The prevalence of stunting (i.e. low length- or height-for-age) from birth to 5 years has been decreasing worldwide, from 40 % in 1990 to 27 % in 2010( Reference de Onis, Blossner and Borghi 1 ), and this rate is expected to fall further to 22 % by 2020. In Latin American countries, for instance, the prevalence of stunting in 2010 was 13·5 % while a prevalence of 11·6 % is predicted by the year 2015( Reference de Onis, Blossner and Borghi 1 ).
In Brazil, there has been a 50 % decline in stunting among children aged <5 years, from 13·4 % in 1996 to 6·7 % in 2006, according to the most recent National Demographic and Health Survey( 2 ). Several contributory factors may explain this decline in the stunting prevalence, such as improvements in maternal schooling, increased purchasing power of low-income families, broader access to health services and the expansion of basic sanitation and water treatment( 3 ). However, owing to social inequalities among Brazilian regions, the north of the country, which encompasses the Amazon area, has notable financial difficulties and administrative, educational and nutritional deficiencies presenting important social and health inequities, explaining why the prevalence of stunting remains higher, affecting around 15 % of children in this age group( 2 ). Concurrently, the incidence of overweight and obesity in children and adolescents is rising rapidly, becoming a major public health problem. Secular trend data on overweight status for Brazilian children aged 5–9 years, conducted by the Brazilian Institute of Geography and Statistics using BMI and the WHO classification as the diagnostic criteria, have shown that the prevalence of overweight in boys during the 1970s was 10·9 %, increasing to 15·0 % in the late 1980s and attaining 34·8 % in 2008–2009. The prevalence of overweight among girls was 8·6 %, 11·9 % and 32·0 % for the same time periods, respectively. It is noteworthy that this trend of increasing overweight occurred across all income groups in both sexes( 4 ).
The pattern of shifts in nutritional status, characterized by persistence of stunting and increasing rates of overweight, typify the nutritional transition scenario found in many developing countries( Reference Kimani-Murage, Kahn and Pettifor 5 , Reference Custodio, Descalzo and Roche 6 ). This transition process may be considered a particular public health concern when affecting children, since poor child growth can have important economic implications. Stunting in childhood is associated with fewer years of schooling and consequently low productivity at work( Reference Grantham-McGregor, Cheung and Cueto 7 ). Overweight and obesity in childhood and adolescence have also been associated with increased risk of both premature mortality and adult morbidity( Reference Reilly and Kelly 8 ). Therefore, assessing nutritional status in childhood is essential for planning preventive actions, as evidenced by several studies showing that early exposure of children to malnutrition could be associated with changes in body composition during adolescence( Reference Sterling, Miranda and Gilman 9 , Reference Bénéfice, Garnier and Simondon 10 ) and adulthood( Reference Reilly and Kelly 8 ).
Studies investigating the determinants of stunting and overweight have been conducted in developed and developing countries( Reference Savva, Tornaritis and Chadjigeorgiou 11 , Reference El Taguri, Betilmal and Mahmud 12 ), highlighting the importance of socio-economic status and maternal health factors. However, little is known about the influence of micronutrient deficiencies, inflammation indicators and dyslipidaemias on stunting or overweight among children( Reference Anderson, Jack and Monchy 13 , Reference Best, Neufingerl and van Gell 14 ).
Therefore, in the present study, socio-economic and maternal characteristics as well as biochemical indicators were assessed in relation to both outcomes – stunting and overweight – in childhood. To our knowledge, it is the first population-based, cross-sectional study that describes the prevalence and factors associated with stunting and overweight in children <10 years of age living in an urban area of a town in the western Brazilian Amazon.
Methods
Study design and population
The present population-based, cross-sectional study on child nutrition and health was performed in December 2007 in Acrelândia, a frontier town located 112 km from Rio Branco, the capital of the state of Acre, in the western Brazilian Amazon region. At the time of the study, Acrelândia had 11 520 inhabitants (44 % residing in the urban area), predominantly migrants from south-eastern and southern regions of Brazil, engaged in commercial agriculture and raising cattle. Infant mortality was estimated as 71/1000 live births in 2000, substantially higher than the national average (28/1000 live births). The human development index (for ranking municipalities in terms of health, education and income levels, reported by the United Nations Program for Development) in Acrelândia was estimated at 0·680 according to data from 2000 (ranged from 0·359 to 0·919 for Brazilian cities)( 15 ).
Sampling strategies and field procedures were reported previously( Reference Cardoso, Scopel and Muniz 16 ). Briefly, all households from the urban area with children up to 10 years of age (n 749) were identified with the assistance of local health workers of the Family Health Program of the Brazilian Ministry of Health. Thus, 1225 eligible children living in 734 households were enrolled (98·0 % of households identified). A structured questionnaire was administered through face-to-face interview to the mothers or guardians of 1151 children (94·0 % of those eligible). The questionnaire was pilot-tested previously and covered the following topics: demographic and socio-economic status; environmental conditions; reproductive health variables; history of infant feeding practices (duration of total and exclusive breast-feeding, child's age at introduction of weaning foods); and the occurrence of morbidities, i.e. diarrhoea (three or more liquid stools within 24 h), cough or fever up to 15d prior to the interview, episodes of malaria and wheezing in the past 12 months and past hospitalization. After the interview, mothers and children were invited to visit the local family health clinic, where research assistants carried out a physical examination and trained phlebotomists obtained a venous blood sample from all children.
All parents or guardians of participating children provided written informed consent prior to enrolment. The study protocol was approved by the institutional review board of the School of Public Health, University of São Paulo, Brazil (no. 1681/07).
Anthropometric assessment
Length/height and weight were measured by trained research assistants following standardized procedures using calibrated equipment( Reference Lohman, Roche and Martorell 17 ). Among children aged <2 years, recumbent length was measured to the nearest millimetre with a locally made 1·3m-long infant measuring board with increments of 1 mm; weight was measured to the nearest 10 g on an electronic paediatric scale (model 1583; Tanita, Tokyo, Japan). Children aged ≥2 years were measured to the nearest millimetre with a stadiometer (model 208; SECA, Hamburg, Germany) affixed to the flat surface of a wall without a baseboard, perpendicular to the floor. For readings, children were positioned barefoot in a standing position in the middle of the stadiometer, with their head, shoulders, buttocks and heels against the wall. Weight was measured on an electronic scale (model HS-302; Tanita) and recorded to the nearest 100 g. Maternal weight and height were subsequently measured by the research assistants by following the same standardized procedures( Reference Lohman, Roche and Martorell 17 ). Each measurement was repeated and the mean value of the two measurements calculated. Birth date was recorded directly from birth certificates or child health cards. BMI was computed as weight in kilograms divided by the square of length/height in metres.
Z-scores for length/height-for-age (HAZ) and BMI-for-age (BAZ) were calculated according to WHO Child Growth Standards( 18 ) for children aged 0–5 years and WHO Growth Reference Data( Reference de Onis, Onyango and Borghi 19 ) for children aged >5 years. The cut-off for stunting was defined as HAZ < −2. For the present analysis, overweight was defined as BAZ > 1 including ‘at risk of overweight’, overweight and obesity for children <5 years old. Among children ≥5 years old, overweight was defined as BAZ > 1( Reference de Onis, Onyango and Borghi 19 , Reference de Onis and Lobstein 20 ).
Maternal nutritional status was classified according to BMI categories( 21 ) as non-overweight (BMI < 25 kg/m2) and overweight (BMI ≥ 25 kg/m2), since only twenty-two mothers were underweight (BMI < 18·5 kg/m2). Of the 1151 participants, one child had HAZ>6 and eleven children had missing data on weight or height. These children were excluded from the present analyses giving a final sample of 1139 children.
Laboratory procedures
A sample (approximately 5 ml) of fasting venous blood was collected from 1131 children (98·3 % of those eligible). At the field laboratory in Acrelândia, whole blood aliquots collected in EDTA-containing vacuum tubes were used to perform full blood cell counts and measure Hb concentrations on an ABX Micro60 automated cell counter (Horiba, Montpellier, France). A separate blood sample was protected from light and centrifuged within 1 h of collection. Plasma C-reactive protein (CRP) concentration was measured using the Immulite high-sensitivity chemiluminescent assay (Diagnostic Products Corporation, Los Angeles, CA, USA). The cut-off for high CRP was ≥5 mg/l as an indicator of inflammation( Reference Thurnham, McCabe and Haldar 22 ). Serum lipid fractions were measured enzymatically using an automatic device (ADVIA 1650; Bayer, East Walpole, MA, USA). TAG concentrations ≥100 mg/dl were considered high according to age( Reference Kwiterovich 23 ). Anaemia, iron deficiency (ID) and iron-deficiency anaemia (IDA) were defined according to Hb, serum ferritin (SF), serum transferrin receptor (sTfR) and CRP concentration, respectively( 24 ). The normal range of sTfR concentration, as determined by the immunoassay manufacturer, was 2·9–8·3 mg/l. ID was defined when SF concentrations were low (<12 μg/l for children aged <5 years or <15 μg/l for those aged ≥5 years) or when sTfR concentrations were high (>8·3 mg/l). IDA was defined when ID occurred in anaemic children; the cut-off for Hb concentration considered was 110·0 g/l for children aged 6 months to 5 years, and 115·0 g/l for children aged ≥5 years. Plasma concentrations of vitamin A (retinol) were measured by the standard HPLC method( Reference Gomes, Alves and Sevanian 25 ); concentrations <0·70 μmol/l defined vitamin A deficiency( 26 ). Children with anaemia or nutrient deficiencies detected during the survey received adequate treatment prescribed by the medical team involved in the project.
Stool samples were collected from 1016 children (97·0 % of those eligible) at the time of the interview, for subsequent analysis. Coprotest® cups containing a preservative solution (10 % w/v formalin) were provided for this purpose. All stool examinations were analysed to search for eggs, cysts and larvae of parasites, according to the qualitative technique of sedimentation( Reference Hoffman, Pons and Janer 27 ), as described elsewhere( Reference Cardoso, Scopel and Muniz 16 ). Geohelminths found in this population included Ascaris lumbricoides, Trichuris trichiura and Strongyloides stercoralis. Children with intestinal parasitic infections received free treatment prescribed by the research clinicians.
Statistical analysis
The main dichotomous dependent variables of interest were stunting and overweight. Explanatory variables comprised socio-economic, maternal and child characteristics as well as micronutrient deficiencies, inflammation and dyslipidaemia indicators.
Principal component analysis was used to derive a wealth index representing a proxy of household income( Reference Filmer and Pritchett 28 ), based on the presence of twelve household assets, as described elsewhere( Reference Cardoso, Scopel and Muniz 16 ). To define socio-economic status, information on the wealth index and number of residents in each household was combined to generate variables of wealth concentration. Households were classified as ‘low wealth concentration’ when the wealth index was below the median and the number of residents was above the median for this population. On the other hand, for the overweight outcome, households were classified as ‘high wealth concentration’ when the wealth index was above the median and the number of residents was below the median. This combined variable was used when analysing associations for the stunting and overweight outcomes. Other socio-economic variables investigated were land or livestock ownership and maternal schooling, categorized as <5 years v. ≥5 years. Maternal characteristics before pregnancy were maternal age at child's birth (<20 years, 20–30 years, ≥30 years) and maternal height in tertiles (<1·55 m, 1·55–1·59 m, ≥1·59 m). Maternal characteristics during pregnancy included the number of prenatal visits (<6 v. ≥6), smoking habits and caesarean delivery. Child's birth weight was categorized as ≤2500 g, 2501–3500 g or ≥3500 g. Age at introduction of cow's milk was used as an indicator of infant feeding practices and was classified as <3 months or ≥3 months based on the median age for weaning observed in our study population. Current care to child was assessed through the number of siblings (<1 v. ≥2). Several morbidities were taken into account: duration of diarrhoea <3 d or ≥3 d, geohelminth infection and high CRP concentration. Finally, biochemical indicators of nutritional status included ID, IDA, vitamin A deficiency and high serum TAG concentration.
Children were stratified into two age categories (<5 years and ≥5 years of age) based on sample size and distribution. First, we compared the distribution of baseline characteristics between the two age groups, using the Pearson χ 2 test. Then for each age group, crude analyses were first conducted using Poisson regression models between the dependent variables of interest (stunting and overweight) and the explanatory variables, adjusting for sex and age. Adjusted prevalence ratios (PR) and 95 % confidence intervals were obtained for the factors associated with stunting and overweight using multiple Poisson regression models with robust variance according to a hierarchical conceptual framework( Reference Victora, Huttly and Fuchs 29 ). At each level of determination, covariates were retained in the model if they were associated with the outcome at P<0·10 and for ordinal variables when they followed a dose–response pattern or if their inclusion in the model changed the PR by 10 % or more. Missing observations were included in the multiple models by creating missing-value categories. All analyses were performed using the Stata statistical software package version 11·0.
Results
Among 1139 children studied, the mean age was 5·13 (sd 2·87) years (range: 3·02 months to 9·98 years). The distribution of the children between the age groups is shown in Table 1. The prevalence of stunting among children aged <5 years and ≥5 years was 7·1 % (95 % CI 5·1, 9·6 %) and 3·7 % (95 % CI 2·4, 5·7 %), respectively. The prevalence of overweight was 20·6 % (95 % CI 17·4, 24·2 %) in children <5 years of age and 9·4 % (95 % CI 7·2, 12·1 %) in the older ones. Children aged <5 years were more likely to have mothers who had more years of schooling, achieved more prenatal visits and realized more caesarean delivery. Children <5 years old also had a higher proportion of IDA, ID and high TAG concentrations when compared with children ≥5 years of age (Table 1).
Table 1 Characteristics of the study sample: urban children (n 1139) aged <10 years living in Acrelândia, western Brazilian Amazon, December 2007

ID, iron deficiency; IDA, iron-deficiency anaemia.
Totals may be less than 1139 due to missing values. P values from χ 2 tests for comparisons between age groups.
*Combination of wealth index above the median plus number of residents below the median.
†Combination of wealth index below the median plus number of residents above the median.
‡Defined by three or more liquid stools within 24 h.
§ID was defined when serum ferritin concentration was <12 μg/l for children aged <5 years or <15 μg/l for those aged ≥5 years or when serum transferrin receptor concentration was high (>8·3 mg/l). IDA was defined when ID occurred in anaemic children; the cut-off for Hb concentration was 110·0 g/l for children aged 6 months to 5 years, and 115·0 g/l for children ≥5 years.
The investigation of the factors associated with stunting was performed only for children <5 years of age because of the low prevalence of stunting among older children (3·7 %). In the adjusted analysis, the lowest tertile of maternal height and low birth weight were positively associated with stunting, as were diarrhoea ≥3 d and geohelminth infection (Table 2).
Table 2 Factors associated with stunting in urban children (n 557) aged <5 years living in Acrelândia, western Brazilian Amazon, December 2007

PR, prevalence ratio; IDA, iron-deficiency anaemia; Ref. reference category.
*Variables were adjusted for others in the same or higher levels following the hierarchical conceptual framework.
†Combination of wealth index above the median plus number of residents below the median.
‡Combination of wealth index below the median plus number of residents above the median.
§Diarrhoea was defined by three or more liquid stools within 24 h.
Among children <5 years of age, high wealth concentration and caesarean delivery were positively associated with overweight in multiple models (Table 3). The prevalence of overweight among children with birth weight ≥3500 g was 85 % higher compared with children whose weight was <2500 g at birth (P < 0·001). ID also remained positively associated with overweight after multiple adjustment.
Table 3 Factors associated with overweight according to age group in urban children (n 1139) aged <10 years living in Acrelândia, western Brazilian Amazon, December 2007
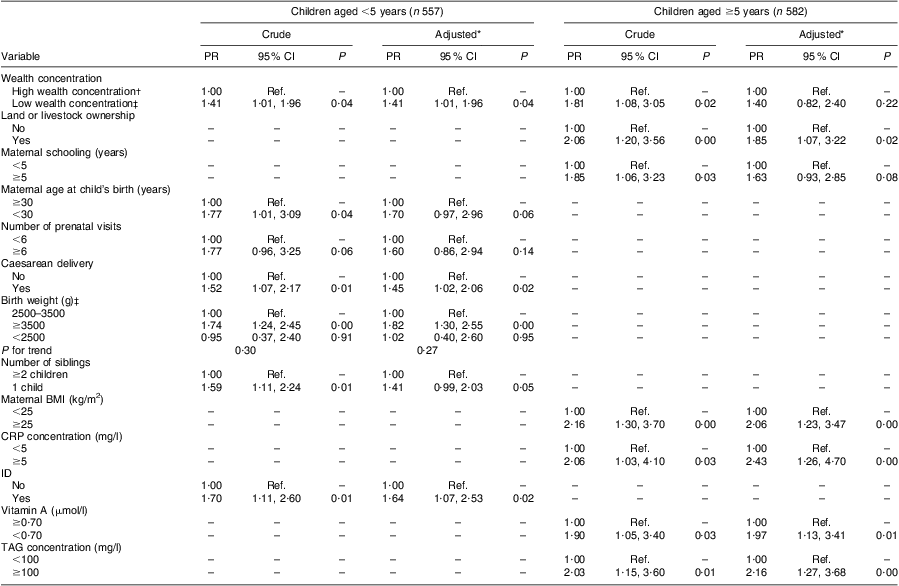
PR, prevalence ratio; CRP, C-reactive protein; ID, iron deficiency; Ref. reference category.
*Variables were adjusted for others in the same or higher levels following the hierarchical conceptual framework.
†Combination of wealth index above the median plus number of residents below the median.
‡Combination of wealth index below the median plus number of residents above the median.
Among children ≥5 years of age, high wealth concentration and maternal schooling were no longer significantly associated with overweight in multiple models. However, prevalence of overweight was 85 % higher among children whose families owned land or livestock compared with children from families not possessing these assets (P = 0·02). After adjusting for this socio-economic variable, overweight among older children remained positively associated with maternal overweight (P < 0·001). Vitamin A deficiency, as well as high CRP and TAG concentrations, was positively associated with the prevalence of overweight in the adjusted analysis (Table 3).
Discussion
Over past years, Acrelândia has shown a decreasing prevalence of stunting (7·1 %) among pre-school children, since Souza et al. ( Reference Souza, Benício and Castro 30 ), studying the same area in 2003, found a prevalence of 10 %. Similar national trends have been observed by the latest Brazilian National Demographic and Health Survey( 2 ). Among children aged 5–10 years, the stunting prevalence of 3·7 % in our study is approximately 50 % lower than the national average found in the latest Brazilian Household Budget Survey (6·8 %)( 4 ).
Brazil has shown declining trends of stunting based on national surveys since the 1970s. According to Monteiro et al. ( Reference Monteiro, Benicio and Conde 31 ), the factors that contributed most to the decline of stunting (between 1996 and 2007) in Brazil were improvements in maternal schooling, increased purchasing power of low-income families and broader access to health services. It is noteworthy that from 2003 Brazil has reactivated the economic growth with improved income distribution, the expanding coverage of cash transfer programmes and also the Family Heath Program. The latter programme is targeted to poor households and covers health prevention and education( Reference Monteiro, Benicio and Conde 31 ).
In the present study, maternal schooling was not associated with stunting related probably to the overall low literacy of the mothers ( ∼40 % with less than 5 years of schooling). We found that low maternal height, low birth weight, diarrhoea ≥3 d and geohelminth infection remained positively associated with stunting in the multiple models.
As previously reported, maternal height is an important determinant of intra-uterine growth restriction( Reference Kramer 32 ) and low birth weight( Reference Black, Allen and Bhutta 33 ). Analysing data from fifty-four developing countries, Ozaltin et al. ( Reference Ozaltin, Hill and Subramanian 34 ) found an inverse association between maternal height and infant mortality, low birth weight and stunting, suggesting that the effects of poor environmental conditions (inadequate nutrition, diseases) to which the mother was exposed can be transmitted to future generations.
Despite the routine distribution of anti-helminthic medication under the Family Health Program of the municipality( Reference Castro, Nunes and Conde 35 ), cases of children with episodes of diarrhoea and geohelminth infection were noted, and these variables were found to be associated with stunting. It has been suggested that this situation could be due to the lack of sanitation and water treatment in the area( Reference Castro, Nunes and Conde 35 ). Moreover, it is known that infections worsen nutritional status especially among undernourished children( Reference Scrimshaw 36 ).
In our study, we did not find an association between stunting and risk for overweight in the children. Meanwhile, prospective studies from developing countries have shown that adolescents who were stunted in childhood tended to gain less lean body mass and more fat mass than their non-stunted counterparts( Reference Martins, Hoffman and Fernandes 37 ). However, the body fat distribution is more likely to be central, a significant important risk factor for chronic diseases( Reference Hoffman, Martins and Roberts 38 ). One of the potential underlying mechanisms involved in the maintenance of energy balance is the low rate of fat oxidation, which means in turn that the oxidized fat must be stored, thus favouring fat accumulation in the body( Reference Hoffman, Martins and Roberts 38 ). This is of great concern, especially in countries undergoing nutrition transition.
In the present study, the socio-economic indicators of high wealth concentration and land or livestock ownership were positively associated with overweight among children aged <5 years and ≥5 years, respectively. In their study of Colombian children from low- and middle-income families, McDonald et al. ( Reference McDonald, Baylin and Arsenault 39 ) found that overweight was strongly associated with socio-economic status indicated by the possession of household assets. Similarly, in other developing countries, the best indicators of income were positively associated with overweight( Reference Wang 40 ). However, the opposite occurs in developed countries where socio-economic status is inversely associated with overweight( Reference Shrewsbury and Wardle 41 ).
Other factors significantly associated with overweight in younger children in our study included caesarean delivery, high birth weight and ID. Children with caesarean delivery had greater risk of developing obesity in adulthood, as recently discussed in a study conducted by Goldani et al. ( Reference Goldani, Bettiol and Barbieri 42 ). The factors underlying this relationship remain unknown, but it has been suggested that the absence of breast-feeding in the first hours of life as a consequence of surgical delivery may be a risk factor for early weaning( Reference Vieira, Vieira and Giugliani 43 ) and consequently for the introduction of inadequate foods during the first years of life.
A significantly higher adjusted prevalence ratio for overweight was found among children weighing ≥3500 g at birth. High weight at birth has been associated with obesity in childhood( Reference Yu, Han and Zhu 44 ) and also with metabolic syndrome in older children( Reference Hirschler, Bugna and Roque 45 ). Obesity may be transmitted across generations as recently demonstrated by Cnattingius et al. in Swedish women( Reference Cnattingius, Villamor and Lagerros 46 ). Those authors found that the combination of an obese woman born large for gestational age increased the risk of her having a large-for-gestational-age infant compared with a non-obese woman whose birth weight was appropriate for gestational age( Reference Cnattingius, Villamor and Lagerros 46 ).
The association between ID and overweight in children <5 years of age found in the present study has been previously documented( Reference Nead, Halterman and Kaczorowski 47 ). Several hypotheses have been proposed to explain this association, such as the increased plasma volume in obese individuals and inadequate intake of nutrients( Reference Pinhas-Hamiel, Newfield and Koren 48 ). Another study however suggested that higher levels of hepcidin might be involved, since obesity is considered a low-grade inflammatory disease( Reference del Giudice, Santoro and Amato 49 ). It is noteworthy that in the present study, sTfR concentration (in addition to SF) was used as a differential diagnosis of ID because this parameter is not affected by infectious processes( Reference Cook, Baynes and Skikine 50 ). The poor quality of diet among the young Amazonian children studied is proposed as a likely hypothesis explaining this association. A previous study assessing the characteristics of diet among children <2 years of age in the same region indicated low consumption of fruit and vegetables, low consumption of Fe-rich foods and excessive consumption of cow's milk and porridge( Reference Garcia, Granado and Cardoso 51 ).
For children aged ≥5 years, having an overweight mother was strongly associated with overweight. This result is in line with the findings of other studies( Reference McDonald, Baylin and Arsenault 39 , Reference Dubois and Girard 52 ). The association may be due to the intergenerational transmission of obesity( Reference Jääskeläinen, Pussinen and Nuutinen 53 ), as well as environmental and/or behavioural factors, since parents play an important role in the development of their children's food preferences and energy intake( Reference Salvy, Elmo and Nitecki 54 ).
Our results also indicated that higher CRP concentrations were positively associated with overweight. Besides the hypothesis of low-grade inflammatory disease caused by excess weight( Reference Toprak, Toprak and Chen 55 ), this association may be explained by the presence of recurrent infections to which these Amazonian children are exposed.
In the present study, overweight children ≥5 years of age were also more likely to have higher TAG concentration than their counterparts. This may be an indication of inadequacies in diet from an early age( Reference Garcia, Granado and Cardoso 51 ). Abnormal levels of TAG have been well documented in the literature among children and adolescents with overweight status as an independent risk factor for CVD( Reference Reilly and Kelly 8 ).
Finally, in the present study vitamin A deficiency was positively associated with overweight in children aged ≥5 years, after adjusting for high CRP and TAG concentrations. It is well known that vitamin A deficiency compromises growth and is associated with low resistance to infections( 26 ). This may be a relevant concern among children residing in the Brazilian Amazon region, where there is high exposure to numerous aetiological agents along with insufficient services of basic sanitation and water treatment. In addition, akin to the explanation for the association of ID and high TAG levels with overweight, the association of vitamin A deficiency and overweight could also be attributed to the poor quality diet of the study population. From an early age, this group has a low intake of fruit and vegetables and substantial consumption of unhealthy foods such as processed foods high in sodium, preservatives, sugars and fats, according to previous partial analysis from our data( Reference Garcia, Granado and Cardoso 51 ). Changes in diet quality are characteristic of the nutritional transition faced by countries undergoing rapid economic development and improvement in living standards( Reference Custodio, Descalzo and Roche 6 , Reference Vio, Albala and Kain 56 ). Overall, Brazilians have decreased their consumption of staple foods such as rice (decline of 40·5 %) and beans (decline of 26·4 %) from 2002 to 2008. The current consumption of fruits and vegetables reaches only one-quarter of WHO recommended levels. On the other hand, the consumption of soft drinks has risen by 39·3 %( 57 ). Dietary patterns are established in childhood, persist into adulthood and are significantly associated with chronic disease risk factors( Reference Mikkila, Räsänen and Raitakari 58 ). Interestingly, a Chinese study suggested that mothers’ nutritional knowledge, health consciousness and exposure to the media may influence their children's diet beyond the determining role of family resources and access to foods available to the community in developing countries undergoing rapid social and economic transition( Reference Wang, Bentley and Zhai 59 ).
Our study has several limitations that should be considered. Because of its cross-sectional design, caution should be taken in interpreting the present findings, since no causal assumptions can be made. Furthermore, the current analysis included no evaluation of indicators of dietary consumption and physical activity in the population studied due to related logistic and methodological issues. In Acrelândia, seasonality of dietary habits has been reported, requiring more recall days, spread across seasons and a period of months to lead a more accurate dietary estimates among school-aged children( Reference Scagliusi, Garcia and Indiani 60 ); however, unfortunately, this was not feasible in the present study due to the field conditions and cross-sectional design. Despite these limitations, the present population-based study used direct and standardized anthropometric measurements in both children and their mothers and employed a comprehensive set of biochemical indicators, thus contributing to the understanding of several socio-economic, maternal and individual factors associated with stunting and overweight in children.
Concerted efforts have been made to increase access to food and to improve the health of Brazilian children through conditional cash transfer programmes and expansion of basic health care. While national programmes are in place to help prevent micronutrient deficiencies and promote healthy eating habits, there remains much work to be done – from infrastructure improvements to the integration of governmental organizations, non-governmental organizations and civil society – in promoting and implementing healthy child growth initiatives.
Conclusion
The prevalence of overweight was higher than that of stunting among pre-school children in our study. Factors positively associated with stunting in children aged <5 years were low maternal height, low birth weight, diarrhoea episodes and geohelminth infection. For overweight, higher wealth concentration, caesarean delivery, high birth weight and ID proved important factors among children <5 years of age, whereas land or livestock ownership, maternal overweight, high CRP and TAG concentrations and vitamin A deficiency were associated factors among older children. These results point to the importance of intensifying the promotion of maternal and child health practices, as well as sustainable access to healthy food, in a bid to reduce short- and long-term complications in this population against a scenario of nutritional transition.
Acknowledgements
Sources of funding: The study was funded by the National Council for Scientific and Technological Development of Brazil (CNPq; grant nos. 551359/2001-3, 502937/2003-3, 307728/2006-4 and 470573/2007-4), the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant no. 2007/53042-1) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Ministry of Education of Brazil). F.C. and R.A.A. received postdoctoral scholarships from CNPq and CAPES, respectively. B.H.L. received a PhD scholarship from FAPESP (grant no. 2008/57796-3). Conflicts of interest: The authors have no conflicts of interest to declare. Authors’ contributions: M.A.C. and P.T.M. designed and conducted the research; F.C. and R.A.A. analysed the data; F.C., R.A.A. and B.H.L. wrote the paper. All authors read and approved the final manuscript. Acknowledgements: The authors are profoundly grateful to all children and their families who participated in the study, to the Municipality Health Service of Acrelândia, and to the field work research team for valuable assistance.





