Consequences of obesity include insulin resistance, dyslipidaemia, hypertension and the development of overt diabetes and CVD with reduced life expectancy(Reference Olshansky, Passaro and Hershow1). There are multiple obesity-mediated pathways that are known and/or suspected to contribute to the influence of adiposity on metabolic disturbances such as excessive NEFA released from adipose tissue and its inhibitory effect on tissue insulin sensitivity, or a low-grade inflammation related to obesity(Reference Ronti, Lupattelli and Mannarino2). Fatty acids, particularly highly unsaturated fatty acids (HUFA), are considered as important aetiological factors for a variety of obesity-related illnesses. HUFA are important for the maintenance of normal biomembrane structure and function(Reference Torrejon, Jung and Deckelbaum3) and are precursors of eicosanoids that have important physiological properties affecting numerous cardiovascular, immune and cellular secretory functions(Reference Shils, Shike and Ross4–Reference Jung, Torrejon and Tighe7). Additionally, HUFA are key regulators of many genes involved in controlling lipid homeostasis and may thereby help to lessen dyslipidaemia(Reference Torrejon, Jung and Deckelbaum3). Further, the anti-arrhythmic action of n-3 HUFA is considered to be protective against myocardial infarction and IHD via increasing cell membrane fluidity(Reference Harris, Miller and Tighe5), influencing membrane ion channel function and regulating cytosolic sodium ion and L-type Ca ion levels in cardiac myocytes(Reference Harris, Miller and Tighe5, Reference Russo6).
As HUFA are derived from both exogenous and endogenous sources, both dietary intake of fatty acids and factors influencing endogenous synthesis could contribute to disease risk. Desaturase 5 (Δ5) is the key enzyme in the endogenesis pathways of n-3 and n-6 HUFA. Although there is no direct measurement of Δ5, its activity can be estimated by the ratio of precursor fatty acid to the subsequent ‘daughter’ fatty acids present in serum(Reference Warensjo, Ohrvall and Vessby8, Reference Warensjo, Riserus and Vessby9), plasma(Reference el Boustani, Causse and Descomps10), erythrocytes(Reference Rodríguez and Christophe11) and skeletal muscle membranes(Reference Pan, Lillioja and Milner12). In that regard, the plasma 20 : 4n-6:20 : 3n-6 ratio in human studies is typically used as a surrogate measure of Δ5 activity. The 20 : 5n-3:20 : 4n-3 ratio is also reflective of enzymic Δ5 activity; however, this latter ratio is not used in human studies due to the low or undetectable amounts of 20 : 4n-3 in human plasma.
Indigenous peoples residing in the Canadian North have a paradoxical coexistence of obesity with low CVD risk(Reference Young, Moffatt and O'Neil13), which has been related to a relatively high intake of n-3 fatty acids from traditional foods. Foods that are traditionally consumed by Cree include lake trout, whitefish, burbot, walleye and sturgeon, which contain significant amount of DHA and EPA(Reference Blanchet, Dewailly and Ayotte14–16). Also, Cree typically consume wild fish, which has higher levels of n-3 fatty acids than fish from aquaculture sources(Reference Dewailly, Blanchet and Gingras17). On the other hand, indigenous peoples are undergoing rapid dietary transition that could diminish n-3 HUFA intake leading to increased chronic disease risk(Reference Robinson18, Reference Brassard, Robinson and Lavallee19). Thus, assessment of plasma HUFA status among indigenous peoples undergoing dietary transition is important. The present study has focused upon the Cree of James Bay as they have continued to undergo dietary transition away from traditional food rich in n-3 fatty acids(Reference Dewailly, Blanchet and Gingras20).
Among the endogenous regulatory factors of Δ5, insulin is of particular interest as its administration in a wide age range of healthy subjects increases activity of Δ5(Reference el Boustani, Causse and Descomps10). Also, an inverse association has been observed between insulin resistance levels and Δ5 activity in cross-sectional studies of apparently healthy individuals(Reference Warensjo, Ohrvall and Vessby8, Reference Pan, Lillioja and Milner12) and in a 20-year prospective follow-up study of middle-aged men(Reference Warensjo, Riserus and Vessby9). The data from these latter studies suggest that, in addition to dietary intake of HUFA, altered insulin resistance levels can modify HUFA tissue profile via the regulation of Δ5 activity. Independent of insulin action, adiposity could also impact upon Δ5 activity and tissue HUFA profiles, as clinical studies have demonstrated strong negative correlations between adiposity measures and Δ5 activity(Reference Warensjo, Ohrvall and Vessby8, Reference Warensjo, Riserus and Vessby9, Reference Pan, Lillioja and Milner12). In that regard, obese Zucker rats demonstrated reduced affinity of hepatic Δ5 to its substrates as compared with their lean rat counterparts, which was not explainable by differences in insulinaemia(Reference Blond, Henchiri and Bezard21).
In the present study, we explored the associations of Δ5 activity and erythrocyte levels of HUFA with measures of adiposity and insulin resistance in a James Bay Cree population undergoing dietary transition away from traditional food.
Methods
Location and subjects
A cross-sectional study was conducted in the community of Mistissini, Québec in the summer of 2005 and was coordinated by the Cree Board of Health in collaboration with McMaster, Laval and McGill universities. The community survey was the first in an ongoing study entitled ‘Nituuchischaayihitaau Aschii: A Multi-Community Environment-and-Health Longitudinal Study in Iiyiyiu Aschii’. Background information on selected aspects of the methodology and study population is provided elsewhere(Reference Egeland, Denomme and Lejeune22). Briefly speaking, local publicity were employed to raise public awareness on the project. A stratified random sampling consisting of four age strata was used to select the residents from a municipal list of the community of Mistissini. Pregnant women were excluded. The recruitment and interviews were conducted with assistance from qualified local bilingual Iiyiyiuch interviewers. Among 359 adults (age ≥ 20 years) who were randomly selected, seventy-nine of them were out of town or not able to be contacted. For the 280 potential participants, 62 % of them participated in the study (n 172). Ethics approval was obtained from participating institutions. While the community study included children, the current analyses were restricted to adults.
Among the 172 adult participants, fasting blood samples were available from 166 individuals (ninety-eight women and sixty-eight men) aged 20–88 years for the measurement of erythrocyte membrane fatty acids, serum glucose and insulin levels. Weight and body fat percentage were measured using a bioelectrical impedance scale (Tanita, Tokyo, Japan). Height was measured without shoes to the nearest cm using a stadiometer with the patient standing on a hard surface. BMI was calculated (kg/m2). Waist circumference was measured at the end of exhalation with the tape placed horizontally between the last floating rib and the iliac crest. Obesity was defined by BMI ≥ 30 kg/m2 and abdominal obesity was defined by the waist (waist ≥ 102 cm in men and ≥ 88 cm in women)(23).
Laboratory analyses
Fatty acids
Blood concentrations of fatty acids were determined in erythrocyte membranes by GLC. Erythrocytes (300 μl) were thawed and lysed in 1 ml water. Membranes were isolated by centrifugation (21 000 g for 15 min) and washed twice with a 0·9 % NaCl solution. The pellet was spiked with an internal standard of phosphatidylcholine 15 : 0 (Avanti Polar Lipids, Alabaster, AL, USA) and lipids were liquid–liquid extracted using chloroform and methanol (2:1, v/v) according to a modified Folch method(Reference Shaikh and Downar24). Fatty acids from membrane phospholipids were methylated in methanol–benzene (4:1, v/v) mixed with acetyl chloride according to previously described methods(Reference Lepage and Roy25). Fatty acid profiles were obtained by capillary GC using a temperature gradient on a HP5890 gas chromatograph (Hewlett Packard, Toronto, Canada) equipped with an HP8823 capillary column coupled with a flame ionisation detector. The elution gas used was He (split ratio 1:72). Fatty acids were identified according to their retention time on the column, using a standard mixture of thirty-seven fatty acids as a basis for comparison (FAME 37 mix; Supelco Inc., Bellefonte, PA, USA), which contained the fatty acid standard 15 : 0, as well as a mixture of thirty-one fatty acids (GLC-411; NuCheck Prep Inc., Elysian, MN, USA) and a mixture PUFA-3 (Matreya Inc., Ontario, Canada). Results were expressed as percentage of total fatty acids.
Blood glucose and insulin
Plasma glucose was measured enzymically, and fasting insulin concentrations were measured with a commercial double-antibody RIA as described by Dewailly et al. (Reference Dewailly, Blanchet and Gingras17).
Statistical analysis
Data for continuous variables are presented as mean values and standard deviations if normally distributed and as the geometric mean with 95 % CI if not normally distributed. Categorical variables were calculated as proportions. Fatty acid content in erythrocyte membranes was expressed as a percentage of total fatty acids. SFA, MUFA, n-3 PUFA, n-6 PUFA and trans-fatty acids were calculated by summing the concentrations of individual acids if they were detectable. The activity of Δ5 was estimated by the 20 : 4n-6:20 : 3n-6 ratio. Insulin resistance was estimated by the homeostasis model assessment of insulin resistance (HOMA-IR), calculated as follows:
As the age span of the present study population ranged from 20 to 88 years, subjects were divided into four age groups. Linear regression was applied to detect linear trends of the anthropometric measures, HOMA-IR, fatty acid profiles of erythrocyte membranes and Δ5 activity across age groups. Interactions between HOMA-IR and anthropometric measures were tested with general linear models. The general linear model was conducted to test linear trends of continuous variables across age groups. The generalised regression model was used to test linear trends of categorical variables across age groups. As there were no observed interactions of sex on Δ5-related associations, the results of associations of Δ5 with anthropometric measures and HOMA-IR were presented in a combined manner for all subjects. Spearman correlations were conducted for the correlations of Δ5 with adiposity measures, HOMA-IR and age. Age-adjusted multivariable linear regression was used to: (1) separately evaluate the associations of Δ5 with HOMA-IR; (2) separately assess the associations of Δ5 with BMI, body fat percentage and waist circumference. Because disease state may influence the association of HOMA-IR with Δ5, the interaction term was included in the age-adjusted linear regression analyses to test the interaction of fasting glucose status on the association between HOMA-IR and desaturase Δ5. All P values were obtained from two-sided tests. Data were analysed with SAS software (version 9.1; SAS Institute Inc., Cary, NC, USA).
Results
Subject characteristics
The present study population was composed of ninety-eight females (aged 40·1 (sd 15·8) years; BMI 34·9 (sd 6·8) kg/m2) and sixty-eight males (mean age 40·7 (sd 16·1) years; mean BMI 31·5 (sd 5·5) kg/m2). There were no significant differences among age groups in adiposity measures (P for trend NS) (Table 1). The geometric mean HOMA-IR increased slightly and non-significantly (P < 0·1) with increasing age, whereas the proportion of subjects with impaired fasting glucose (i.e. fasting glucose defined as ≥ 5·7 mmol/l)(26) increased significantly with advancing age (P < 0·0001).
Table 1 Clinical characteristics of the study population
(Mean values and standard deviations)
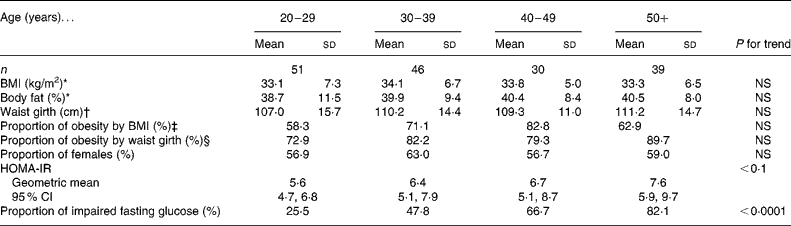
HOMA-IR, homeostasis model assessment of insulin resistance.
* Age group 18–29 years, n 48; age group 30–39 years, n 45; age group 40–49 years, n 29; age group 50+ years, n 35.
† Age group 18–29 years, n 48; age group 30–39 years, n 45; age group 40–49 years, n 29; age group 50+ years, n 39.
‡ Proportion of subjects with BMI ≥ 30 kg/m2.
§ Proportion of subjects with waist girth ≥ 88 cm (female) or ≥ 102 cm (male).
Erythrocyte fatty acid content
Fatty acid classes of erythrocyte membranes showed distinctive associations with age (Table 2). In particular, total n-3 fatty acids increased significantly across age groups (P < 0·0001). The latter observed association was driven mainly by two HUFA fatty acids, 20 : 5n-3 and 22 : 6n-3, which consistently and significantly increased with age (P < 0·0001). Conversely, total n-6 fatty acids decreased significantly across age groups (P < 0·0001). Almost all individual n-6 fatty acids showed the same strong and inverse associations with age (P < 0·0001) except for 20 : 4n-6 whose percentage in the fatty acid profile of erythrocyte membranes differed only slightly between different age groups (P = 0·05).
Table 2 Proportion (% of total fatty acids) of fatty acids in erythrocytes of the study population
(Mean values and standard deviations)
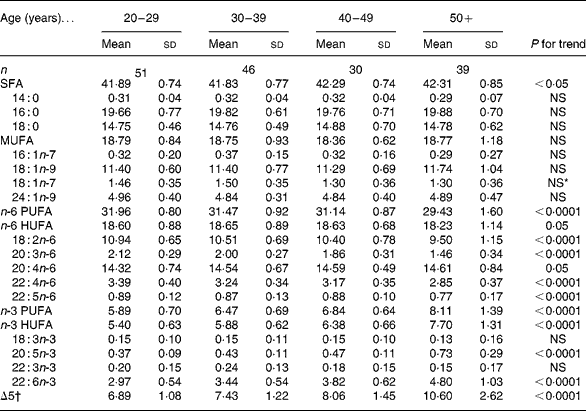
HUFA, highly unsaturated long-chain fatty acids; Δ5, desaturase 5.
* No clinical significance, because although P < 0·01 for the general linear model, R 2 is only 4 % and β is too small ( − 0·005).
† Δ5 is estimated as the 20 : 4n-6:20 : 3n-6 ratio.
As expected, n-3 fatty acids were inversely correlated with n-6 fatty acids (r s − 0·716; P ≤ 0·0001). Similarly, 20 : 3n-6 negatively correlated with 20 : 5n-3 (r − 0·463; P < 0·0001) and 22 : 6n-3 (r − 0·503; P < 0·0001). In terms of the numerator and denominator of the ratio representing Δ5 activity, the numerator (20 : 4n-6) increased with age (P = 0·05) while the denominator (20 : 3n-6) decreased with age (P < 0·0001), resulting in significant increases in Δ5 across all age groups (P < 0·0001) (Table 2).
The proportion of total SFA increased with age (P < 0·05), but the increase was subtle and there was no significant difference in individual SFA level by age. In terms of other fatty acid classes, i.e. MUFA and trans-fatty acids, no significant differences were observed across age groups for either the total fatty acid class or for any individual fatty acid.
Desaturase 5, homeostasis model assessment of insulin resistance and adiposity
Negative correlations were observed between Δ5 and adiposity measures, particularly BMI (r s − 0·175; P < 0·05) and percentage body fat (r s − 0·168; P < 0·05). The activity of Δ5 was not correlated significantly with HOMA-IR in the unadjusted analyses (Table 3). Upon further analyses of fasting glucose status, however, a significant interaction term showed differences in the slope of HOMA-IR with Δ5 as the dependent variable (P = 0·03). In that regard, a strong and significant inverse relationship of HOMA-IR with Δ5 was observed among those with normal fasting glucose but not those with impaired fasting glucose. There was no observed significant interaction of fasting glucose status on the associations between adiposity and Δ5. Thus, all the subjects were combined together in separate age-adjusted models evaluating BMI, percentage body fat and waist circumference for their associations with Δ5. The adjusted model using Δ5 as the dependent variable showed for BMI: β − 0·037, se 0·019, P = 0·05; for percentage body fat: β − 0·041, se 0·013, P = 0·002; and for waist: β − 0·020, se 0·009, P = 0·03 (Table 4).
Table 3 Spearman correlation coefficients among adiposity, homeostasis model assessment of insulin resistance (HOMA-IR) and age

Δ5, desaturase 5.
*P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001.
†0·1 < P < 0·05.
Table 4 Age-adjusted regression coefficients of adiposity measures and homeostasis model assessment of insulin resistance (HOMA-IR) and their individual associations with desaturase 5*
(β Coefficients with their standard errors)

* Adjusted for age and separately assessed.
† R 2adj refers to the whole model.
Discussion
In the present study, HUFA n-3 fatty acids, especially 22 : 6n-3, increased significantly with age, while n-6 fatty acids showed an opposite trend. These latter findings are consistent with previous observations that older Cree consume more traditional food rich in HUFA n-3 fatty acids, while young Cree consume more market foods that are rich in n-6 fatty acids(Reference Dewailly, Blanchet and Gingras20). In that regard, plasma EPA and DHA were shown to increase significantly with age in an earlier study of James Bay Cree(Reference Dewailly, Blanchet and Gingras20). High consumption of traditional food, including fish, has been reported among older community members throughout northern indigenous communities in the three Canadian territories(Reference Kuhnlein, Receveur and Soueida27). In the present study, 20 : 3n-6 negatively correlated with the two n-3 fatty acids, 20 : 5n-3 and 22 : 6n-3, that are abundant in fish and sea food(Reference Appavoo, Kubow and Kuhnlein28, Reference Kuhnlein, Kubow and Soueida29). As only trace amounts of 20 : 3n-6 are present in food, this fatty acid is primarily indicative of endogenous metabolic activity. Since n-3 fatty acids and n-6 fatty acids compete with each other within membrane phospholipids(Reference Khan, Elherik and Bolton-Smith30) and for the desaturases(Reference Escudero, Montilla and Garcia31), increased intake of fatty acids from one class leads to the reduced presence of fatty acids in membranes from the other classes. Hence, a reasonable explanation is that higher consumption of fish in older Cree inhibits elongation and desaturation of n-6 fatty acids in these subjects, leading to the negative relationship between n-3 HUFA and 20 : 3n-6 observed in the erythrocyte membrane fatty acid profiles.
The fatty acid composition of erythrocyte membranes reflects the dietary intake of fatty acids which are mainly exogenously originated, such as HUFA, trans-fatty acids and odd-number fatty acids. Although the fatty acid profile of erythrocyte membranes is modulated by both endogenous pathways and dietary intake, the efficiency of endogenous synthetic pathways of n-3 HUFA is recognised to be rather low. Based on clinical trials using isotopically labelled tracer fatty acids or analysing blood fatty acid profile after dietary supplementation, a recent review concluded that only about 5 % of 18 : 3n-3 consumed is transformed to 20 : 5n-3 and less than 0·5 % of 18 : 3n-3 is converted into 22 : 6n-3(Reference Plourde and Cunnane32). Hence, the erythrocyte membrane n-3 HUFA content reflects primarily dietary intake. The measurement of fatty acid profiles of erythrocyte membranes used in the present study has been extensively tested in dietary PUFA intervention studies using dietary sources of 20 : 5n-3 and 22 : 6n-3(Reference Brown, Roberts and Pritchard33, Reference Kuriki, Nagaya and Tokudome34) and is commonly used as an evaluation of HUFA intake in epidemiological studies(Reference Baylin, Kim and Donovan-Palmer35). Normally the endogenous pathway of HUFA biosynthesis exerts a relatively minor influence on the HUFA content of erythrocyte membranes. When dietary HUFA intake is low, however, the contribution of endogenous HUFA biosynthesis towards erythrocyte HUFA content becomes physiologically significant. Since dietary fatty acid intake was closely related to age in the present study, an adjustment for age was performed to eliminate the confounding influence of fatty acid intake on Δ5 activity, which was estimated by the 20 : 4n-6:20 : 3n-6 ratio in erythrocyte membranes. Thus, the impact of other metabolic influences on Δ5 activity, such as obesity and insulin resistance, was assessed by adjusting for the confounding effects of age and dietary fatty acid intake.
Interestingly, in the present study population, there was no significant difference of obesity prevalence between age groups in both sexes. Obesity was prevalent in the study population as shown by the mean BMI of >33 kg/m2, including more than one-third of subjects having BMI of ≥ 35 kg/m2 (data not shown) and a mean waist circumstance >106 cm in all of the age groups. Both prevalence of obesity and the average BMI level among all age groups were much higher than previously reported in a recent Canadian national survey of the non-indigenous population(36) or data from an earlier national survey of multiple ethnic populations in the USA(37).
A significant proportion of Cree subjects had impaired fasting glucose level ( ≥ 5·7 mmol/l) and this proportion increased with age. At normal fasting glucose conditions ( < 5·7 mmol/l), a positive association exists between levels of insulin and glucose (r 0·417, P = 0·0001; data not shown) among the Cree. This correlation, however, disappeared in the subgroup with impaired fasting glucose, indicating that insulin's regulation was disrupted. Hence, it was not surprising that an inverse association between HOMA-IR and Δ5 was observed among subjects with normal fasting glucose, while this association became non-significant among subjects with impaired fasting glucose.
In contrast, no significant interaction was observed for the effects of adiposity on Δ5 between normoglycaemic and impaired glucose-tolerant individual, suggesting that gradients of adiposity continue to play an important role in Δ5 activity regardless of the degree of disease progression. In the present study, body fat percentage was a more significant negative predictor of Δ5 than waist girth, which may be related to the unusually high adiposity of the Cree study population. In the presence of high adiposity, effects of abdominal adiposity on metabolic consequences may be diluted, since Health Canada suggests that waist girth is not to be considered as an additive risk for individuals with BMI ≥ 35 kg/m2(23). Similar to the Cree, inverse associations between adiposity level and Δ5 have been reported in several studies including Pima Indians(Reference Pan, Lillioja and Milner12), obese Hungarian children(Reference Decsi, Molnar and Koletzko38) and Caucasians(Reference Warensjo, Ohrvall and Vessby8). Among Pima Indians, the 20 : 4n-6:20 : 3n-6 ratio in skeletal muscle membranes was significantly and strongly inversely related to adiposity measures(Reference Pan, Lillioja and Milner12). A study on Hungarian children showed that the 20 : 4n-6:20 : 3n-6 ratio in plasma phospholipids was reduced in obese children compared with normal controls and this ratio was lowest among obese subjects with the metabolic syndrome(Reference Decsi, Molnar and Koletzko38). The latter observation may suggest that obesity and other metabolic syndrome components such as insulin resistance may have additive effects on suppressing Δ5 activity. A recent study on apparently healthy middle-aged Caucasians reported a negative correlation between the 20 : 4n-6:20 : 3n-6 ratio in serum cholesteryl esters and sagittal abdominal diameter that was independent of age(Reference Warensjo, Ohrvall and Vessby8). Evidence from in vitro studies and rodent models also suggests a direct link between adiposity and Δ5 activity. In one in vitro study, fresh microsomes taken from obese and lean Zucker rats were incubated with the substrate 20 : 3n-6 at physiological concentrations. Δ5 activity, measured as 20 : 4n-6 formed per time unit per weight unit of liver microsomal protein, was lower in freshly isolated microsomes from obese Zucker rats at all age stages, which correlated with in vivo observations of Δ5 activity (as assessed by hepatic 20 : 4n-6:20 : 3n-6 ratios) being lower in obese than in lean Zucker rats(Reference Blond, Henchiri and Bezard21). The lower Δ5 activity in the hepatic microsomes in the obese Zucker rats was not accountable for the hyperinsulinaemic condition of the obese Zucker rats. Similar findings have been reported(Reference Cunnane, Manku and Horrobin39) in some but not all(Reference Hughes and York40) studies involving ob/ob mice. In the present work, no significant interaction was observed for the effects of adiposity on Δ5 between normoglycaemic and impaired fasting glucose individuals, suggesting that gradients of adiposity continue to play an important modulatory role in Δ5 activity regardless of the degree of disease progression involving glucose intolerance.
In most populations, advancing age typically leads to increasing adiposity and reduced insulin sensitivity with a consequent decrease in Δ5 activity. Among the Cree, however, adiposity was not related to age and age was only weakly and non-significantly related to insulin resistance. Therefore, age cannot account for the observed relationships of the adiposity measures with Δ5 seen among the studied population of Cree subjects or the observed association between HOMA-IR with Δ5 observed among Cree subjects with normal fasting glucose. Additionally, the statistical adjustment for age used in the present study adjusts for the observed age-related differences in dietary intake. To date, animal studies show inconsistent findings regarding the importance of age on Δ5 activity(Reference Maniongui, Blond and Ullmann41Reference Takahashi and Horrobin,42), indicating that future studies are warranted in this regard.
Several limitations should be recognised in the present study. The causal relationships between the variables of interest cannot be determined based on the cross-sectional data. In addition, more precise measurements of body composition such as dual-energy X-ray absorptiometry could be considered to detect subcutaneous adipose tissue and visceral adipose tissue in future studies, which would clarify which part of adipose tissue has more significant influence on fatty acid metabolism. Future studies are needed to observe whether the results from the current studies can be generalised to other populations.
The results reported herein indicate that the high prevalence of obesity among Cree of James Bay concomitant with a low n-3 HUFA intake could exacerbate chronic disease risks through reduction in Δ5 activity. The activity of Δ5 may be important for disease prevention, as this enzyme is critical for the synthesis of optimal n-3 HUFA tissue profiles that are associated with anti-inflammatory and anti-arrhythmic cardioprotective effects. These findings also illustrate that several disease-related factors such as dietary changes, obesity and insulin resistance can work in tandem to elevate disease risk in indigenous peoples undergoing rapid transitions.
Acknowledgements
The present study was supported by the Niskamoon Corporation. The authors thank the community research staff and participants. The authors' responsibilities were as follows: Y. E. Z. analysed the data and drafted the manuscript; S. K. supervised the manuscript drafting; E. D. designed the study; P. J. carried out the fatty acid analysis; G. M. E. designed the study and supervised the data analysis. All authors contributed to the revision of the manuscript.
None of the authors have any financial or personal conflict of interest to disclose.






