Twin–twin transfusion syndrome (TTTS), a severe complication of monochorionic (MC) twin pregnancy, leads to high morbidity and mortality if untreated (Berghella & Kaufmann, Reference Berghella and Kaufmann2001). The standard treatment option for TTTS is fetoscopic laser surgery (FLS) to photocoagulate placental vascular anastomoses, which are the primary etiology of TTTS (Rossi & D’Addario, Reference Rossi and D’Addario2008; Sago et al., Reference Sago, Ishii, Sugibayashi, Ozawa, Sumie and Wada2018; Sago &Wada, Reference Sago and Wada2020; Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004). The goal of FLS is to ablate all intertwin anastomoses; however, when anastomoses remain patent, recurrent TTTS or twin anemia polycythemia sequence (TAPS) can occur after FLS (Robyr et al., Reference Robyr, Lewi, Salomon, Yamamoto, Bernard, Deprest and Ville2006).
The incidence of residual anastomoses after FLS using the standard selective technique was reported to be up to 30% (Lopriore et al., Reference Lopriore, Middeldorp, Oepkes, Klumper, Walther and Vandenbussche2007); thus, the Solomon technique, in which the entire vascular equator is ablated, was developed to reduce residual anastomoses (Lopriore, Slaghekke et al., Reference Lopriore, Slaghekke, Middeldorp, Klumper, Oepkes and Vandenbussche2009; Slaghekke et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014). Since a randomized controlled trial revealed that the Solomon technique reduced the risk of complications associated with residual anastomoses (Slaghekke et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014), this technique has been widely adopted at fetal treatment centers around the world (Akkermans et al., Reference Akkermans, Peeters, Klumper, Lopriore, Middeldorp and Oepkes2015).
Initial studies concerning the Solomon technique found no adverse effects associated with the technique (Baschat et al., Reference Baschat, Barber, Pedersen, Turan and Harman2013; Dhillon et al., Reference Dhillon, Hillman, Pounds, Morris and Kilby2015; Slaghekke et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014). However, concerns remain regarding adverse events associated with the Solomon technique as it involves coagulation of the entire vascular equator, including many normal areas of the placenta that lack vascular anastomoses. There have been only two reports describing the adverse events associated with the Solomon technique: one regarding placental abruption (Lanna et al., Reference Lanna, Faiola, Consonni and Rustico2017) and the other regarding preterm premature rupture of the membranes (pPROM; Akkermans et al., Reference Akkermans, de Vries, Zhao, Peeters, Klumper, Middeldorp and Lopriore2017). The adverse effects associated with the use of the Solomon technique in the treatment of TTTS remain unclear.
In the present study, we evaluated the outcomes and adverse events after FLS to treat TTTS using the Solomon technique for the coagulation of placental anastomoses in comparison to those occurring in association with the use of the selective technique.
Methods
We conducted a retrospective analysis of a consecutive cohort of MC twin cases diagnosed with TTTS, in which FLS was performed at the National Center for Child Health and Development (NCCHD) from 2010 to 2017. In this study period, we performed FLS using the selective technique until July 2014 and then using the Solomon technique from August 2014. The definition of TTTS was based on the following criteria: polyhydramnios with a maximum vertical pocket (MVP) ≥8.0 cm in the recipient and oligohydramnios with an MVP ≤2.0 cm in the donor regardless of gestational age.
We compared the pregnancy outcomes and adverse events of cases treated with each technique in the study period. Cases in which FLS was discontinued because completion was not feasible due to visual or performance-related issues were excluded from the analysis. The following antenatal variables were analyzed: gestational age (GA) at FLS, Quintero stage (Quintero et al., Reference Quintero, Morales, Allen, Bornick, Johnson and Kruger1999), placenta location and operation procedure details (operation time, total laser energy). The pregnancy outcome and adverse events were as follows: incidence of preterm birth, pPROM, placental abruption, recurrent TTTS, TAPS following FLS, GA at delivery, interval from FLS to delivery, birth weight, fetal death and neonatal survival at 28 days. The definition of recurrent TTTS was the occurrence of TTTS at least 1 week after FLS. The presence of TAPS was defined by the antenatal or postnatal diagnostic criteria (Slaghekke et al., Reference Slaghekke, Kist, Oepkes, Pasman, Middeldorp, Klumper and Lopriore2010). The antenatal criteria were Doppler ultrasound abnormalities showing an increased peak systolic velocity in the middle cerebral artery (MCA-PSV) >1.5 multiples of the median (MoM) in one fetus, suggestive of anemia, and a decreased MCA-PSV <1.0 MoM in the other fetus, suggestive of polycythemia. The postnatal criteria were intertwin Hb difference >8.0 g/dl and reticulocyte count ratio >1.7 and/or placenta with only small vascular anastomoses. Placentas of cases with abnormal clinical findings were sent to NCCHD. A placenta study with colored dye was performed. Placental abruption was diagnosed based on clinical findings of vaginal bleeding and sudden-onset abdominal pain accompanied by uterine contractions, and/or a clot adhering to the maternal side of the placenta after delivery (Elsasser et al., Reference Elsasser, Ananth, Prasad and Vintzileos2010). Placental abruption was defined as displaying at least one severe maternal complication, such as disseminate intravascular coagulation, blood transfusion, hypovolemic shock, hysterectomy or death, or one fetal/neonatal complication, such as non-reassuring cardiotocography, preterm delivery, or death (Ananth et al., Reference Ananth, Lavery, Vintzileos, Skupski, Varner, Saade and Wright2016). Cases of pPROM <28 and <32 weeks were diagnosed based on the obvious clinical leakage of amniotic fluid from the vagina with subsequent delivery before 28 or 32 weeks of gestation, respectively.
FLS was performed similarly to previous reports (Sago et al., Reference Sago, Hayashi, Saito, Hasegawa, Kawamoto, Kato and Murakoshi2010; Sago et al., Reference Sago, Ishii, Sugibayashi, Ozawa, Sumie and Wada2018). Under local and regional anesthesia, a 3.8-mm cannula was percutaneously inserted into the recipient’s sac. A 2-mm fetoscope with a 3-mm sheath (Karl Storz, Tuttlingen, Germany) was used through the cannula. If required, amnioinfusion of warmed saline solution was administered into the recipient’s sac. All chorionic vascular anastomoses between the fetuses were selectively coagulated using a neodymium-doped yttrium aluminum garnet (Nd:YAG) laser. In the Solomon technique, we additionally coagulated a thin line of tissue at the placental surface to connect the sites of selective ablation from edge to edge. We usually used 20−30 watts of laser power; however, a higher power setting (up to 50 watts) was used if required. The amniotic fluid was subsequently drained until MVP ≤6 cm was achieved. Four operators were involved initially, and two had been changed before 2014, while one was added in 2017, thus resulting in a total of five operators being involved. One senior surgeon (HS) attended almost all operations as an instructor or a surgeon. The operation time was defined as the time from skin incision to skin closure, including the time of amnioinfusion and amnioreduction before and after laser coagulation. Perioperative management with prophylactic tocolysis and antibiotics and postoperative management for 2 weeks were provided at our center. Therapeutic abortion for fetal reasons is not allowed in Japan. Most patients returned to the referring perinatal centers, which were part of the national neonatal network, at 2 weeks after FLS. Prenatal management with weekly ultrasound, including pulsed Doppler of MCA, and delivery and neonatal managements were provided by each perinatal center. Request sheets for clinical data on pregnancy, delivery and children, which remained almost the same with only minor revisions during this cohort study, were brought to each perinatal center with patients. Clinical data on cases that were managed at the referring perinatal centers were sent to our center at 1 month after delivery or following discharge of children. Contact was maintained through our staff to perform follow-up.
Statistical Analyses
All statistical data were analyzed using the Stata software program, version 14.0 (Stata Corp, College Station, TX, USA). Where applicable, Fisher’s exact test, Student’s t test or the Mann–Whitney U test was used for comparisons between groups; p values of <.05 were considered to indicate statistical significance. Group differences in the risk of each pregnancy outcome or adverse event were compared using univariable logistic regression. A multivariate logistic regression analysis of risk factors for pPROM in all cases was conducted using factors that showed a significant association in the univariate analysis. The results of the logistic models were expressed as the odds ratio (OR) and 95% confidence interval (CI).
Results
Among 400 FLS cases, FLS was performed using the selective coagulation method in 230 cases managed between January 2010 and July 2014 (selective group) and using the Solomon technique in 170 cases managed between August 2014 and December 2017 (Solomon group). Three cases in which FLS was discontinued were excluded from the selective group. One case in which FLS was discontinued and one case in which the patient was lost to follow-up were excluded from the Solomon group. Thus, the 227 cases in the selective group and 168 cases in the Solomon group were included in the analysis (Figure 1).
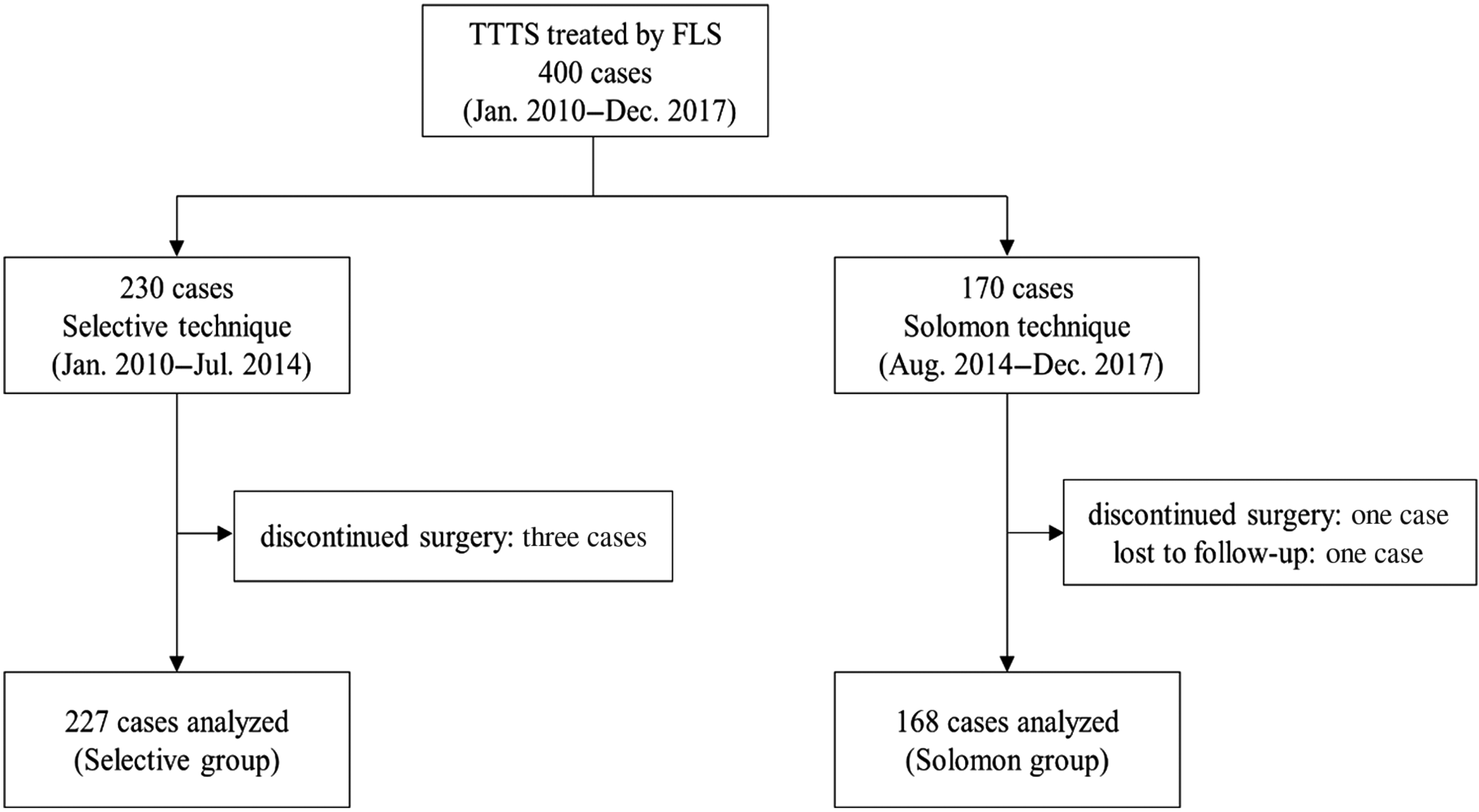
Fig. 1. A flow diagram of cases of twin–twin transfusion syndrome treated by fetoscopic laser surgery.
Note: FLS, fetoscopic laser surgery.
Table 1 shows the patient characteristics and procedure details of each group. There were no significant differences between the groups in characteristics such as the maternal age, BMI, GA at FLS (21.1 ± 2.4 weeks in the selective group vs. 20.8 ± 2.7 weeks in the Solomon group, p = .245), Quintero stage or placenta location. There were significant differences between the groups in the procedure findings. In the Solomon group, the operation time was significantly shorter (52.7 ± 18.4 vs. 59.6 ± 19.6 minutes, p < .001) and the total laser energy was higher (median 12.2 vs. 9.6 kJ, p < .001) in comparison to the selective group.
Table 1. Patient characteristics and procedure details

Note: Data are shown as the mean ± standard deviation, median (interquartile range), or n (%).BMI, body mass index; GA, gestational age; FLS, fetoscopic laser surgery.
* Fisher’s exact test, Student’s t test, or Mann–Whitney U test.
Table 2 shows the adverse events in the selective and the Solomon groups. There were no significant differences between the groups in the incidence of preterm birth. The incidence of pPROM was significantly higher in the Solomon group than in the selective group at the time points of before 32 weeks (20.2% vs. 7.1%, p < .001) and before 28 weeks (6.6% vs. .9%, p = .008). The incidence of placental abruption in the Solomon group was also significantly higher than that in the selective group (10.7% vs. 3.5%, p = .007). However, the incidence rates for the maternal events of placental abruption were not high in both groups with no significant differences (.6% vs. 1.3%, p = .487). The differences between the two groups in gestational age at placental abruption did not reach statistical significance (median 33.1 weeks vs. 28.9 weeks, p = .056). The incidence rates of recurrent TTTS and TAPS were very low in both groups and did not differ to a statistically significant extent (recurrent TTTS: .9% in the selective group vs. 0% in the Solomon group; TAPS: .4% vs. .6%).
Table 2. Adverse events in the selective and Solomon groups

Note: Data are shown as median (range) or n (%).CI, confidence interval; GA, gestational age; pPROM, preterm premature rupture of membranes; OR, odds ratio; TTTS, twin–twin transfusion syndrome; TAPS, twin anemia-polycythemia sequence.
* Fisher’s exact test or logistic regression analysis.
Table 3 compares the perinatal outcomes of the selective and the Solomon groups. There were no significant differences in the GA at delivery (median 33.7 weeks in the selective group vs. 33.3 weeks in the Solomon group, p = .801), interval from FLS to delivery or rate of preterm birth. There were no significant differences in the birth weight of the donor between the selective and Solomon groups (median 1504 g vs. 1548 g, p = .984); however, the birth weight of the recipient was significantly smaller in the Solomon group than in the selective group (median 1790 g vs. 1933 g, p = .049). No significant differences in the percentage of birth weight discordance were noted between the selective and Solomon groups (median 13.7% vs. 15.9%, p = .161). No significant differences were observed in the double survival rate (74.0% in the selective group vs. 79.2% in the Solomon group, p = .235); however, the rate of survival of at least one twin was significantly higher in the Solomon group than in the selective group (98.2% vs. 93.8%, p = .046). There were no therapeutic abortions. The rate of delivery before viability (<22 weeks) was lower in the Solomon group; however, the difference did not reach statistical significance (.9% vs. 2.4%, p = .058). The overall death rate in the Solomon group was lower than that in the selective group; however, the result did not reach statistical significance (11.3% vs. 16.1%, p = .058). The neonatal death rate in the Solomon group was significantly lower than that in the selective group (1.3% vs. 0%, p = .041).
Table 3. Perinatal outcomes in the selective and Solomon groups

Note: Data are shown as the mean ± standard deviation, median (interquartile range), or n (%).BW, birth weight; CI, confidence interval; GA, gestational age; FLS, fetoscopic laser surgery; OR, odds ratio.
* Fisher’s exact test, Student’s t test, Mann–Whitney U test, or logistic regression analysis.
Table 4 compares the background characteristics of the cases with and without placental abruption or pPROM (<32 weeks) in the Solomon group. Cases with placental abruption showed no remarkable background characteristics, including procedure parameters. In cases with pPROM (<32 weeks), the operation time was significantly longer (60.2 ± 18.3 vs. 50.8 ± 18.0 min, p = .012), and the total laser energy was higher (median 15.9 vs. 12.0 kj, p = .001) than in cases without pPROM. The GA at delivery in cases of pPROM was significantly earlier than in cases without pPROM (median 29.0 vs. 34.4 weeks, p < .001).
Table 4. The background characteristics of placental abruption and pPROM (<32 weeks) in the Solomon group

Note: Data are shown as the mean ± standard deviation, n (%) or median interquartile range. pPROM, preterm premature rupture of membranes; BMI, body mass index; GA, gestational age; FLS, fetoscopic laser surgery.
* Fisher’s exact test, Student’s t test or Mann–Whitney U test.
The results of multivariate analyses of procedure factors associated with pPROM (<32 weeks) are shown in Table 5. The Solomon technique (OR 2.64, 95% CI [1.32, 5.28], p = .006] and total laser energy (OR 1.07, 95% CI [1.01, 1.13], p = .014) were significant risk factors for pPROM (<32 weeks); however, the operation time (OR 1.01, 95% CI [.84, 1.21], p = .909) was not.
Table 5. Multivariate-adjusted OR of procedure factors associated with pPROM (<32 weeks) in all cases

Note: CI, confidence interval; OR, odds ratio; pPROM, preterm premature rupture of membranes.
* Multivariate logistic regression analysis.
Discussion
In this study, we demonstrated that the Solomon technique was associated with an increased the risk of adverse events after FLS. The rates of placental abruption and pPROM (at both <28 weeks and <32 weeks) were significantly higher in the Solomon group than in the selective group. The Solomon technique and total laser energy were risk factors for pPROM. The rate of survival of at least one twin in the Solomon group (98.2%) was significantly higher than that in the selective group (93.8%). The Solomon technique led to superior survival outcomes but increased risks of placental abruption and pPROM. The birth weight of the recipient was smaller in the Solomon group than in the selective group. Close attention should be paid to adverse events during the perinatal management of MC pregnancies after FLS using the Solomon technique for TTTS.
Since the Solomon technique has been shown to reduce postoperative residual anastomoses, many institutions have introduced this technique, including our own center (Akkermans et al., Reference Akkermans, Peeters, Klumper, Lopriore, Middeldorp and Oepkes2015). However, little evidence is available concerning the adverse effects associated with the Solomon technique, which coagulates the entire vascular equator of the placenta, including the normal area without vascular anastomoses. Lanna et al. (Reference Lanna, Faiola, Consonni and Rustico2017) reported that placental abruption occurred more frequently in MC twins who underwent FLS, especially when the Solomon technique was used (3% [9/287] vs. Solomon 14% [12/86], p < .001). Theirs was the first report to indicate the association between the Solomon technique and placental abruption. The present study also showed that placental abruption was significantly more frequent in the Solomon group than in the selective group; however, severe maternal events were not frequently observed as significant clinical complications.
Our study showed that pPROM was also significantly more frequent in the Solomon group than in the selective group and the Solomon technique was a significant risk factor for pPROM. While FLS is known to be associated with an increased risk of pPROM (Chalouhi et al., Reference Chalouhi, Essaoui, Stirnemann, Quibel, Deloison, Salomon and Ville2011; Egawa et al., Reference Egawa, Hayashi, Yang, Sakamoto and Sago2013), there were no marked differences in the incidence of pPROM between the Solomon and selective groups in initial reports (Baschat et al., Reference Baschat, Barber, Pedersen, Turan and Harman2013; Dhillon et al., Reference Dhillon, Hillman, Pounds, Morris and Kilby2015; Slaghekke et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014). Indeed, the incidence of pPROM after FLS using the selective technique was 34% (46/135), while that after FLS using the Solomon technique was 42% (57/137), which did not amount to a statistically significant difference (OR 1.38, 95% CI [.84, 2.26]), probably due to the small sample size (Slaghekke et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014). Placental damage is reportedly more frequent in patients undergoing FLS using the Solomon technique than in those using selective technique, and greater placental damage was associated with a higher incidence of pPROM before 32 weeks (Akkermans et al., Reference Akkermans, de Vries, Zhao, Peeters, Klumper, Middeldorp and Lopriore2017). Our study additionally confirmed that the Solomon technique was associated with an increased risk of pPROM after FLS.
Since the incidence of placental abruption and pPROM was higher in the Solomon group than in the selective group, we searched for factors that might influence the occurrence of placental abruption or pPROM in the Solomon group. The operation time and total laser energy were found to be associated with the occurrence of pPROM. In contrast, neither the operation time nor the total laser energy was associated with the occurrence of placental abruption. Multivariate analyses revealed that total laser energy, but not operation time, was a significant risk factor for pPROM. The use of greater laser energy is reportedly associated with greater placental damage, which can lead to an increased incidence of pPROM (Akkermans et al., Reference Akkermans, de Vries, Zhao, Peeters, Klumper, Middeldorp and Lopriore2017). Therefore, reducing total laser energy would help to reduce the occurrence of pPROM. These findings are useful for improving the surgical techniques used for FLS.
The Solomon technique coagulates the entire vascular equator, which involves many normal areas of the placenta. The placental function is well known to be associated with fetal growth, so we suspected that the birth weight might be a potential point of difference between the two groups. Indeed, while no significant differences were observed between two groups in the birth weight of the donor, the birth weight of the recipient was significantly smaller in the Solomon group than in the selective group (median 1790 g vs. 1933 g, p = .049). These results imply that the Solomon technique may influence the fetal growth of the recipient. Most of the normal placental area coagulated by the Solomon technique belonged to the recipient, and coagulation of the normal placental area might disturb fetal growth. No marked effects of the Solomon technique on birth weight were noted in a previous report (Baschat et al., Reference Baschat, Barber, Pedersen, Turan and Harman2013), although other reports comparing the two techniques lacked information on birth weight (Slaghekke et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014; Ruano et al., Reference Ruano, Rodo, Peiro, Shamshirsaz, Haeri, Nomura and Belfort2013). A further study will be required to assess the effect of the Solomon technique on fetal growth.
In the present study, the rate of survival of at least one twin was significantly higher in the Solomon group than in the selective group, which is consistent with previous reports (Baschat et al., Reference Baschat, Barber, Pedersen, Turan and Harman2013; Dhillon et al., Reference Dhillon, Hillman, Pounds, Morris and Kilby2015; Ruano et al., Reference Ruano, Rodo, Peiro, Shamshirsaz, Haeri, Nomura and Belfort2013). Although the incidence of recurrent TTTS or TAPS was very low in the Solomon group, there were no significant differences between the two groups, since the incidence of these conditions was already very low in the selective group, and their incidence was lower in comparison to our previous study (Taniguchi et al., Reference Taniguchi, Sumie, Sugibayashi, Wada, Matsuoka and Sago2015). The incidence may have been underestimated in both groups, since placental examinations were not performed. In addition, the criteria for TAPS were published in 2010 (Slaghekke et al., Reference Slaghekke, Kist, Oepkes, Pasman, Middeldorp, Klumper and Lopriore2010), so underestimation was suspected in the selective group. Many cases were managed at referring perinatal centers, so awareness of TAPS was suspected to be low in the early term of this cohort. Considering these factors, the superior survival rate in the Solomon technique may be the result of reducing recurrent TTTS or TAPS after FLS.
There were no marked differences between the two groups in the incidence of preterm birth, which is the most relevant prognostic factor (Lopriore, Ortibus et al., Reference Lopriore, Ortibus, Acosta-Rojas, Le Cessie, Middeldorp, Oepkes and Lewi2009). This finding is a bit strange, since the incidences of placental abruption and pPROM were higher in the Solomon group than in the selective group. Placental abruption was not associated with preterm birth, since the gestational age at placental abruption (median 33.1 weeks) was close to that at delivery (median 33.3 weeks) in the Solomon group. In addition, the difference in the incidence of pPROM between the two groups might have had relatively little impact on preterm birth. Similar observations were found with regard to chorioamniotic membrane separation (CMS) after FLS. Although CMS was strongly correlated with pPROM, it had no measurable impact on the gestational age of delivery (Egawa et al., Reference Egawa, Hayashi, Yang, Sakamoto and Sago2013).
One notable difference between the two groups was the operation time, as the operation time in the Solomon group was significantly shorter than that in the selective group. This finding was unexpected, since the Solomon technique adds a coagulation line from one edge to another edge after the completion of selective coagulation. The reduction in the operation time in the Solomon group may be due to a learning curve effect; the selective group underwent procedures from January 2010 to July 2014, while the Solomon group underwent procedures from August 2014 to December 2017. The rate of survival of at least one twin in the selective group was higher than that previously reported by our group (91.2%) between 2002 and 2006 (Sago et al., Reference Sago, Hayashi, Saito, Hasegawa, Kawamoto, Kato and Murakoshi2010). The outcomes of FLS are thought to depend on the experience and caseload of a center (Diehl et al., Reference Diehl, Diemert, Grasso, Sehner, Wegscheider and Hecher2017). Thus, a learning curve effect may have influenced the improvement in the rate of survival of at least one twin in the Solomon group in this study.
The influence of the quality of neonatal care on the outcomes of fetal therapy cannot be ignored. In this study, the rate of the survival of at least one twin in the Solomon group was 98.2%, which seems remarkably high compared to that in other published studies (Baschat et al., Reference Baschat, Barber, Pedersen, Turan and Harman2013; Ruano et al., Reference Ruano, Rodo, Peiro, Shamshirsaz, Haeri, Nomura and Belfort2013; Slaghekke et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014). Furthermore, there were no neonatal deaths in the Solomon group, which was also an extraordinary result; the neonatal death rate in the Solomon group was significantly lower than that in the selective group (0% vs. 1.3%, p = .041). All neonates in this study were managed at perinatal centers included in the national neonatal network. The overall survival in very-preterm infants reportedly ranged from 78% to 93% among 10 national neonatal networks, with the difference being highest at 24 weeks’ gestation (range 35% to 84%; Helenius et al., Reference Helenius, Sjors, Shah, Modi, Reichman, Morisaki and Lehtonen2017). Our national neonatal network showed the highest survival rate in the world. The availability of nationwide high-quality neonatal care in our country may have contributed to the remarkable high survival rate after FLS, especially in the Solomon group in this study.
The present study was associated with some limitations. First, this was a retrospective analysis of a consecutive cohort involved in many referring centers. The study period of each group differed, which may have led to some difficulties in comparing the outcomes due to changes in operators and perinatal care. The reliability of data is limited, since most patients were managed at referring perinatal centers after FLS and perinatal clinical data were obtained there. pPROM was limited to cases with subsequent preterm delivery in the present study, since available clinical data were limited. Second, we lacked placental studies, including color-dye injection to evaluate residual anastomoses and pathological examinations to evaluate placental damage. Residual anastomoses were not evaluated in patients without clinical symptoms in this study. Residual anastomoses are reportedly not rare, even following the introduction of the Solomon technique (Knijnenburg et al., Reference Knijnenburg, Slaghekke, Tollenaar, van Klink, Zhao, Middeldorp and Lopriore2019). Although the incidence of recurrent TTTS and TAPS was very low in both groups, the presence of residual anastomoses in some cases might have been missed in this study, especially in the selective group. An analysis of the placental damage may aid in evaluating the underlying etiology of the adverse events associated with the Solomon technique. Third, we only reported the short-term outcomes with some procedural parameters. The long-term outcomes and the results of neurological examinations are very important for thoroughly assessing the surgical technique (Matsushima et al., Reference Matsushima, Ozawa, Sugibayashi, Ogawa, Tsukamoto, Miyazaki and Sago2020; Rossi et al., Reference Rossi, Vanderbilt and Chmait2011). One strength of our study is the relatively large cohort for comparing the rates of adverse events. Indeed, our sample size was the largest among studies comparing outcomes between the selective and Solomon techniques.
In conclusion, this study showed that the Solomon technique increased the incidence of placental abruption and pPROM. An adverse effect of the Solomon technique on fetal growth of the recipient was suspected. The rate of survival of at least one twin in the Solomon group was significantly higher than that in the selective group, although the possibility of a learning curve effect and improvements in neonatal care, which cannot be excluded in a consecutive cohort study, should be considered. Given the present findings, the Solomon technique remains the preferable choice of surgery for TTTS. However, close attention should be paid to adverse events during the perinatal management of MC twin pregnancies treated by FLS using the Solomon technique. A further study to investigate placental damage in relation to laser energy, fetal growth and residual anastomoses is required to optimize and further improve FLS for TTTS.
Acknowledgments
We thank Dr N Nakamura and Dr M Shibata for their assistance. We thank the doctors who referred the TTTS patients to us for FLS and cared for these patients after FLS at their perinatal centers.
Financial support
The Grant of National Center for Child Health and Development 30-5 of Japan.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. This study was approved by the Ethics Committee at the National Center for Child Health and Development (No. 972).








