Introduction
Reintroduction of a species aims to re-establish a viable population within the indigenous range of the focal species (IUCN 1998, 2013). The measure of success of a reintroduction programme is the establishment of a self-sustaining population (Scott and Carpenter Reference Scott and Carperter1987), which ultimately relies on the survival ability of the released animals at the release site. A population is considered self-sustaining if it shows fidelity to the release area, long-term survival and breeding success (Armstrong et al. Reference Armstrong, Castro, Alley, Feenstra and Perrott1999). The success of reintroduction programmes depends on the quality of the habitat in which the reintroduction takes place, the adaptive ability of the birds to new conditions, the total number and the age structure of the released birds (Griffith et al. Reference Griffith, Scott, Carpenter and Reed1989, Wolf et al. Reference Wolf, Griffith, Reed and Temple1996, Armstrong et al. Reference Armstrong, Davidson, Dimond, Perrott, Castro, Ewen, Griffiths and Taylor2002, Tweed et al. Reference Tweed, Foster, Woodworth, Monahan, Kellerman and Lieberman2006). To establish a new population, it is essential for the released birds to survive during the establishment phase, to settle in the new reintroduced area, to pair and nest successfully in the release habitat and to produce a sufficient number of young individuals to balance mortality (Yu et al. Reference Yu, Li and Huo2015). In addition, the age structure of a reintroduced population should be considered to see whether the population will increase continuously because a population with more adults has a potential to grow faster (Komers and Curman Reference Komers and Curman2000). Among all reintroduction programmes for Ciconiiformes species, the reintroduction project for the White Stork Ciconia ciconia in Switzerland was relatively successful (Schaub et al. Reference Schaub, Pradel and Lebreton2004), and the results provided useful insight for the protection of the Crested Ibis Nipponia nippon.
The Crested Ibis is now extirpated in almost all of its former range except in China (BirdLife International 2001). The reasons for the rapid disappearance of the species in most of its historical range remains unclear, but deforestation, illegal hunting, and the excessive use of pesticides were generally believed to be largely contributable to the successive extirpation during the 20th century (Shi et al. 1991). The rediscovery of the last seven individuals (two pairs and three nestlings) in a remote mountainous village in Yangxian gave new hope for the survival of the species (Liu Reference Liu1981).
Over the past three decades, a great deal of conservation effort, both in situ and ex situ, has been undertaken by researchers and the local government. The wild ibis population has increased to approximately 1,100 (Wang et al. Reference Wang, Liu, Qing, Ding, Cui, Ye, Lu, Yan, Ke and Ding2014) and more than 1,000 captive-bred individuals were raised in 12 conservation breeding centres or zoos in China, Japan and South Korea (Yu et al. Reference Yu, Li and Huo2015). The great success in multiple conservation projects (BirdLife International 2001) enabled the reintroduction of the Crested Ibis within its former ranges (Yu et al. Reference Yu, Chang, Li, Chen and Shi2009, Nagata and Yamagishi Reference Nagata and Yamagishi2013). By October 2011, 56 captive-bred individuals (29 males and 27 females) were reintroduced into the wild in four subsequent releases (Huo et al. Reference Huo, Guo, Li and Yu2014, Yu et al. Reference Yu, Li and Huo2015). All released individuals and wild-born fledglings were monitored over an eight-year period (Wang et al. Reference Wang, Ye, Li, Huo, Li and Yu2016).
In this article, we aim (i) to quantify age structure and adult sex ratio (ASR) of the reintroduced population, (ii) to employ a modern capture-recapture analysis method to estimate the survival rates of different cohorts in the reintroduced population, and (iii) to propose suggestions for subsequent releases of the Crested Ibis and other endangered species.
Methods
Study area
Our study area is located in Ningshan County (33°07′–33°50′ N, 108°02′–108°56′ E), (Shaanxi Province) on the southern slope of the Qinling Mountains (Figure 1). The area is used by the ibis for breeding and foraging and the landscape mosaic is characterised by intermontane basins, often developed for rice fields, interspersed with hills. Numerous shallow streams flow into the Changan River, where most individuals of the population bred and foraged. The specific geographical location and natural conditions have been reported in details by Yu et al. (Reference Yu, Li and Huo2015).
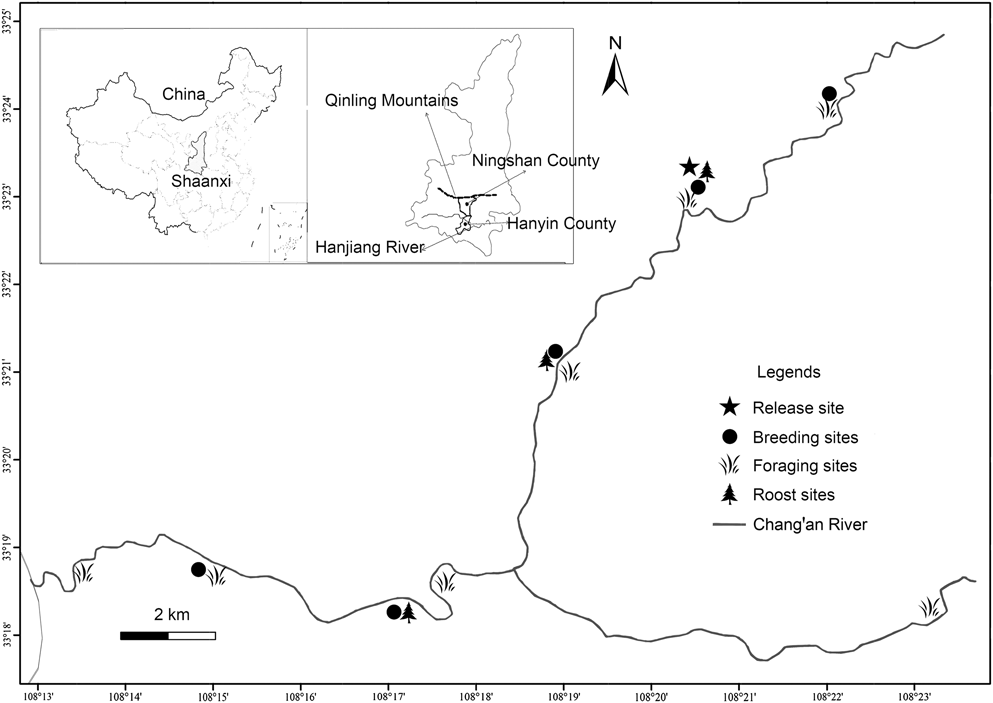
Figure 1. Map of released region showing the location of the release site (pentagram), breeding sites (solid circle), foraging sites (grass) and roost sites (tree) for the reintroduced population. An adult male of the reintroduced population travelled south-east (to Hanyin County) and paired with another female from the wild population of Yangxian County.
Study birds and field observations
Up to the present study, a total of 56 captive-reared individuals have been reintroduced into the study area. From May to October in 2007 (13 females, 13 males); 2008 (four females, two males); 2009 (six females, eight males), and 2011 (three females, seven males), were released, respectively. Two breeding pairs formed and fledged three nestlings successfully immediately after release in 2008 (Yu et al. Reference Yu, Chang, Li, Chen and Shi2009). New breeding pairs were formed continuously, and the number of fledglings increased during the following years (Yu et al. Reference Yu, Li and Huo2015). By June 2015, 26 breeding pairs have produced 88 fledglings over eight breeding seasons (Yu et al. unpubl. data). In addition, three females immigrating into the released area from the wild population in Yangxian County mated with the released birds or their offspring.
For post-release monitoring, all released birds were marked individually by numeric metal rings and coloured alphanumeric plastic bands (provided by the National Bird Banding Centre of China) on their legs to allow resighting after release. Since 2008, all wild-born fledglings were marked at 23–25 days of age when the wings were still inadequate for flying freely but their tarsi were developed enough for ringing. Eleven captive-bred males and five of the strongest nestlings (typically the eldest) of 23–25 days old were fitted each with a radio transmitter weighing 15g, approximately 1% of the body weight (model RI-2D; frequency 216.368-216.691 MHz; battery life ∼18 months; Holohil Systems, Carp, Ontario, Canada).
The annual cycle of the resident ibis can be divided into three stages: breeding period (March to June), wandering period (July to October) and wintering period (November to February) (Shi and Cao Reference Shi and Cao2001). The annual resighting and recovery data was obtained using banding and radio tracking from 2007 to 2014. At 1-week intervals in the breeding period, all breeding pairs in the study area were checked for their unique leg bands by spotting scopes and binoculars. We monitored females throughout the breeding season and measured reproduction in terms of the number of fledglings produced per female per year (Armstrong et al. Reference Armstrong, Davidson, Dimond, Perrott, Castro, Ewen, Griffiths and Taylor2002). All ibises, as well as the radio-marked individuals generally formed wandering flocks in the non-breeding period (autumn and winter) for approximately five months. We observed the different groups at dusk (17h40–18h40) when all members of a wandering flock perched in a night-roosting site and the individuals observed in a fledgling group between 07h00 and 12h00 hours on a rotational basis at 2-week intervals.
Age structure and adult sex ratio of the reintroduced Crested Ibis population
Age structure, defined as the number of individuals in different age groups, is valuable in reflecting the quantity configuration of different age groups, in order to understand the population’s history, and to forecast population dynamics (Sun et al. Reference Sun, Li, Niu and Lou2005). The dynamics of the wild Crested Ibis population was illustrated by an age pyramid (Lu et al. Reference Lu, Zhai, Zhang and Geng1997). In the present study, age pyramids of the wild-born ibises were used to determine the overall age distribution, indicating the fecundity and probability of survival of our focal population.
The proportion of the sexes during the period of production was calculated as the female/male ratio (Komers and Curman Reference Komers and Curman2000) and defined as adult sex ratio (ASR) (Donald Reference Donald2007). We estimated ASR of the population using a method of direct field observation (Donald Reference Donald2007) to roughly estimate the population sex ratio. Although all birds were individually marked, the estimation of ASR was difficult due to the lack of external sexual differences (Baumgardt et al. Reference Baumgardt, Goldberg, Reese, Connelly, Musil, Garton and Waits2013) in the Crested Ibis. Therefore, the sexes of all wild-born individuals were determined only by observation of copulation behaviour when they became sexually mature at least ≥ 2 years old (Yu et al. Reference Yu, Li and Huo2015). The statistical significance of departure from an expected sex ratio of 0.5 was assessed using Pearson’s χ2 goodness-of-fit statistic. Results were considered statistically significant at P = 0.05 and values were reported as mean ± SE.
Estimation of the survival probabilities of the reintroduced population
We used capture-recapture data collected from 2008 to 2015 to estimate the correlative annual survival probabilities of the reintroduced population using Cormack-Jolly-Seber (CJS) model in the program MARK 7.1, which was especially developed for analysing capture-recapture data (Lebreton et al. Reference Lebreton, Burnham, Clobert and Anderson1992). The basic CJS model estimate survival and recapture (resighting) probability for each interval and assumes no differences among animals. The CJS can be modified by dividing animals into groups, introducing age structure, and creating relationships among intervals (Armstrong and Ewen Reference Armstrong and Ewen2002). We conducted survival analyses for released individuals, all individuals combined, and individuals of different age classes of recruitment from 2008 to 2015, respectively. Thus, we prepared three detailed encounter histories for different cohorts at the beginning of the year of release. The encounter history files are matrices of ones and zeros showing which individuals were encountered in each survey. Crested Ibis generally forage and roost in pairs and/or flocks and radio-tagged individuals can be the resighting cue for the others. Hence we assumed that all individuals had the same probabilities of resighting and survival.
We developed a set of candidate models: a model with full-time dependence in both survival and recapture (φ (t) p (t)); a model with constant in both survival and probability of recapture (φ (.) p (.)); and a model with constant survival (φ (.) p (t)) and constant probability of recapture (φ (t) p (.)). We selected the most parsimonious one based on Akaike’s Information Criterion corrected for small sample sizes (AICc). The model with the lowest AICc was considered as the best supported model (Anderson et al. 2000, Burnham and Anderson Reference Burnham and Anderson2002). We considered models with differences of AICc (ΔAICc) values ≤ 2 from the best-fit model equally parsimonious (Burnham and Anderson Reference Burnham and Anderson2002), and in such cases included variables from all models with ΔAICc ≤ 2 in the analysis. We also calculated AICc weights for each candidate model (Buckland et al. Reference Buckland, Burnham and Augustin1997), which represent the weight of evidence in support of each model (Alisauskas and Lindberg Reference Alisauskas and Lindberg2002). The most general model was tested with the bootstrap goodness-of-fit test in MARK. The variance inflation factor (ĉ) was also estimated for detecting multicollinearity (Burnham and Anderson Reference Burnham and Anderson1998).
Results
Reproduction status, age structure and adult sex ratio
Since 2008, a total of 26 pairs were recorded during an eight-year period (two in 2008, five in 2009, seven in 2010, seven in 2011, six in 2011, nine in 2013, ten in 2014 and seven in 2015). By 2015, a total of 88 birds had fledged successfully, and two second generation birds formed a breeding pair and fledged one young bird (the third generation) successfully in the wild. Additionally, annual counts of the individuals indicated that the reintroduced population grew each year over the course of our research period.
By the end of the breeding season in 2015, the minimal estimated population size was 108 individuals (Figure 2), which included 11 yearlings (0–1 year old), 22 juveniles (1–2 years old), 59 adults (≥ 2 years old) and 16 old individuals (> 10 years old). The percentage of yearlings and juveniles, breeding adults and old individuals were 30.6%, 54.6% and 14.8%, respectively (Figure 3a). For wild-born birds, the percentage of mature individuals accounted for 66.8% of our focal population. The large proportion of mature individuals in our focal population indicated a gradually growing population (Figure 3b). The released individuals were mainly composed of adults and juveniles whose mean age at release was 5.2 ± 2.5 years (range 2–10, n = 27) for females and 6.4 ± 2.9 years (range 2–11, n = 29) for males, indicating higher fecundity of the founder population (Figure 3c).
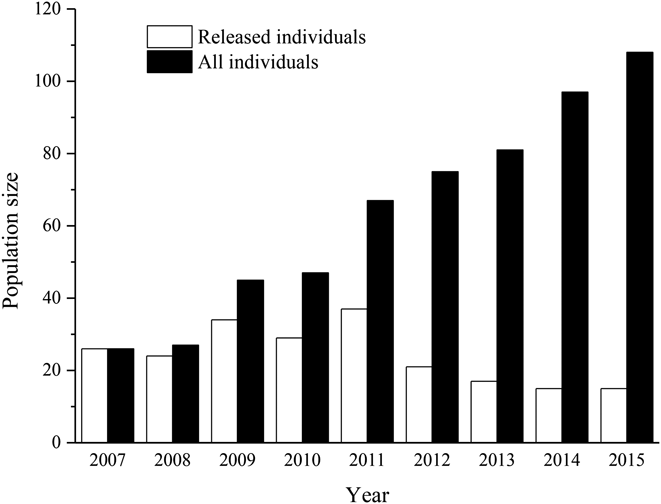
Figure 2. The population size of the reintroduced Crested Ibis between 2007 and 2015.

Figure 3. The age structure of different cohorts for the reintroduced populations of the Crested Ibis. The age of all individuals (a) and wild-born individuals (b) show the values for 2015 and released birds for years at release.
The ASR of the released ibises (29 males and 27 females) was 0.536 in order to reintroduce an initial population with non-skewed ASR. There was a slight bias towards males in the ASR with the increase of wild-born offspring. By 2015, 57 mature individuals including 28 females and 29 males were observed in the reintroduced population (Table 1). The bias of ASRs (0.33–0.51) was not statistically significant, indicating a population with a balanced ASR at the present stage.
Table 1. Adult sex ratio (ASR) estimates from wild-born individuals of the focal Crested Ibis population.

Survival rates
During our study, 56 captive-bred ibises and 88 wild-born fledglings were included in the survival analysis. The model with temporal variation in the survival rate and no temporal variation in the recapture (φ (t) p (.)) was more parsimonious overall given the lowest AICc (Table 2). Estimate of survival rates for the released individuals ranged from 0.472 (95% CI: 0.289–0.663) in 2008 to 0.832 (95% CI: 0.414–0.972) in 2015 (Table 3). The annual survival rates of released individuals increased without further release since 2011, indicating a progress in acclimatisation of the individuals to the release site.
Table 2. Model selection for the survival (φ) and resighting probability (p) of the released and all individuals in the reintroduced Crested Ibis population in Ningshan County (2007-2015), based on a variance inflation factor (ĉ) of 0.96 (P=0.51) and 0.99 (P=0.47), respectively.
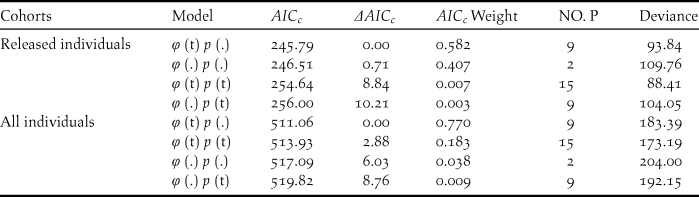
Survival rates (φ) and the probability of recapture (p) were modeled as constant over time (.) or as an annual function of time (t). The number of estimated parameters is given by (NO. P).
Table 3. Annual survival estimates (φ), SE, 95% confidence intervals and relative important values for the best model of the released and all individuals.
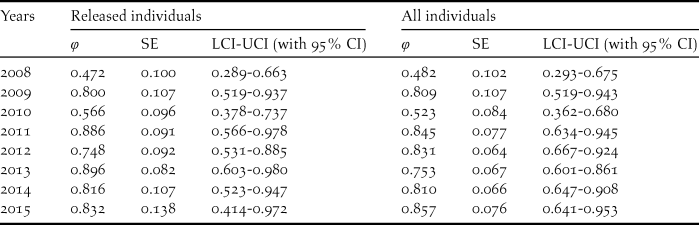
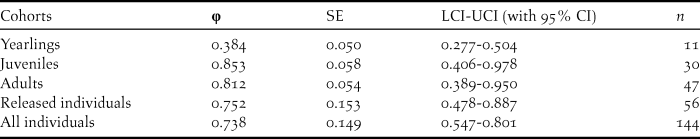
The survival rates for all individuals with the best model φ (t) p (.) averaged 0.738 (95% CI: 0.547–0.801), fluctuating between 0.482 and 0.857 (Tables 3, 5). The model φ (a3- ./ ./ .) p (.) was more parsimonious overall for different age classes (Table 4). As shown in Table 5, the survival rates for adults (0.757; 95% CI: 0.389–0.950) was much higher than that for yearlings (0.384; 95% CI: 0.277–0.504) but slightly lower than that of juveniles (0.830; 95% CI: 0.406–0.978).
Table 4. Model selection for the survival rate (φ) and resighting probability (p) for the different age classes of the reintroduced Crested Ibis in Ningshan County (2008-2015), based on a variance inflation factor (ĉ) of 0.97 (P= 0.47).
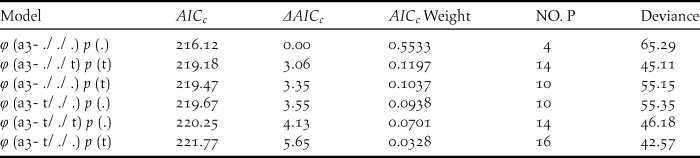
Six relatively good models describing survival rate (φ) and resighting probability (p) were selected. The number of estimated parameters is given by (NO. P).
Table 5. Survival estimates (φ), SE, 95% confidence intervals and relative important values for the best model of the different age classes, and mean survival probabilities for released and all individuals.
Discussion
Since the subsequent release of 56 captive-bred individuals from 2007 to 2011, reproduction (Yu et al. Reference Yu, Li and Huo2015), post-fledging dispersal (Huo et al. Reference Huo, Guo, Li and Yu2014), mortality (Li et al. Reference Li, Huo and Yu2013) and survival rate (present study) of the reintroduced population have been studied over an eight-year period. Observations indicate that the number and the range of the reintroduced population increased gradually without further releases (Wang et al. Reference Wang, Ye, Li, Huo, Li and Yu2016). Although there is no general agreement on what constitutes a successful reintroduction, there are several criteria of success that can be referred to (Sarrazin and Barbault Reference Sarrazin and Barbault1996, Seddon Reference Seddon1999, Robert et al. Reference Robert, Colas, Guigon, Kerbiriou, Mihou, Saint-Jalme and Sarrazin2015). Additionally, the largest proportion of mature individuals and a balanced ASR in our focal population may be a positive indicator of population trends. Based on these criteria, we conclude that the primary goal of establishing a self-sustaining population in one of the species’ former ranges has been achieved. Our reintroduction programme could be a good example for future conservation of other endangered species.
The establishment probability of a released population can be affected by the size and composition of the release group (Armstrong and Seddon Reference Armstrong and Seddon2007). The age of introduced animals appeared to be important for the success of reintroductions owing to the higher fecundity of mature females (Bronson Reference Bronson1989, Komers and Curman Reference Komers and Curman2000). As shown in Figure 3c, the released birds were mainly composed of adults and juveniles. Compared with the wild population (Lu et al. Reference Lu, Zhai, Zhang and Geng1997), wild-born offspring were exclusively young birds including yearlings, juveniles and breeders no more than eight-years old (Figure 3b). These may be contributable to the rapid growth of our focal population (Wang et al. Reference Wang, Ye, Li, Huo, Li and Yu2016).
The population-level offspring sex ratio (OSR) generally does not differ significantly from equality (Yamaguchi et al. Reference Yamaguchi, Kawano, Eguchi and Yahara2004, Eraud et al. Reference Eraud, Lallemand and Lormée2006), but skewed ASRs with a bias towards males are common in wild bird populations, especially in globally threatened species (Donald Reference Donald2007). There is currently no quantitative evidence that a male-biased ASR skew occurs either in the wild (Lu et al. Reference Lu, Zhai, Zhang and Geng1997) or in a released population (present study; Table 1). It is generally believed that a skewed ASR can be explained by higher mortality of females (Githiru et al. Reference Githiru, Lens, Bennun and Perrins2006, Székely et al. Reference Székely, Thomas and Cuthill2006). For the Crested Ibis, the sexual differences in survival rates and offspring sex ratio remain poorly studied. Therefore we cannot explain the current balanced ASRs of the ibis population. There is evidence that a general correlation exists between the ASR and the population trend, or the habitat quality (Zanette Reference Zanette2001, Wilkinson et al. Reference Wilkinson, Langston, Gregory, Gibbons and Marquiss2002, Johnson et al. Reference Johnson, Sherry, Holmes and Marra2006). The ASR might be a useful indicator of a species’ population trajectory or conservation status (Donald Reference Donald2007). In monogamous mating systems (e.g. Crested Ibis), the extinction probability of small populations is likely to be lowest when the sex ratio is balanced (Bessa-Gomes et al. Reference Bessa-Gomes, Legendre and Clobert2004). This might be one of the causes resulting in the rapid growth of wild and reintroduced ibis populations.
Since the release, the reintroduced population has been monitored extensively with particular attention being paid to the survival of wild-born birds over an eight-year period (Yu et al. Reference Yu, Li and Huo2015). With exception of yearlings, the average survival estimates for each cohort such as released individuals, juveniles and adults were relatively high (Table 5). The survival rate of adults (0.812) was slightly higher than that of the wild population (71%; Ding Reference Ding2004). The survival rate of yearlings may be underestimated because some fledglings emigrated outside our study area (Yu et al. Reference Yu, Li and Huo2015).
Most reintroduction projects aimed at saving endangered animals in the 1970s and 1980s failed to establish viable populations, partly due to poor planning and subsequent monitoring (Griffith et al. Reference Griffith, Scott, Carpenter and Reed1989, Wolf et al. Reference Wolf, Griffith, Reed and Temple1996). The success of reintroduction programmes is influenced by the quality of the habitat where the reintroductions took place, the adaptive ability of the birds to new conditions, as well as the number and age structure of the released birds (Griffith et al. Reference Griffith, Scott, Carpenter and Reed1989, Wolf. et al. Reference Wolf, Griffith, Reed and Temple1996, Armstrong et al. Reference Armstrong, Davidson, Dimond, Perrott, Castro, Ewen, Griffiths and Taylor2002, Tweed et al. Reference Tweed, Foster, Woodworth, Monahan, Kellerman and Lieberman2006). Small release groups could fail to establish a self-sustaining population due to random factors (demographic stochasticity), low survival rates at low densities (Allee effects) (Caughley Reference Caughley1994). Our modelling results indicated that the initial population showed lower survival rate (0.472; Table 3) due to poor acclimatisation and high level of post-release dispersal immediately after release. Of 20 mortalities from 56 released individuals, eight (40%) died from food shortage, especially in winter, six (30%) from flight collision, five (25%) from electrocution on power lines, and one (5%) from unknown cause (Li et al. Reference Li, Huo and Yu2013). It is often believed that dispersal and/or mortality can be reduced by holding animals at the release site for a specified period. For example, housing animals in an enclosure could reduce dispersal and mortality for mammals (Tuberville et al. Reference Tuberville, Buhlmann, Bjorkland and Booher2005, Shier and Owings Reference Shier and Owings2006, Hamilton et al. Reference Hamilton, Kelley, William, Kelt and Wittmer2010), yet it was useless for bird species. Therefore, a method was put forward to broadcast animal calls in the vicinity of the release sites (Molles et al. Reference Molles, Calcott, Peters, Delamare, Hudson, Innes, Flux and Waas2008). For the reintroduced Crested Ibis, subsequent releases were conducted in Ningshan County in the southern Qinling Mountains (Yu et al. Reference Yu, Chang, Li, Chen and Shi2009, Reference Yu, Li and Huo2015), Tongchuan City (Wang Reference Wang2016) and Qianhu Wetland Park in the northern Qinling Mountains (Yu et al. unpubl. data). These showed that soft release and food supplementation are useful to hold the individuals around the release site. For example, most of the breeders nested near the release site in Tongchuan (Wang Reference Wang2016) and Qianyang (Yu et al. unpubl. data) ibis populations.
The success of a reintroduction can be affected by several factors including population size, post-release management, habitat conditions and genetic makeup at population level (Armstrong and Seddon Reference Armstrong and Seddon2007). It is generally believed that there is a positive relationship between release group size and establishment success (Griffith et al. Reference Griffith, Scott, Carpenter and Reed1989, Wolf et al. Reference Wolf, Griffith, Reed and Temple1996). A study on even-toed ungulate (Artiodactyla) species showed that the variation in the rate of increase of reintroduced animals was larger when < 20 animals were released (Komers and Curman Reference Komers and Curman2000). Where post-release dispersal and mortality are low, populations can potentially establish successfully from < 10 released individuals (Taylor et al. Reference Taylor, Jamieson and Armstrong2005). However, high post-release dispersal and mortality would create a large disparity between the release group size and the effective initial population size. For example, in spite of a larger release group size (107 captive-bred individuals), the average breeding success of the Sado ibis population in Japan is much lower than that of our focal population (Wang et al. Reference Wang, Ye, Li, Huo, Li and Yu2016). Therefore, we conclude that the effective initial population size may vary with habitat conditions in different release sites.
In addition to factors mentioned above, habitat conditions such as food availability and native predators are crucial to population survival (Huo et al. Reference Huo, Guo, Li and Yu2014). In the wild and our focal populations, Crested Ibises mainly forage in rice fields, shallow rivers, arid croplands and grasslands during different seasons (Shi and Cao Reference Shi and Cao2001, Huo et al. Reference Huo, Guo, Li and Yu2014). However, in the northern Qinling Mountains, the rice fields in the vast areas along the Wei River almost disappeared over the past three decades, so we speculated that the other two reintroduced populations (Tongchuan and Qianhu) will be increasingly difficult to sustain because they would have to forage exclusively in shallow rivers. Another factor that has significantly decreased the survival rate is nest predation, such as by snakes (especially the king rat snake Elaphe carinata) and birds of prey (e.g. the Goshawk Accipiter gentilis). They usually attack eggs and chicks; in contrast, weasels Mustela spp. may also prey on adults as well as on eggs and nestlings (Cao et al. Reference Cao, Lu and Zhai1995, Xi et al. Reference Xi, Lu, Geng, Zhai and Zhang1997, Lu et al. Reference Lu, Zhai, Zhang and Geng1997, Zhang et al. Reference Zhang, Lu, Zhai, Xi and Wang2000, Li et al. Reference Li, Huo and Yu2013). The king rat snake was a significant nest predator that usually preyed on nidicolous nestlings of the Crested Ibis. By 2014, four nestlings from the reintroduced population (Li et al. Reference Li, Huo and Yu2013) and 15 nestlings from the wild population by 2006 (Yu et al. Reference Yu, Liu, Xi and Lu2006) had died from predation by this dangerous reptile. The predation of the Goshawk on chicks resulted in complete failure of two breeding pairs in 2013 (Li et al. Reference Li, Huo and Yu2013).
Although the reintroduction project in Ningshan County, as well as in other places, has great promise with some necessary interventions, threats to the population remain. As a consequence, the following proposals are made. First, more efforts are needed to reduce post-release mortality and dispersal with a focus on pre-release anti-predator training and habitat restoration. Another approach is usually to model the relationship based on the available data on survival, reproduction and dispersal rates for the species (Komers and Curman Reference Komers and Curman2000, Towns and Ferreira Reference Towns and Ferreira2001). Second, in the case of highly endangered flagship species, high intensity post-release intervention (e.g. food supplementation, veterinary care or predator control) is clearly warranted (Seddon Reference Seddon1999). Food supplementation has been proved to be more effective for enhancing reproductive success both in wild and reintroduced populations of the Crested Ibis (Yu el al. Reference Yu, Liu, Xi and Lu2006, Reference Yu, Li and Huo2015). Some approaches to prevent the birds from dying of predation (e.g. the king rat snake) have not been very effective. Therefore, there is an urgent need for a cost-effective management technique that can be applied at an ecosystem level (Yu et al. Reference Yu, Li and Huo2015). Third, although habitat conditions will be the main drivers of population growth, this will also be affected by the genetic diversity of the organism. Great care should be taken in the selection of reintroduction sites, as species with low genetic diversity are thought to be more limited in their ability to tolerate a wide range of environmental factors (Zhang et al. Reference Zhang, Fang and Xi2004). Hence, research is urgently needed, not only to predict how management will affect genetic diversity of reintroduced populations, but also to predict the effects on population growth and persistence. Finally, a long-term and effective international cooperation on ibis conservation among China, Japan, Korea, and Russia should be established.
Acknowledgements
We are very grateful to the reintroduction base of Crested Ibis in Ningshan County for providing logistical help and research aid. We also thank Cao Hai-xin, Guo Jun-feng and Ma Xiao-chun for obtaining the survival data and Liu Chao for drawing the maps. This work was completely supported by the National Nature Science Foundation of China under grant number 31172103 and 31572282.










