Traditional Chinese medicine has been widely used for thousands of years to treat fractures and joint diseases. Many herbs shown to have kidney-tonifying activities are used in traditional Chinese medicine formulae for prevention and treatment of osteoporosisReference Yang, Xu, Yang, Lin, Liu and Yuan1. Although these herbal medicines are regarded as cost-effective alternatives by their traditional users, their international acceptance as a major regimen for prevention and treatment of osteoporosis require extensive research using modern science.
The source of the crude drug, Fructus Ligustri Lucidi (FLL, Chinese name, Nvzhenzi), is the fruit of Ligustrum lucidum Ait 2. It has been used in traditional Chinese medicine for over 1000 years, mainly to treat ailments such as menopausal problems, blurred vision, tinnitus, rheumatic pains, palpitations, backache, insomnia as well as to alleviate age-related symptomsReference Liang3–Reference Li, Lau and Fung5. In the theory of traditional Chinese medicine, it has the effects of maintaining healthy energy and nourishing the liver and kidneys. It is therefore a commonly prescribed herbal material in a number of formulae used to tonify the kidneys and strengthen boneReference Wang, Zeng, Xu and Xie6, Reference Huang and Yang7.
Modern research has shown that FLL is useful for prevention of bone-marrow loss in cancer patients receiving chemotherapyReference Bown8 and could increase estrogen receptor (ER)ß mRNA expression in amygdale of male ratsReference Cai, Du and Cao9. Hao et al. observed that FLL induced ultrastructural changes on the corticotrophs of rat pituitary gland and provided morphological evidence for the action of FLL in modulating endocrine functionReference Hao, Bi, Yu, Sun and Ding10. Our previous study indicated that Ca balance was improved and Ca absorption rate was elevated in ovariectomized (OVX) rats treated with FLLReference Zhang, Wong, Chen, Leung, Wu and Yao11, Reference Zhang, Lai, Leung, Wu, Yao and Wong12.
Ca homeostasis is regulated by complex hormonal signals in vivo, including parathyroid hormone, 1,25-dihydroxyvitamin D3 and calcitoninReference Zhang, Lai, Wu, Favus, Leung and Wong13–Reference Lips15. Organ systems that are actively involved in maintaining Ca homeostasis include the parathyroid gland for sensing serum Ca level, duodenum for active Ca absorption from diet, kidney for Ca reabsorption, as well as bone for maintaining extracellular fluid Ca levelReference Zhang, Lai, Wu, Favus, Leung and Wong13–Reference Lips15. Our previous results demonstrated that FLL could improve Ca balance, suggesting that FLL might act directly on the axis of Ca homeostasis. However, it is unclear whether the improvement of Ca balance by FLL extract would result in bone-protective effects and whether the effects of FLL extract will be influenced by dietary Ca levels. Thus, the current study was designed to investigate the protective effect of FLL extract on bones in oestrogen-replete and oestrogen-depleted rats fed diets containing different level of Ca.
To study the action of FLL on bone formation, rat osteoblastic UMR-106 cells were used. UMR-106 cells share a number of phenotypic properties with mature osteoblasts. These similarities include morphological appearance, responsiveness to calciotropic agents, and a relative high level of expression of cell surface alkaline phosphatase activityReference Stanford, Jacobson, Eanes, Lembke and Midura16. The formation of a calcified extracellular matrix in a controlled cell-mediated manner signifies the final stage of osteoblast differentiation and bone formationReference Declercq, Van den Vreken, De Maeyer, Verbeeck, Schacht, De Ridder and Cornelissen17, Reference Declercq, Verbeeck, De Ridder, Schacht and Cornelissen18. Thus, to determine whether FLL induced osteoblastic differentiation and bone formation, its effects on mineralized matrix formation in UMR-106 cells were studied.
The present study was conducted to investigate whether FLL extract exerts protective effects on bone mass and bone strength in aged sham-operated and OVX rats fed diets with different dietary Ca level. The direct effects of FLL extract on mineralization and extracellular mineral content in UMR-106 cells were also evaluated.
Materials and methods
Preparation of Fructus Ligustri Lucidi extract
FLL was obtained from the Jiangsu province of China. A voucher specimen was deposited in the school of Chinese medicine, the Chinese University of Hong Kong. The dried and powdered (25 kg) of the crude plant was extracted with 70 % ethanol twice, the preparation was filtered and concentrated under vacuum to produce a viscous residue at a yield of 38·1 %, by weight of the starting materials. For in vivo studies 15 ml portions of extract were stored at − 20°C until use when they were diluted tenfold with distilled water. For in vitro studies the extract powder was prepared by freeze drying, stored in the desiccator and reconstituted on the day of use with sterilized water.
Oleanolic acid and ursolic acid are the two main active compounds in FLL and oleanolic acid is a commonly used marker for authentication of FLL according to the Chinese Pharmacopoeia. Fig. 1 shows a typical chromatographic profile including standard samples and FLL extract. Oleanolic acid and ursolic acid were adequately resolved from other unknown compounds and could be clearly identified by retention time, confirming the identity of the FLL extract.

Fig. 1 Reverse-phase HPLC for the qualitative analysis of oleanolic acid and ursolic acid in the ethanol extract of Fructus Ligustri Lucidi (FLL). The chromatograms shown are for standard samples (oleanolic acid (![]() .) and ursolic acid (
.) and ursolic acid (![]() ) and FLL extract (
) and FLL extract (![]() ).
).
Animal experiment
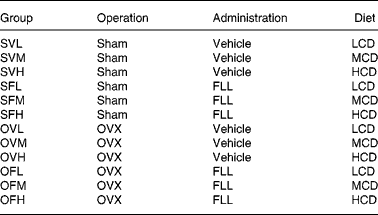
Female, retired breeder Sprague-Dawley rats (Experimental Animal Centre of Guangdong province, Guangzhou, China) were used in this study. The rats had six previous reproductive cycles and were about 11 months of age at the beginning of the study. The rats were housed in a room which provided alternating 12 h periods of light and dark with the room temperature at 23 ± 1°C and humidity 55 ± 5 %. Rats (n 120) were divided randomly into two groups and subjected to operation as follows: sham-operation (Sham, n 60) and ovariectomy (OVX, n 60). During recovery from operation, all rats were fed medium-Ca diet (MCD, TD 98 005, 0·6 % Ca, 0·65 % P) for 10 d before the treatment regimen was initiated. Both the Sham and OVX rats were then randomly and equally divided into three groups and fed with diets containing different levels of Ca: low-Ca diet (LCD, TD 05 004, 0·1 % Ca, 0·65 % P), MCD and high-Ca diet (HCD, TD 05 005, 1·2 % Ca, 0·65 % P). The nutritional composition of the different diets is shown in Table 1. Sham and OVX rats fed with diets containing different level of Ca were treated orally with either FLL extracts (700 mg/kg) or its vehicle for 12 weeks. Animal grouping is shown in Table 2. The body weight of the animals was recorded weekly for adjusting administration of FLL. At sacrifice, the tibias and femurs were collected, cleaned of all soft tissue, wrapped together with intact lumbar vertebra in saline-soaked towels, and stored at − 20°C for further analysis. The experimental protocol was approved by the Animal Ethic Committee of the Hong Kong Polytechnic University.
Table 1 Diet composition
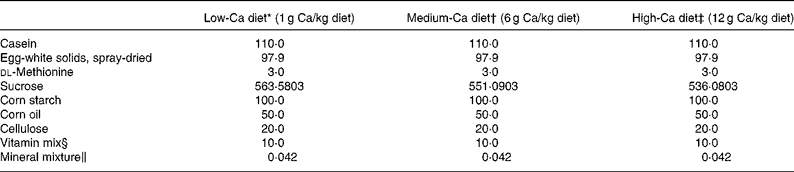
* Harlan Teklad, Madison, WI. Low Calcium Diet (LCD, TD #05 004). This formula is a modification of TD #98 005 to reduce Ca to 0·1 %.
† Harlan Teklad, Madison, WI. Control Diet (MCD, TD #98 005). This formula is a modification of TD #86 464.
‡ Harlan Teklad, Madison, WI. High Calcium Diet (HCD, TD #05 005). This formula is a modification of TD #98 005 to increase Ca to 1·2 %.
§ Vitamin mixture from Harlan Teklad (#40 060) provided (mg/kg diet): p-aminobenzoic acid, 110·1; ascorbic acid, coated, 1016·6; biotin, 0·44; vitamin B-12, 29·7; calcium pantothenate, 66·1; choline dihydrogen citrate, 3496·9; folic acid, 1·98; inositol, 110·1; menadione, 49·5; niacin, 99·1; pyridoxine HCl, 22·0; riboflavin, 22·0; thiamin HCl, 22·0; dry vitamin A retinyl palmitate (500,000 U/g), 39·65; dry cholecalciferol (500,000 U/g), 4·4; dry vitamin E dl-α-tochopherol acetate (500 U/g), 242·3; cornstarch (diluent), 4666·9.
‖ Mineral mixture from Harlan Teklad provided (mg/kg diet): potassium phosphate, monobasic, 24·6; calcium carbonate, 14·74 for MCD, 2·25 for LCD and 29·75 for HCD; potassium chloride, 5·6; sodium bicarbonate, 4·62; magnesium oxide, 3·83; sodium chloride, 3·7; sodium selenite, 0·5; ferric citrate, 0·21; manganous carbonate, 0·123; zinc carbonate, 0·056; chromium potassium sulfate (12H2O), 0·0193; cupric carbonate, 0·011; and potassium iodate, 0·0004.
Table 2 Animal grouping, according to operation (OVX, ovariectomized), administration (FLL, Fructus Ligustri Lucidi) and diet (LCD, low-Ca diet; MCD, medium-Ca diet; HCD, high-Ca diet)
Bone mass determination in rat tibia, femur and lumbar vertebra
Tibias, femurs and lumbar vertebra were thawed at room temperature before testing, and bone mass was measured by peripheral quantitative computerized tomography with an XCT2000 machine (Norland Stratec Medizintechnik GmbH, Birkenfeld, Germany) aided by specially designed software for small animals. Cone Phantom (Slice 1, 787 mm2; Slice 2, 0·539 1/cm; Slice 3, 0·333 1/cm; Slice 4, 0·436 1/cm) and Standard Phantom (0·436 1/cm) were scanned to calibrate the instrument. The exact position of the tibia within the gantry core of the XCT machine was visualized with a fan beam from a 58 kV X-ray source with a current of 0·178 mA. The site chosen for scanning was composed mainly of trabecular bone and was located 2·5 mm distal to the proximal end and the distal end, and the intermediate scan for cortical bone was centred on the mid-shaft of long bone. The intact lumbar vertebra 2 (LV-2) was also measured. Analysis of these scans produced measurement of volumetric bone mineral density (BMD) and bone mineral content (BMC). Humidity was maintained at 50 % to 60 % during the measurements. CV obtained for BMD of the same long bone and spine bone after repositioning five times were 0·78 % and 0·89 %, respectively.
Bone strength of rat tibia
Rat tibias then underwent a three-point bending mechanical test on Hounsfield material testing machine (Model H10KM, Hounsfield Test Equipment Limited, Surrey, UK) to determine maximal load (N, a measure of the maximum force that the bone withstood before fracture) and stiffness (N/mm, a measure of the extrinsic rigidity of the bone). Each specimen was placed on two supports spaced 15 mm apart and the load was applied to the bone midway between the supports at a deformation rate of 2 mm/min until fracture occurred. The tibias were positioned such that bending occurred about the medial–lateral axis. Load-deformation curves were recorded during the bending process.
Cell culture
UMR-106 cells, a clonal osteoblastic cell line derived from rat osteosarcoma (ATCC no. CRL-1661), were routinely cultured in Dulbecco's modified Eagle medium supplemented with 5 % fetal bovine serum, penicillin (100 U/ml) and streptomycin (100 μg/ml). Dulbecco's modified Eagle medium, fetal bovine serum, and penicillin–streptomycin were purchased from Life Technologies Inc (Carlsbad, California, USA). At about 80 % confluence, cells were seeded in a 96-well microtiter plate (Falcon, Becton-Dickinson, Franklin Lakes, NJ, USA) and 12-well plate (NUNC, Roskilde, Denmark) at a density of 5 × 103 and 6 × 104 cells/well, respectively. Upon confluence in the 96-well plate, cells were starved in serum-free Dulbecco's modified Eagle medium for another 24 h. Cells were then treated with FLL extract at 0·1 μg/ml, 1 μg/ml, 10 μg/ml and 100 μg/ml for 24 h or 48 h. Cells were cultured at 37°C in a humidified atmosphere of 95 % air and 5 % CO2.
Cell- proliferation assay
The medium was removed and 10 μl of the combined 3-(4,5-dimethy1-2-yl)-5-(-3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS)/phenazine methosulfate (PMS) (Promega, Madison, WI, USA) solution was added to each well of the 96-well plate containing 90 μl PBS. The plate was incubated for 1 h at 37°C in a humidified humidified atmosphere of 95 % air and 5 % CO2. The absorbance at 490 nm was recorded using an ELISA plate reader.
Nodule formation
Upon confluence in 6-well multiplates, cells were cultured in mineralizing medium (Dulbecco's modified Eagle medium supplemented with 5 % fetal bovine serum, 10 mm sodium ß-glycerophosphate, 50 ug/ml ascorbic acid and FLL extracts; the final concentration was the same as for cell-proliferation tests) and were refreshed at 3-d intervals. Nodule was stained with Alizarin red (Chemical Reagents Corp., Beijing, China) for detection of Ca deposit on day 3 and day 6. Briefly, cells were washed twice with PBS, fixed for 1 h at 4°C in 70 % ethyl alcohol, and subjected to staining with Alizarin red solution (0·1 %) for 10 min at room temperature. Nonspecific staining was removed by repeated washing using water.
Determination of extracellular Ca and P deposits
Upon medium removal, cells were washed twice with distilled water. Five hundred microlitres HCl (1m) was added to each well in 6-well plate. The well was rinsed with another 500 μl HCl (1m) and the solutions were pooled. Cell debris was removed by brief centrifugation. Ca and P ion concentrations in the extracellular matrix were determined by commercial kits (Zhongsheng Beikong Bio-technology and Science Inc, Beijing, China).
Statistical analysis
The data from these experiments is reported as means with their standard errors for each group. Analysis of the effects of herb and OVX as grouping variables was performed by two-way ANOVA at each dietary Ca level and the effect of different Ca diets as one intervention was also analyzed by two-way ANOVA both in Sham rats and in OVX rats. Inter-group differences were analyzed by one-way ANOVA, followed by using Tukey's post hoc test to compare the group means (GraphPad PRISM, version 4.0). Values of P < 0·05 were considered statistically significant.
Results
Bone mass in rat tibia
Two-way ANOVA analysis (Table 3) showed that (1) ovariectomy significantly induced bone loss of proximal metaphysis (P < 0·001) and diaphysis (P < 0·05) at all dietary Ca levels and distal metaphysis (P < 0·01) at LCD and MCD; (2) different Ca-containing diets produced marked influence on BMD (P < 0·05) and BMC (P < 0·05) of tibial diaphysis in Sham rats, moreover, there was the interaction (P < 0·05) between Ca diets and FLL administration on the two parameters; (3) dietary Ca restriction resulted in the decline (P < 0·01) of BMD of tibial distal metaphysis in OVX rats; (4) FLL did not alter BMD at either proximal metaphysis or distal metaphysis of tibia in rats fed diet of any Ca level; (5) FLL could significantly increase BMD (P = 0·0355) and BMC (P = 0·0476) of the tibal diaphysis in rats fed LCD, as well as increase BMC (P = 0·0047) of the tibal diaphysis in rats fed MCD. No improvement on bone mass of tibal diaphysis was observed in rats fed HCD. As shown in Fig. 2, FLL significantly increased BMC at tibal diaphysis in Sham rats fed LCD (v. vehicle- treated group, P < 0·05) and MCD (v. vehicle treated group, P < 0·01), whereas FLL did not alter bone mass at any scanning sites of the tibia in all OVX rats. However, it should be noted that both HCD alone (P < 0·05) and co-treatment of FLL and HCD (P < 0·05) could prevent LCD-induced bone loss at the diaphysis and distal metaphysis of tibia in OVX rats (v. vehicle-treated LCD fed OVX rats).
Table 3 Bone mass measured by peripheral quantitative computerized tomography in rat tibia
(Mean values and standard errors for between six and eight rats per group)

LCD, low-Ca diet; MCD, medium-Ca diet; HCD, high-Ca diet; FLL, Fructus Ligustri Lucidi; OVX, ovariectomized; S, Sham; O, OVX; V, Vehicle; F, FLL; L, LCD; M, MCD; H, HCD; BMD, bone mineral density; BMC, bone mineral content.
§ Inter-group differences were analyzed by one-way ANOVA, followed by Tukey's post hoc test.
abcd Mean values in columns of the same dietary-Ca level with unlike superscript letter are significantly different: abP < 0·05; cdP < 0·01.
*P < 0·05,
**P < 0·01,
***P < 0·001, statistical analysis by two-way ANOVA.
† P < 0·05, ††† P < 0·001, compared with rats in OVL group.
‡ P < 0·05, ‡‡P < 0·01, P value in parentheses shows the interaction between Ca diets and FLL feeding.

Fig. 2 (a) Bone mineral density (BMD) and (b) bone mineral content (BMC) of tibial diaphysis. Measurements for treatment groups (S, Sham; O, ovariectomized; V, Vehicle; F, Fructus Ligustri Lucidi) on different diets (LCD, low-Ca diet (L); MCD, medium-Ca diet; HCD, high-Ca diet) shown are: □, SV; ![]() , SF;
, SF; ![]() , OV; ■, OF. Values are expressed as means with standard errors indicated by vertical bars (n 6–8). Inter-group differences were analyzed by one-way ANOVA, followed by Tukey's post hoc test. *P < 0·05; **P < 0·01, v. V-treated group receiving similar treatment; †P < 0·05, v. OVL.
, OV; ■, OF. Values are expressed as means with standard errors indicated by vertical bars (n 6–8). Inter-group differences were analyzed by one-way ANOVA, followed by Tukey's post hoc test. *P < 0·05; **P < 0·01, v. V-treated group receiving similar treatment; †P < 0·05, v. OVL.
Bone mass in rat femur
Two-way ANOVA analysis (Table 4) indicated that (1) ovariectomy significantly decreased BMD of femoral proximal (P < 0·01) and distal (P < 0·001) metaphysis and reduced BMD and BMC of femoral diaphysis (P < 0·01) at all dietary Ca levels; (2) no statistically significant effect of different Ca-containing diet on bone mass at any bone site was found either in Sham rats or in OVX rats; (3) similar to the effects on rat tibia, FLL treatment did not alter the bone mass of the proximal and distal metaphysis of the rat femur; (4) FLL treatment significantly increased BMC (P = 0·0377) of femoral diaphysis in rats fed LCD and increased BMD (P = 0·0121) and BMC (P = 0·0418) of femoral diaphysis in rats fed MCD. As shown in Fig. 3, FLL treatment increased BMC of femoral diaphysis in Sham rats fed LCD (v. SVL group, P < 0·05) and increased BMD of femoral diaphysis in OVX rats fed MCD (v. OVM group, P < 0·05). In contrast, FLL did not alter bone mass in Sham and OVX rats fed HCD, suggesting that high-Ca diet feeding might mask the positive effect of FLL on bone mass.
Table 4 Bone mass measured by peripheral quantitative computerized tomography in rat femur
(Mean values and standard errors for between six and eight rats per group)

LCD, low-Ca diet; MCD, medium-Ca diet; HCD, high-Ca diet; FLL, Fructus Ligustri Lucidi; OVX, ovariectomized; S, Sham; O, OVX; V, Vehicle; F, FLL; L, LCD; M, MCD; H, HCD; BMD, bone mineral density; BMC, bone mineral content.
ab Mean values in columns of the same dietary-Ca level with unlike superscript are significantly different: P < 0·05.
† P < 0·05, compared with rats in OVL group.
*P < 0·05, **P < 0·01, ***P < 0·001, statistical analysis by two-way ANOVA.
§ Inter-group differences were analzed by one-way ANOVA, followed by Tukey's post hoc test.

Fig. 3 (a) Bone mineral densiy (BMD) and (b) bone mineral content (BMC) of femoral diaphysis. Measurements for treatment groups (S, Sham; O, ovariectomized; V, Vehicle; F, Fructus Ligustri Lucidi) on different diets (LCD, low-Ca diet (L); MCD, medium-Ca diet; HCD, high-Ca diet) shown are: □, SV; ![]() , SF;
, SF; ![]() , OV; ■, OF. Values are expressed as means with standard errors indicated by vertical bars (n 6–8). Inter-group differences were analyzed by one-way ANOVA, followed by Tukey's post hoc test. *P < 0·05, v. V-treated group receiving similar treatment.
, OV; ■, OF. Values are expressed as means with standard errors indicated by vertical bars (n 6–8). Inter-group differences were analyzed by one-way ANOVA, followed by Tukey's post hoc test. *P < 0·05, v. V-treated group receiving similar treatment.
BMD in rat lumbar vertebra
The effects of ovariectomy, FLL and different dietary Ca level on BMD of the LV-2 in rats fed diets containing different level of Ca are shown in Table 5. Two-way ANOVA analysis indicated that (1) ovariectomy produced significant decrease of spine BMD in rats at each dietary Ca level (P < 0·05, Table 5); (2) low-Ca feeding decreased BMD of spine in Sham rats (P < 0·01, Table 5); (3) FLL treatment resulted in statistically significant elevation of BMD in LV-2 in rats fed LCD (P < 0·05, Table 5). As shown in Fig. 4, BMD of LV-2 was significantly increased by FLL-treatment in both Sham (P < 0·05) and OVX (P < 0·05) rats fed LCD (v. vehicle-treated rats). However, no obvious change was observed in rats fed MCD and HCD in response to FLL treatment (v. vehicle-treated group, NS), regardless of their levels of oestrogen in vivo. It should be noted that both high-Ca diet alone (P < 0·05) and in combination with FLL (P < 0·01) could significantly elevate BMD of LV-2 in rats regardless of their oestrogen level (v. vehicle-treated rats fed LCD).
Table 5 Two-way ANOVA analysis of the effects of ovariectomy (OVX) and Fructus Ligustri Lucidi (FLL) in rats fed different Ca-containing diets†, and different dietary Ca level in Sham and OVX rats on bone mineral density of lumbar vertebra

LCD, low-Ca diet; MCD, medium-Ca diet; HCD, high-Ca diet.
† Effect of different Ca diets and herbal treatment, which are the two interventions in the analysis of two-way ANOVA, was evaluated in Sham rats and OVX rats, respectively.
*P < 0·05, **P < 0·01, statistical analysis by two-way ANOVA.

Fig. 4 Bone mineral density (BMD) of lumbar vertebra-2. Measurements for treatment groups (S, Sham; O, ovariectomized; V, Vehicle; F, Fructus Ligustri Lucidi) on different diets (LCD, low-Ca diet (L); MCD, medium-Ca diet; HCD, high-Ca diet) shown are: □, SV; ![]() , SF;
, SF; ![]() , OV; ■, OF. Values are expressed as means with standard errors indicated by vertical bars (n 6–8). Inter-group differences were analyzed by one-way ANOVA, followed by Tukey's post hoc test. *P < 0·05, v. V-treated group; †P < 0·05, ††P < 0·01, v. SVL; ‡P < 0·05, ‡‡ P < 0·01, v. OVL.
, OV; ■, OF. Values are expressed as means with standard errors indicated by vertical bars (n 6–8). Inter-group differences were analyzed by one-way ANOVA, followed by Tukey's post hoc test. *P < 0·05, v. V-treated group; †P < 0·05, ††P < 0·01, v. SVL; ‡P < 0·05, ‡‡ P < 0·01, v. OVL.
Bone strength in rat tibia
Two-way ANOVA showed that (1) ovariectomy led to the reduction of the maximal load (P < 0·01, Table 6) and stiffness (P < 0·05, Table 6) at tibial diaphysis in rats fed MCD; (2) dietary Ca deficiency has significant effect on the maximal load (P < 0·01) and stiffness (P < 0·001) of tibial diaphysis in Sham rats, while this effect interplayed with FLL administration (Table 6); (3) FLL treatment increased the maximal load of tibia diaphysis in rats fed MCD (P < 0·05, Table 6), and the stiffness of tibia diaphysis in rats fed LCD (P < 0·05, Table 6) and MCD (P < 0·01, Table 6). As shown in Fig. 5, in response to FLL treatment, the maximal load and stiffness of Sham rats fed MCD were elevated by 12·0 % (P < 0·05) and 32·6 % (P < 0·01), respectively. Similarly, FLL increased the maximal load and stiffness of OVX rats fed LCD by 20·3 % (P < 0·05) and 22·2 % (P < 0·05), respectively. In contrast, FLL treatment did not alter the bone strength of tibial diaphysis in Sham rats and OVX rats fed HCD.
Table 6 Two-way ANOVA analysis of the effects of ovariectomy (OVX) and Fructus Ligustri Lucidi (FLL) in rats fed different Ca-containing diet, and different dietary Ca level in Sham and OVX rats on bone strength of tibial diaphysis‡

LCD, low-Ca diet; MCD, medium-Ca diet; HCD, high-Ca diet.
‡ Effect of different Ca diets and herbal treatment, which are the two interventions in the analysis of two-way ANOVA, was evaluated in Sham rats and OVX rats, respectively.
*P < 0·05, **P < 0·01, ***P < 0·001, statistical analysis by two-way ANOVA.
†P < 0·05, †††P < 0·001, P value in parentheses showed the interaction between Ca diets and FLL feeding.

Fig. 5 (a) Maximal load and (b) stiffness of rat tibial diaphysis in three-point bending test. Measurements for treatment groups (S, Sham; O, ovariectomised; V, Vehicle; F, Fructus Ligustri Lucidi) on different diets (LCD, low-Ca diet (L); MCD, medium-Ca diet; HCD, high- Ca diet) shown are: □, SV; ![]() , SF;
, SF; ![]() , OV; ■, OF. Values are expressed as means with standard errors indicated by vertical bars (n 10). Inter-group differences were analyzed by one-way ANOVA, followed by Tukey's post hoc test. *P < 0·05; **P < 0·01, v. V-treated group receiving similar treatment.
, OV; ■, OF. Values are expressed as means with standard errors indicated by vertical bars (n 10). Inter-group differences were analyzed by one-way ANOVA, followed by Tukey's post hoc test. *P < 0·05; **P < 0·01, v. V-treated group receiving similar treatment.
Effect of Fructus Ligustri Lucidi treatment on osteoblastic cell functions
The effect of FLL on osteoblastic cell proliferation was studied by treatment of rat osteoblastic UMR-106 cells with FLL extract at the concentration of 0·1–100 μg/ml for 24 and 48 h. The results showed that FLL did not alter cell proliferation, and higher doses did not result in cytotoxic effect in UMR-106 cells (data not shown). To evaluate the effects of FLL on mineralization, UMR-106 cells were incubated in mineralizing medium upon confluence. Upon staining with Alizarin red for extracellular calcified matrix, it was found that mineralization began to occur after 3d incubation and became nearly complete after 6d incubation. The result clearly showed that the densities of calcified deposits as observed under light microscopy increased with increasing concentration of FLL extracts (data not shown). As shown in Fig. 6, extracellular Ca and P levels of UMR-106 cells treated by FLL extract (10 μg/ml) were both significantly elevated by day 3 and their levels were significantly increased by 1-2 fold in UMR-106 cells treated with 1–100 μg/ml by day 6. The increase in extracellular mineral content of UMR-106 cells by FLL extract (0·1 μg/ml to 10 μg/ml) was dose-dependent with a maximum at 10 μg/ml.

Fig. 6 The extracellular levels of (a) Ca deposit and (b) phosphate (P) deposit in rat osteoblast-like UMR 106 cells. Cells were cultured in mineralizing medium supplemented with vehicle (C) and Fructus Ligustri Lucidi (FLL) extracts at the concentration of 0·1, 1, 10, 100 μg/ml for 3 d (□) and 6 d (■). Values are expressed as means with standard errors indicated by vertical bars. Results are obtained from three independent experiments. *P < 0·05; **P < 0·01; ***P < 0·001, v. C.
Discussion
The present study is the first to evaluate the effect of the ethanol extract of FLL on bone properties in aged normal and OVX rats. Our results indicated that significant improvement of bone mass by FLL could be found at the diaphysis of tibia and femur in aged rats fed either LCD or MCD, but not in those fed HCD. These results suggest that the effect of FLL on bone mass was influenced by the level of dietary Ca intake. Our results also indicated that the effects of FLL extract on bone were site-specific as it could only preserve bone mass and bone strength at the diaphysis in the appendicular bones as well as BMD in the lumbar spine, but not at the proximal metaphysis and distal metaphysis of the appendicular bones in aged rats. In addition, the loss of bone mass at the diaphysis and distal metaphysis of appendicular bones induced by LCD and ovariectomy in aged rats could also be protected by co-treatment with HCD and FLL. Most importantly, the results from our in vitro studies provide additional evidence for the protective effects of FLL on bone as enhancement of matrix ossification was found in rat osteoblast-like UMR-106 cells treated with FLL extract.
Appendicular bone loss induced by ovariectomy was much more prominent than that by Ca-deplete diet in this study, demonstrated by the significant reduction of BMD of cancellous bone and cortical bone in OVX rats shown in Tables 3 and 4, which was inconsistent with our previous reportsReference Zhang, Lai, Wu, Favus, Leung and Wong13, Reference Zhang, Lai, Chow, Wu and Wong19. In addition, detrimental effects of Ca restriction on appendicular bone quality were more serious in OVX rats than that in Sham rats, which could be attributed to, at least partially, our recent observation that ovariectomy worsens secondary hyperparathyroidism in mature rats during LCDReference Zhang, Lai, Wu, Favus, Leung and Wong13.
In addition, it appeared that appendicular bone loss at the tibia diaphysis caused by ovariectomy in rats fed all levels of dietary Ca only resulted in the change of bone strength in rats fed MCD, but not in those fed LCD or HCD. These results suggest that the significant change in bone mass by ovariectomy might not lead to a change in bone strength in rats. As bone mass is not the only determinant of bone strength, our results suggest that dietary Ca intake might alter other determinants such as bone microarchitecture that results in the protection against the loss of bone strength in rats fed LCD or HCD.
In our previous study, we demonstrated that FLL extract could suppress the induction of bone turnover markers by ovariectomy and improve Ca balance in young OVX rats. Based on these results, we hypothesized that FLL could protect against bone loss via its actions on the axis of Ca homeostasis. To determine whether the actions of FLL are mediated through the Ca homeostasis axis, both Sham and OVX aged Sprague-Dawley rats were subjected to different dietary regimen containing low, medium or high level of Ca content. The results in the present study clearly demonstrated that FLL did not offer additional benefit to HCD in protection against bone loss in aged intact and OVX rats, suggesting that the bone protective effects of FLL extract are influenced by dietary Ca levels. These results support our hypothesis that the actions of FLL on bone are mediated through its positive effect on Ca balance as diets containing high level of Ca alone (HCD) could improve Ca balance in aged intact and OVX rats to a level that prevent further improvement of Ca balance and bone mass that could otherwise be achieved by FLL treatments in rats fed MCD and LCD. It is also interesting to note that FLL treatment can increase bone mass and bone strength at different sites in both Sham and OVX aged rats fed MCD and LCD, suggesting that the effect of FLL on bone are independent of the oestrogen status of the animals.
The results in the present study also indicated that the actions of FLL on bone were site-specific. It could protect against the loss of bone mass and improve mechanical property at the diaphysis, but not at the epiphysis, of appendicular bones in aged rats. The results indicated that FLL preferentially increased cortical bone mass, but not trabecular bone mass, in appendicular bones in aged rats. In fact, similar bone-compartment-specific actions were previously reported in other studies when animals were treated with the complex of rhIGF-I/IGFBP-5Reference Bauss, Lang, Dony and Kling20, retinoidReference Kneissel, Studer, Cortesi and Šuša21, long-chain n-3 PUFAReference Shen, Yeh, Rasty, Li and Watkins22, or daidzeinReference Fonseca and Ward23. All of these agents act solely on cortical bone, but not on trabecular bone, in their experimental conditions.
However, it should be noted that FLL improved spine BMD in rats fed LCD, suggesting that FLL could also increase trabecular bone content in animals when the dietary Ca level was low. The different effects of FLL observed in trabecular bone of spine and appendicular bone might be due to the differences in sensitivity of the trabecular bone at these two sites to Ca restriction. A previous study reported that redistribution of bone mass from axial to appendicular skeleton occurred in rats receiving Ca restrictionReference Geng, DeMoss and Wright24, suggesting that trabecular bone mass in spine would be preferentially reduced in response to LCD feeding. Thus, the positive effect of FLL on BMD in both Sham and OVX rats demonstrated at the spine, but not at the appendicular sites, could be due to the much reduced trabecular bone mass found at the spine in response to LCD.
Our results indicated that either HCD alone or in combination with FLL could enhance bone mass at both the corticial bone-rich diaphysis and trabecular bone-rich distal metaphysis of tibia and femur in OVX rats when compared to vehicle-treated OVX rats fed LCD. The results suggest that Ca has a greater protective effect on bone than FLL because treatment of aged OVX rats fed LCD with FLL alone (OFL group) did not exhibit protective effects on bone. The lack of bone protective effects of FLL extract in OVX rats fed LCD indicate that the action of FLL on bone requires the presence of sufficient supply of dietary Ca to compensate for the bone loss due to the negative Ca balance created by oestrogen deficiency in OVX rats. Nonetheless, the effects of FLL cannot be disregarded as FLL, when combined with HCD, preserved bone mass more favourably than HCD alone. Similarly, BMD of lumbar vertebra in aged Sham and OVX rats treated with FLL and HCD were higher than those in corresponding group when fed LCD alone (Fig. 4). These findings suggest that the combination of FLL and HCD can positively influence bone mass and bone strength in aged rats.
The fact that FLL treatment could promote calcification of the extracellular matrix of osteoblastic cells suggests that FLL could act directly on osteoblastic cells and promote bone formation. Our results, however, showed that FLL extract did not alter osteoblastic cell proliferation, suggesting that its positive actions on bone might be at the later stage of osteoblastic cell development. The observed enhancement of mineralization may, at least partially, account for the increase of cortical bone mass in the appendicular bones by promoting new bone formation.
The present study is the first to reveal the bone protective effects of FLL and its direct effects on the process of bone mineralization. The fact that its bone protective actions are not affected by oestrogen status suggests that its action might be different from other phytooestrogen-containing herbs. In addition, its actions on bone appear to be masked by the consumption of HCD, suggesting that it might directly work on the axis of Ca homeostasis. Future study will be required to delineate the molecular mechanism involved in bone protective effects of this herb and to identify the active fractions or constituents that could account for its biological actions. Taken together, FLL could be considered as a potential alternative regimen for prevention and treatment of osteoporosis. It could increase bone mass and bone strength in aged rats possibly through its actions on Ca homeostasis and bone mineralization.
Acknowledgements
The authors thank the State Key Laboratory of Chinese Medicine and Molecular Pharmacology for providing the support in carrying out this study. This work was supported by the Areas of Excellence Scheme established under the University Grants Committee of the Hong Kong Special Administrative Region, China (AOE/P-10/01) as well as by the CERG grant (PolyU 5402/04M) from the Hong Kong Research Grant Council, HKSAR.














