alabama, respectively, found aminocyclopyrachlor to show strong suppression of approximately 80% at 6 mo after treatment (MAT), with little to no suppression after 12 MAT. However, Greis (Reference Greis2012) found no reduction in rhizome biomass at 9 or 16 MAT, while Enloe et al. (Reference Enloe, Belcher, Lowenstein, Aulakh and van Santen2012) found a 28% reduction in rhizome biomass at 12 MAT, which was significantly different from the untreated controls. This may suggest underlying differences in long-term I. cylindrica control in south Alabama, near the original Japanese material introduction, versus central peninsular Florida, near the original Philippine material introduction site.
Flumioxazin is a herbicide used in many crop and non-crop settings for control of a wide range of broadleaf and grass weeds. It inhibits protoporphyrinogen oxidase, resulting in rapid membrane disruption and subsequent death in susceptible species (Duke et al. Reference Duke, Lydon, Becerril, Sherman, Lehnen and Matsumoto1991). Flumioxazin has been widely used as a herbicide tank-mix partner in many agronomic studies addressing glyphosate resistance (Beckie Reference Beckie2011), as it provides effective control of many weed species. It is also widely used in aquatic systems, where it controls free-floating species such as water lettuce (Pistia stratiotes L.) (Mudge and Haller Reference Mudge and Haller2012; Mudge and Netherland Reference Mudge and Netherland2016). Tank mixes of glyphosate and flumioxazin are also widely used in nursery production and landscape maintenance (Wehtje et al. Reference Wehtje, Gilliam and Marble2010) due to a lack of impact on many desirable ornamental species. This selectivity might make it a potentially useful tank-mix partner with glyphosate for I. cylindrica control.
Given the need for additional tools for I. cylindrica control with current concerns for glyphosate resistance, our objective was to evaluate glyphosate and aminocyclopyrachlor as stand-alone treatments and potential tank-mix combinations across a broad range of I. cylindrica populations collected throughout Florida. We also evaluated a tank mix of glyphosate and flumioxazin to assess its utility compared with glyphosate alone. We hypothesized that differences in I. cylindrica susceptibility to glyphosate from populations sampled throughout Florida would help explain variation in glyphosate performance reported by land managers.
Materials and Methods
Greenhouse studies were conducted during the spring and summer of 2016 at the University of Florida Center for Aquatic and Invasive Plants in Gainesville, FL. Imperata cylindrica rhizomes were collected from 12 populations originating from both the peninsular and western panhandle regions of Florida (Figure 1). Collections encompassed USDA plant hardiness zones 8a in the far northern panhandle near Laurel Hill, FL, to 10b in the southern peninsular region near Coral Gables, FL. Additional details of the collection are provided in Hiatt (Reference Hiatt2016). Individual rhizomes from each population were planted in 100-L stock pots filled with construction sand. These were fertilized and watered as needed until the new plants were well established and produced sufficient rhizome biomass for experimentation. The process of rhizome propagation for I. cylindrica is rapid, and sufficient material was readily generated from the previous growing season. In mid-April 2016, rhizome segments 10 cm in length were harvested from the stock pots. For each population, two rhizome segments were planted into each of 25 (3.8-L) pots for study. Pots were filled with a 1:1 mix of construction sand and potting media (Professional Top Soil, Margo Garden Products, Folkston, GA) mixed with a complete (15-9-12) slow-release fertilizer (Osmocote Plus, Scotts, Maryville, OH). Rhizome segments were planted in each pot at a depth of 2.5 cm. These were watered daily and grown for approximately 75 d until plants were well established. Established plants were those that had produced several new shoots per pot, and new rhizomes were present throughout the pot.
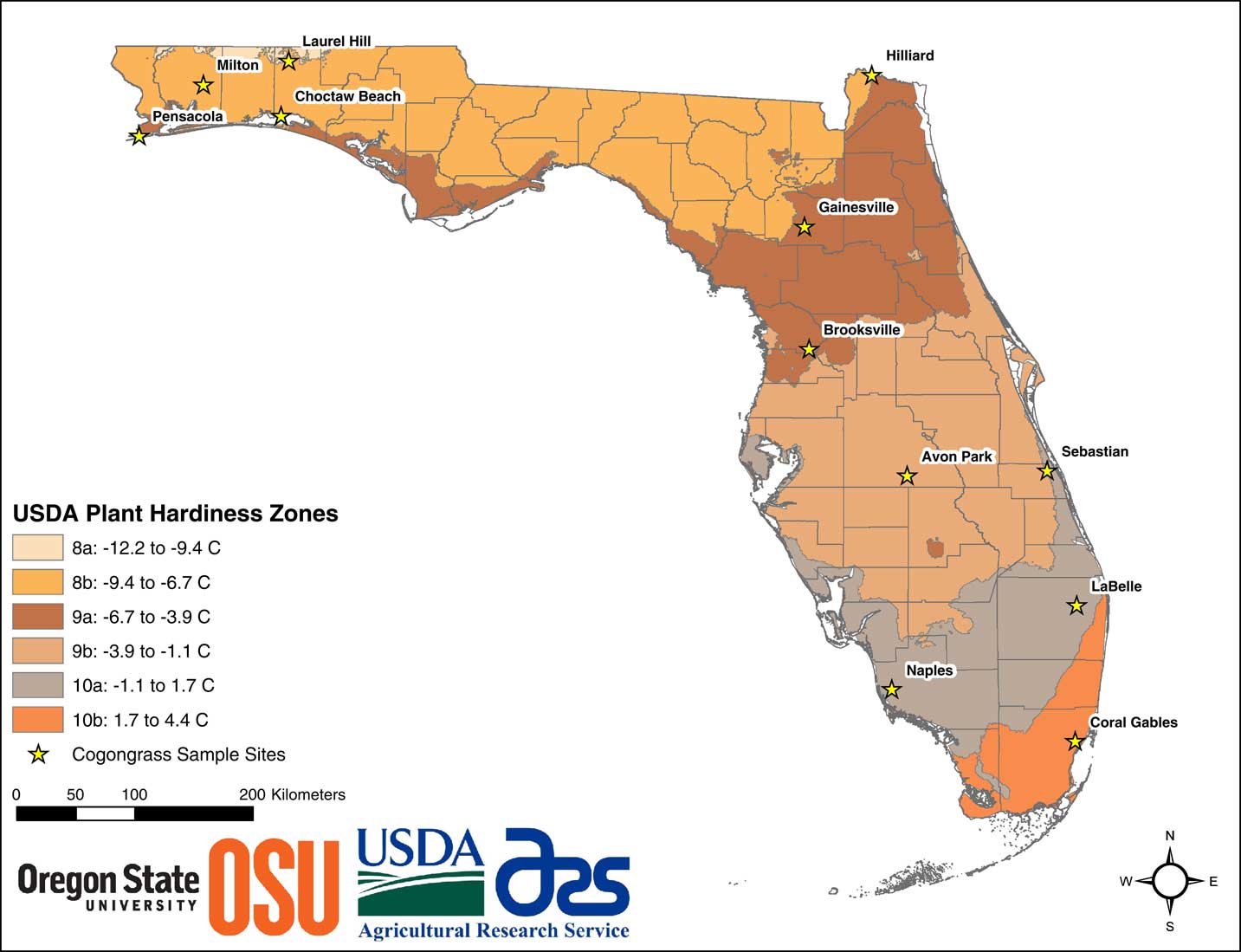
Figure 1 Collection locations for the 12 Imperata cylindrica populations used in the study. USDA plant hardiness zone map layer accessed with permission at: http://planthardiness.ars.usda.gov/PHZMWeb (accessed January 17, 2018).
Treatments included glyphosate applied at 0.28 kg ae ha−1 (Rodeo® herbicide, Dow AgroSciences, Indianapolis, IN), aminocyclopyrachlor applied at 0.28 kg ae ha−1 (Method® herbicide, Bayer Crop Science, Research Triangle Park, NC), glyphosate+aminocyclopyrachlor applied at 0.28+0.28 kg ae ha−1, glyphosate+flumioxazin (Clipper® herbicide, Valent USA, Walnut Creek, CA ) applied at 0.28 kg ae ha−1+0.14 kg ai ha−1, and an untreated control. A nonionic surfactant (Induce, Helena Chemical, Collierville, TN) was added to each herbicide treatment at 0.5% v/v. Treatments were applied with a CO2-pressurized backpack boom sprayer equipped with four 8002 flat-fan nozzles at a pressure of 276 kPa and an application volume of 178 L ha−1.
Baseline maximum shoot height was measured for each pot (N=300) just before herbicide treatment. At 30 d after treatment (DAT), shoots were clipped to the soil surface and all live shoots were separated, oven-dried at 65 C for 72 h, and weighed. Plants were then allowed to grow for an additional 30 d. At 60 DAT, all shoot regrowth was harvested as previously described. Additionally, all belowground biomass, which included roots and rhizomes, was harvested, oven-dried, and weighed. The entire experiment was repeated in mid-July 2 wk later in the summer of 2016 on a second set of planted pots and was completed by mid-September. Ambient greenhouse conditions were similar over both experimental runs. The mean daily maximum, mean, and mean minimum temperatures were 32.5, 26.3, and 20.1 C, respectively, for run 1, and 32.4, 26.3, and 20.2 C, respectively, for run 2 (Southern Regional Climate Center accessed January 23, 2018).
Statistical Analysis
For each experimental run, five replicate pots for each herbicide by I. cylindrica population combination were arranged in a completely randomized design. Imperata cylindrica growth by population, as measured by pretreatment shoot height, 30 DAT shoot biomass, and 60 DAT shoot regrowth biomass, was compared using ANOVA for the untreated controls. Additionally, belowground biomass and total biomass (aboveground+belowground biomass) at 60 DAT were compared using ANOVA for all treatments. Herbicide efficacy was compared in terms of a percent reduction in the 60 DAT belowground biomass and total biomass (shoot+belowground) compared with the untreated control. Shoot biomass alone at 60 DAT was not analyzed, because more than 90% of observations were zero following herbicide treatments. This also precluded useful estimation of root to shoot ratios. The analysis of treatment efficacy was performed using the 60 DAT belowground or total biomass averaged for each experimental run, herbicide treatment, and population combination.
Average 60 DAT plant biomass per pot is not a typical continuous variable, because it includes growth, necrosis, and mortality. Other characteristics of this experimental data are that the mass of the untreated control has no defined upper bound, a treatment mean could be zero, treatments have a multiplicative effect with respect to percent reduction, and variance was found to be proportional to the mean. These characteristics lend themselves to analysis methodology applied to count data. Although plant mass is not strictly a count, the analysis of efficacy was modeled using Poisson distributional assumptions with a log-link function as outlined by McCullagh and Nelder (Reference McCullagh and Nelder1989) for the biological assay of data from Fisher (Reference Fisher1949). The analysis was performed as a generalized linear mixed model using SAS PROC GLIMMIX (Littell et al. Reference Littell, Milliken, Stroup, Wolfinger and Schabenberger2006). This approach allowed treatments to be compared in terms of percent reduction relative to the untreated control and provided tests of antagonism (see Appendix 1 in the Supplementary Material). The test of antagonism is significant if the difference of tank-mix percent control minus “expected” percent control differs significantly from zero. Expected tank-mix percent control was calculated as 100× (1−(1−proportion control from herbicide 1 used alone)×(1−proportion control from herbicide 2 used alone). Proportion or percent control are not the dependent variables in the analysis or tests of hypotheses, but these results are presented in terms of percent control calculated from biomass treatment means. The rationale for this approach in this analysis is that the difference between a treatment and the untreated check in the linear predictor part of this generalized linear model (that uses a log-link function) equates to treatment biomass divided by the untreated check biomass after applying the inverse link function.
Treatment effects were partitioned using contrast statements to construct a two-way ANOVA for glyphosate and aminocyclopyrachlor herbicides and to test whether glyphosate+flumioxazin and glyphosate+aminocyclopyrachlor tank mixes differed. The test of the aminocyclopyrachlor main effect is that the average of aminocyclopyrachlor and aminocyclopyrachlor+glyphosate treatments differ from the average of the untreated check and glyphosate-alone treatments. The glyphosate main effect is that the average of glyphosate and glyphosate+aminocyclopyrachlor treatments differ from the average of the untreated check and aminocyclopyrachlor treatments. Main effect means were compared using Tukey’s adjustment for multiplicity at P=0.05 when applicable.
Results and Discussion
Pretreatment I. cylindrica shoot heights were not different among 9 of the 12 populations and ranged from 104.4 to 123.4 cm (Table 1). Populations from Milton and Pensacola were significantly shorter at 47 and 67.8 cm, respectively. Imperata cylindrica from Laurel Hill averaged 89 cm in height and was not different from any other population.
Table 1 Comparison of Imperata cylindrica panhandle and peninsular Florida population shoot parameters for the untreated controls with means considered normally distributed.

a Population means within columns followed by the same letter are not significantly different using Tukey’s mean comparison adjustment (P=0.05).
b Standard error of population means from analysis that considers treatment run a random effect.
Across all populations, there were also limited differences in I. cylindrica shoot biomass for the untreated controls at 30 DAT and for regrowth at 60 DAT (Table 1). There were no significant differences in 10 of the populations for shoot weight at 30 DAT, and these ranged from 12.7 to 23.1 g. Only Milton (5.4 g) produced significantly lower biomass than Brooksville and Choctaw Beach at 30 DAT. Subsequent shoot regrowth at 60 DAT also yielded few differences among populations, as 10 out of 12 populations were not different (Table 1). However, Brooksville had significantly greater regrowth than either Milton or Pensacola. Root plus rhizome weights at 60 DAT for the untreated controls did not differ among any populations and ranged from 9.7 to 23.3 g (Table 2). Total weight (root+rhizome+shoot regrowth) at 60 DAT did not differ for 11 out of 12 populations and ranged from 17.9 to 30.5 g. Only Milton (12.2 g) was significantly lower than Choctaw Beach or Coral Gables. These pretreatment height data and biomass data from 30 and 60 DAT for the untreated controls indicate similar performance across most populations in our study. Only the Milton population from the panhandle resulted in significantly lower heights and weights than any other population. Explicit reasons for this are unclear but may have been related to possible genetic differences, as Burrell et al. (Reference Burrell, Pepper, Hodnett, Goolsby, Overholt, Racelis, Diaz and Klein2015) found a high degree of genetic variation among I. cylindrica populations sampled within the panhandle region of Florida.
Table 2 Comparison of Imperata cylindrica panhandle and peninsular Florida population biomass parameters (standard errors in parenthesesFootnote a ) for the untreated controls at the final harvest (60 d after treatment).

a Approximate standard error of population means from the generalized linear mixed-model analysis that considers treatment run a random effect.
b Population means within columns followed by the same letter are not significantly different using Tukey’s mean comparison adjustment (P=0.05).
There were no significant I. cylindrica population or population by herbicide treatment interactions (P>0.39 for both root and total biomass) for all herbicide treatments tested at 60 DAT. This was similar to a lack of differences in shoot height and biomass between source populations for the untreated controls. The lack of I. cylindrica population either as a significant main effect or in the interaction with herbicide treatment indicates that the herbicide treatments were not influenced by inherent variation among populations tested. This suggests that other factors are contributing to variable I. cylindrica control reported by many land managers in Florida.
Across all I. cylindrica populations, the herbicide treatment main effect was highly significant for root plus rhizome biomass and total biomass at 60 DAT (P<0.001). Glyphosate and aminocyclopyrachlor reduced belowground biomass by 78% and 76%, respectively, and were not significantly different (Table 3). Additionally, the tank mix of these two herbicides reduced belowground biomass by 91% and was significantly greater than either herbicide alone. This increase of 13% to 15% in control would appear promising for improved, local I. cylindrica management and increased chemical options for land managers. However, when tests of antagonism were applied to the data, actual control was significantly lower than projected for both belowground biomass and total biomass. This is statistically noteworthy but may be biologically questionable, as the differences were less than 5 percentage points between actual and projected. This pattern was also consistent for total biomass. The tank mix increased percent control by 10% to 11% compared with either herbicide applied alone but was significantly less than projected control (Table 3). Finally, the tank mix of glyphosate+flumioxazin did not improve I. cylindrica control compared with glyphosate alone for belowground or total biomass. No test of antagonism was performed for the glyphosate+flumioxazin tank mix, since flumioxazin was not included as a stand-alone treatment.
Table 3 Comparison of 60 DAT root and total biomass (standard error of means in parentheses) and percent control.

a Percent control followed by the same letter does not differ at P=0.05 using Tukey’s adjustment for multiplicity.
b Expected control was significantly greater than actual control (P=0.022).
c Expected control was significantly greater than actual control (P=0.001).
These data indicate a significant impact of aminocyclopyrachlor on I. cylindrica biomass, and our data are congruent with other published studies. In field studies conducted in Alabama, Enloe et al. (Reference Enloe, Belcher, Lowenstein, Aulakh and van Santen2012) found aminocyclopyrachlor applied at the same rate in this study (0.28 kg ha−1) reduced rhizome biomass by 28% at 12 MAT. This rate also reduced vegetative I. cylindrica cover by 84% and 54% at 3 and 5 MAT, respectively, but resulted in cover higher than the untreated control at 12 MAT. Additional field dose–response studies reported in Enloe et al. (Reference Enloe, Belcher, Lowenstein, Aulakh and van Santen2012) indicated that aminocyclopyrachlor applied at 0.56 kg ha−1 resulted in approximately 80% control at 7 to 8 MAT. Similarly, we observed a reduction in I. cylindrica belowground and total biomass of near 80% at 60 DAT across our populations.
Although aminocyclopyrachlor primarily controls many broadleaf weeds, certain grasses have also exhibited susceptibility to aminocyclopyrachlor. Parker et al. (Reference Parker, Wehtje, McElroy, Flessner and Panizzi2015) found I. cylindrica was more susceptible to aminocyclopyrachlor than tall fescue [Lolium arundinaceum (Schreb.) Darbysh.], bahiagrass (Paspalum notatum Fluegg), and bermudagrass [Cynodon dactylon (L.) Pers.]. Additionally, Flessner et al. (Reference Flessner, McCurdy and McElroy2011b) found differential susceptibility among six Japanese lawngrass (Zoysia japonica Steud.) cultivars to aminocyclopyrachlor. The physiological basis for sensitivity in certain grasses is not completely understood. However, in St. Augustinegrass [Stenotaphrum secundatum (Walter) Kuntz], Flessner et al. (Reference Flessner, Dute and McElroy2011a) found injury consistent with other synthetic auxin-type herbicides that included growth stimulation and vascular inhibition.
Our data demonstrated consistent susceptibility to aminocyclopyrachlor in both panhandle and peninsular I. cylindrica populations from across a wide geographic range in Florida. One major consideration with aminocyclopyrachlor that needs to be addressed is potential non-target injury in natural areas. Aminocyclopyrachlor has resulted in injury to non-target species through soil residual activity (Kniss and Lyon Reference Kniss and Lyon2011) and release from treated plants (Lewis et al. Reference Lewis, Richardson, Yelverton and Wentworth2013). Although not classified as purely nonselective, a better understanding of nontarget impacts in southeastern ecosystems where I. cylindrica is prominent is critical to developing potential use patterns.
Regarding the glyphosate+flumioxazin tank mix, we found no evidence to suggest flumioxazin improved I. cylindrica control with glyphosate. The tank mix resulted in an 89% reduction in total biomass, while glyphosate alone resulted in an 83% reduction, and these were not significantly different. Byrd (Reference Byrd2007) tested flumioxazin at 0.07, 0.14, and 0.28 kg ha−1 with ammonium sulfate at 0.51 kg ha−1 and glyphosate at 1.68 kg ha−1. He reported no difference in I. cylindrica control when glyphosate was applied with or without any rate of flumioxazin. Glomski (Reference Glomski2013) examined flumioxazin tank mixes with glyphosate for control of Japanese knotweed (Reynoutria japonica Houtt.) at 0.05, 0.11, and 0.21 kg ha−1 with and without 2.0 kg ha−1 glyphosate. In that study, they reported no difference in treatment performance when flumioxazin was applied alone or with glyphosate. Our study found a comparable outcome, as flumioxazin was not an effective tank additive.
In conclusion, our findings suggest that variability in efficacy, as suggested by managers, is unlikely due to conferred resistance. Other abiotic factors such as drought, shade, and seasonality of treatment may be important. Additionally, water quality, which is well known to impact glyphosate performance, may be important (Nalewaja and Matysiak Reference Nalewaja and Matysiak1991). Variation in herbicide application methods for broadcast- and spot-treatment approaches should also be examined, as they likely vary considerably in dose among applicators, especially for spot treatments. While no indication of glyphosate tolerance or resistance was observed, the ecological implications of detected growth differences among some I. cylindrica populations, albeit minor, also indicates a need for additional studies to better understand the invasion ecology of I. cylindrica in the southeastern United States. Finally, aminocyclopyrachlor, which demonstrated considerable activity on I. cylindrica, should be further examined, especially from a non-target perspective. Identifying the susceptible species range to determine potential collateral impacts of this active ingredient in a resistance management rotation would be very useful.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2018.12
Acknowledgments
The authors would like to thank Drew Hiatt for collecting and sharing all I. cylindrica populations used in this study. This research was supported by the University of Florida Center for Aquatic and Invasive Plants and by the USDA Forest Service Southern Research Station (14CA1130129051).






