Physician’s support and endorsement of preventive health behaviours are important predictors of adherence to medical regimens among individuals diagnosed with cancer (Bellizzi et al., Reference Bellizzi, Rowland, Jeffery and Mcneel2005). Common reactions to a melanoma diagnosis, both immediate and during the 10-year follow-up period, include ‘trusting my doctors’ and ‘following the medical advice exactly’ (Zschocke et al., Reference Zschocke, Augustin and Muthny1996). Melanoma survivors have a ninefold increased risk for developing a subsequent primary melanoma compared to the general population (Bradford et al., Reference Bradford, Freedman, Goldstein and Tucker2010). Most practice guidelines for follow-up care recommend skin self-examination (SSE) for melanoma survivors (Marciano et al., Reference Marciano, Merlin, Bessen and Street2014; Watts et al., Reference Watts, Dieng, Morton, Mann, Menzies and Cust2015), as a means of facilitating early detection and timely treatment. Early detection is a key contributor to reductions in melanoma-related mortality (Katalinic et al., Reference Katalinic, Waldmann, Weinstock, Geller, Eisemann, Greinert, Volkmer and Breitbart2012). Research has found that upwards 61% of melanomas are self-detected as opposed to physician-detected (Carli et al., Reference Carli, De Giorgi, Palli, Maurichi, Mulas, Orlandi, Imberti, Stanganelli, Soma and Dioguardi2003; Geller, Reference Geller2009), and those who practice SSE are diagnosed with thinner (earlier disease) melanoma compared to those who do not (Carli et al., Reference Carli, De Giorgi, Palli, Maurichi, Mulas, Orlandi, Imberti, Stanganelli, Soma and Dioguardi2003; Swetter et al., Reference Swetter, Pollitt, Johnson, Brooks and Geller2012). Further, analyses from a recent randomized controlled trial (Robinson et al., Reference Robinson, Wayne, Martini, Hultgren, Mallett and Turrisi2016) found that over 24 months, melanoma survivors who had been instructed to check their skin identified 43 new melanomas, whereas survivors who had not specifically been trained or instructed to check their skin did not identify any new melanomas. Among melanoma survivors, reported rates for thorough (entire body) and regular SSE (at least every two months) range from 14 to 39% (Loescher et al., Reference Loescher, Harris, Lim and Su2006; Manne and Lessin, Reference Manne and Lessin2006; Mujumdar et al., Reference Mujumdar, Hay, Monroe-Hinds, Hummer, Begg, Wilcox, Oliveria and Berwick2009; Pollitt et al., Reference Pollitt, Geller, Brooks, Johnson, Park and Swetter2009; Coups et al., Reference Coups, Manne, Stapleton, Tatum and Goydos2016).
Physician recommendation for SSE and live demonstration of how to inspect the skin for problematic lesions play a crucial role in the initiation and maintenance of SSE. For example, individuals with a personal history of melanoma who have been instructed or shown how to check their skin by physicians rated this behaviour as more important (Zschocke et al., Reference Zschocke, Rhein, Grimme, Stein, Muthny and Augustin2000) and were more likely to perform SSE compared to those who did not receive such instructions (Manne et al., Reference Manne, Fasanella, Connors, Floyd, Wang and Lessin2004; Coups et al., Reference Coups, Manne, Stapleton, Tatum and Goydos2016). Currently, it is unknown what the rates for physician recommendation of SSE to newly diagnosed patients are across medical settings. A small pilot conducted by the current authors in a melanoma clinic in Quebec, Canada found that among 41 newly diagnosed patients, 71% (n=29) reported that a physician had recommended they perform regular SSE post diagnosis compared to only 37% (n=15) who reported receiving similar recommendations prior to diagnosis (Körner et al., Reference Körner, Coroiu, Martins and Wang2013a). In another study conducted in the United States with melanoma survivors (n=177), 37% of patients reported having been shown by a healthcare professional how to correctly perform SSE, but the rates of healthcare professional recommendations for SSE were not reported (Coups et al., Reference Coups, Manne, Stapleton, Tatum and Goydos2016). Failure to recommend SSE to patients at high-risk is a missed opportunity for skin cancer prevention at the level of primary healthcare, with important and potentially devastating ramifications for patients and their families, as well as the medical system, as substantially more resources are allocated to managing metastatic melanoma versus earlier stages (Tsao et al., Reference Tsao, Rogers and Sober1998).
Studies conducted with melanoma patients typically use patient-reported outcomes to assess whether physicians recommended SSE. As no standardized self-report measure of physician support of SSE currently exists, our team developed a measure based on clinical practice guidelines for melanoma follow-up care (for reviews of clinical guidelines, see Marciano et al., Reference Marciano, Merlin, Bessen and Street2014; Watts et al., Reference Watts, Dieng, Morton, Mann, Menzies and Cust2015), which stress the importance of physician recommendation for SSE and demonstration of SSE. The aim of the current study was to report on the preliminary validation of the Physician Support of SSE Scale, including factorial structure, convergent and divergent validity, and internal consistency reliability.
Method
Participants and procedures
This study is a secondary analysis using baseline data collected for a longitudinal study investigating barriers and facilitators of SSE among melanoma survivors. English and French-speaking participants were recruited from two hospitals in Montréal, Canada between 2012 and 2016. Participants completed self-report baseline questionnaires assessing socio-demographic information and psychosocial constructs, including physician’s recommendation and support of SSE, patient distress, intention to perform SSE, and self-efficacy for SSE. Patient health information was extracted from hospital medical charts. Institutional Research Boards of McGill University and participating hospitals approved the study.
Measures
Development of the Physician Support of SSE Scale
A large pool of items, using different phrasing in English, was created to assess physician support of SSE. When choosing the item content, we consulted clinical care guidelines, which suggest that clinicians should recommend SSE to high-risk individuals, but also show how SSE should be performed, and also our pilot data from a study with non-melanoma skin cancer. The final version of the scale included nine items, which encompass various aspects of SSE-related support provided by physicians, such as pragmatic (cognitive) aspects (‘My physician has asked me if I have questions or concerns about examining my skin’), behavioural components (‘My physician has pointed out a lesion(s) that I should keep an eye on’), and perceptions of physician attitudes towards SSE (‘It seems important to my physician that I do skin self-exams’). In addition to recommendations to perform SSE, we also included items assessing recommendations for regular SSE and SSE of the entire body. This is a self-report scale to be completed by patients: patients were instructed to relay their experience with one physician involved in their melanoma care in the previous three months. To minimize recall bias, the items were scored on a Likert scale ranging from 0 (not at all true) to 3 (true) as opposed to a dichotomous scale, which is often used for rating observable behaviours. The questionnaire was developed in English and then translated into Canadian French to reflect the language needs of our Québécois patients. An authorized French translator with prior experience in health research translated the English items into French (forward translation) and one bilingual research assistant who identified as Anglophone (ie, native English speaker) translated the French items back into English (backward translation). Subsequently, two bilingual members of our research group evaluated the forward and backward translations against each other and suggested changes, which were discussed with the French authorized translator, who then finalized the French version. Two independent bilingual reviewers familiar with psychosocial oncology research, and one community member (ie, cancer patient) ensured the clarity and relevance of the items and approved the final English and French versions.
Physician support of SSE was assessed via the newly developed nine-item scale, with responses ranging from 0 (not at all true) to 3 (true), and possible scores between 0 and 27.
Additional measures used for the validation of the Physician Support of SSE Scale
The 15-item Health Care Climate Questionnaire (HCCQ; Williams et al., Reference Williams, Grow, Freedman, Ryan and Deci1996) was used to assess autonomy-supportive attitudes from healthcare providers. The HCCQ responses range from 1 (strongly disagree) to 7 (strongly agree) with possible scores between 15 and 105. The HCCQ is commonly used to measure perceived healthcare provider support in samples of patients with chronic illness, which showed good psychometric properties across multiple samples (Williams et al., Reference Williams, Grow, Freedman, Ryan and Deci1996). The 15-item Skin Cancer Index (SCI) (Rhee et al., Reference Rhee, Matthews, Neuburg, Burzynski and Nattinger2005) was used to assess skin cancer-specific distress. The SCI responses range from 1 (very much) to 5 (not at all), with possible scores between 15 and 75. Intentions to perform SSE were assessed using one item, ‘How likely are you to self-examine your skin on a regular basis in the coming year?’ scored on a range from 1 (very unlikely) to 5 (very likely). Frequency of clinical skin exams, recommendations for SSE, and demonstration of SSE by healthcare professional prior to current melanoma diagnosis (Körner et al., Reference Körner, Drapeau, Thombs, Rosberger, Wang, Khanna, Spatz, Coroiu, Garland and Batist2013b) were assessed using three individual items scored on a scale ranging from 0 (never) to 3 (a few times a year). For all measures, higher scores indicated more of the measured construct, with items of the SCI being reverse coded to reflect this trend.
Data analysis plan
Analyses were conducted with patients diagnosed within five years of study participation, given the more frequent follow-up regimen during this time period at our recruiting hospitals (Wang, Reference Wang2007). Exploratory Factor Analysis (EFA) with weighted least squares parameter estimation and oblique rotation was conducted with the nine-item Physician Support of SSE Scale. As per recommended guidelines for assessing model fit (Hu and Bentler, Reference Hu and Bentler1999), in addition to the χ 2 test, which is highly sensitive to sample size and potentially leading to erroneous rejection of the model fit (Reise et al., Reference Reise, Widaman and Pugh1993), we used a combination of other model fit indices: the Tucker–Lewis index (TLI; Tucker and Lewis, Reference Tucker and Lewis1973), the comparative fit index (CFI; Bentler, Reference Bentler1990) and the root mean square error of approximation (RMSEA; Steiger, Reference Steiger1990). Good fitting models are indicated by a TLI and CFI ⩾0.95 and RMSEA⩽0.06 (Hu and Bentler, Reference Hu and Bentler1999), although a CFI and TLI of 0.90 or above (Kline, Reference Kline2005) and a RMSEA of 0.08 or less (Browne and Cudeck, Reference Browne and Cudeck1993) are regarded as indicators of an adequate model fit. These analyses were conducted using Mplus 7 (Muthén and Muthén, Reference Muthén and Muthén2007), which is a modelling software preferred for factor analysis over SPSS.
Descriptive statistics were computed for the study sample. Cronbach’s α was computed for the Physician Support of SSE scale. Convergent and divergent validity of the scale were assessed through Pearson’s and Spearman correlations with socio-demographic (age, education) and medical (cancer stage) variables and the study measures. The magnitude of the bivariate correlations was interpreted following Cohen’s effect size descriptors, that is, r≤0.10 indicating small, r=0.30 indicating moderate, and r=0.50 indicating large differences (Cohen, Reference Cohen1988). These analyses were conducted using SPSS, version 20.
Results
Sample characteristics
Current secondary analysis included patients diagnosed with melanoma within past five years (n=188; 78% of our total sample). Sample characteristics are included in Table 1.
Table 1 Sample characteristics (n=188)
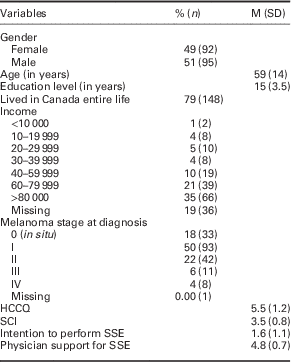
HCCQ=Health Care Climate Questionnaire; SCI=Skin Cancer Index; SSE=skin self-examination.
Factorial structure of the Physician Support of SSE Scale
An EFA with the nine-item scale showed less than ideal, but acceptable fit, χ 2(n=188, df=27)=85.61, P=0.000, RMSEA=0.11, 90% CI [0.08, 0.13], CFI=0.998, TLI=0.997. As a sensitivity analysis, we conducted EFA’s with the French (χ 2(n=94, df=27)=51.00, P=0.004, RMSEA=0.10, 90% CI [0.06, 0.14], CFI=0.997, TLI=0.996) and English (χ 2(n=94, df=27)=47.83, P=0.000, RMSEA=.009, 90% CI [0.05, 0.13], CFI=0.999, TLI=0.998) subsamples and found similar results.
Detailed statistics for the nine-item scale, including factor loadings and inter-item correlations computed across the entire sample, were included in Table 2. We noted high (⩾0.9) inter-item correlations between item #4 (‘My physician has recommended that I do skin exams’) and item #5 (‘My physician has recommended that I examine my skin regularly’), and between item #5 and item #6 (‘My physician has recommended that I examine the skin of my whole body’). A second EFA excluding item #5 showed acceptable fit, χ 2(n=188, df=20)=68.75, P=0.000, RMSEA=0.11, 90% CI [0.09, 0.14], CFI=0.996, TLI=0.995).
Table 2 Factor loadings, confidence intervals (CI), means (and standard deviations), and Pearson’s inter-item (and corrected item-total) correlations
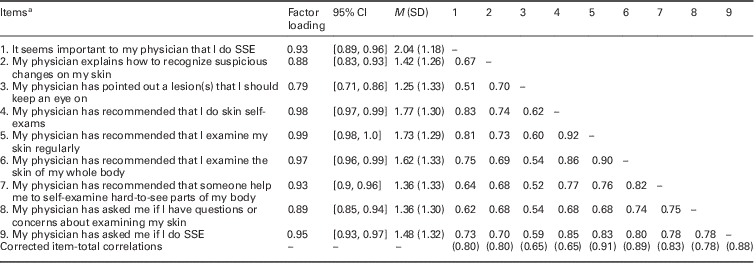
All correlations were significant at P<0.001.
a French items available upon request from main author.
SSE=skin self-examination.
Validity and reliability of the nine-item Physician Support of SSE scale
There were no associations with demographic information, such as age, education, and with medical variables, melanoma stage, time since diagnosis. The Physician Support for SSE Scale was positively associated with the HCCQ (r=0.39, P<0.001), prior healthcare professional (HCP) recommendations for SSE (r=0.36, P<0.001), prior HCP-led skin exam (r=0.18, P=0.014), prior HCP demonstration of SSE (r=0.26, P<0.001), and intention to perform SSE (r=0.22, P=0.003); the scale was not associated with the SCI (distress). Cronbach’s alpha for the combined English and French sample was α=0.96.
Discussion
This paper describes the preliminary validation of a short measure for physician support of SSE among patients already diagnosed with melanoma, who were recruited from an outpatient secondary care clinic. Factor analyses with English, French, and combined samples suggested that the nine items represent a single factor. High inter-items correlations were observed between items assessing recommendations for SSE (item #4) and recommendations for regular SSEs (item #5) and between recommendations for regular SSE (item #5) and recommendations for SSE of the entire body (item #6). These correlations are not unusual, given the overlapping content of the respective items. However, removing item #5 did not result in improved fit for the overall model. The high correlations between these items could be partially explained by the lack of evidence on the optimal frequency of SSE: while there are currently no randomized controlled trials assessing the impact of SSE on melanoma early detection or mortality (Coroiu et al., Reference Coroiu, Moran, Kingsland, Thombs and Körnerin preparation), frequency of SSE included in clinical care guidelines range from monthly to yearly (Watts et al., Reference Watts, Dieng, Morton, Mann, Menzies and Cust2015). This is likely to influence the consistency of physician recommendations for SSE, which are reflected in patients’ self-reports of the recommendations. The physician support of SSE scale had moderate positive associations with measures of perceived support from HCP, previous recommendation and demonstration of SSE and previous skin examination by HCP, and intentions to perform SSE, which lends support for the convergent validity of the measure. The scale was not associated with demographic characteristics or with skin cancer distress, providing evidence for the divergent validity of the physician support for SSE scale. Further, internal consistency reliability of the combined English and French samples was excellent.
This scale was designed primarily as an outcome measure for intervention studies with individuals at high risk for melanoma (and other skin cancers) in order to identify those who have not been instructed to perform SSE or educated about SSE by physicians, and who could benefit from interventions to facilitate SSE uptake. Although the participants recruited in the current study were all secondary care patients, there are other individuals at high risk for whom this scale is appropriate, including first-degree relatives of melanoma patients, survivors of childhood cancers treated with radiation, transplant recipients, for whom clinical care guidelines also recommend SSE (for a review of clinical care guidelines, see Watts et al., Reference Watts, Dieng, Morton, Mann, Menzies and Cust2015). Pending further psychometric evaluation, the items of this scale (English and French versions) could be modified for applicability to other healthcare professionals, including nurses. Given the limited time physicians can allocate to each patient, it might simply be more feasible that the recommendations to check the skin be provided by other professionals on the care team, with nurses being best positioned for this important task.
An important limitation of the current study is the lack of a qualitative component to inform the item development phase of the scale under investigation. While we conducted extensive literature reviews on the topic of physician recommendations for health behaviours, including SSE, and consulted with melanoma prevention experts and clinical dermatologists, we did not conduct interviews or focus groups with patients.
Given the importance of physician recommendations for SSE, that no other scale assessing this construct currently exists, and based on our results, we recommend that the nine-item version of the scale be retained in future research with individuals at high risk for melanoma, for whom SSE is highly applicable. We also recommend that future studies using this scale should focus on further investigating its psychometric properties. Future versions of this scale might specify the optimal frequency of SSE, as more research becomes available, and might incorporate items reflecting recommendations to use aids while performing SSE (eg, mirrors, melanoma pictures, partners); might update the instructions embedded in the scale to include other healthcare professionals, such as nurses; and might add other relevant items, as they become apparent from conversations with patients.
Acknowledgements
We would like to thank our participants who graciously offered their time to complete our study and the numerous research assistants with the Health Psychology Research Group (HPRG) at McGill who collected data for this study.
Conflicts of Interest
None.
Financial Support
The longitudinal study was supported by operating grants from the Fonds de la Recherche en Santé du Québec (FRSQ) and the Canadian Institute for Health Research (CIHR) awarded to Annett Körner, PhD. Adina Coroiu, MA, PhD Candidate is supported by a doctoral training award from CIHR; Chelsea Moran, BA, and Rosalind Garland, BA were supported by master’s training awards from CIHR.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Ethical approval was obtained from the necessary hospital and university research ethics boards.





