INTRODUCTION
Fluoroquinolone (FQ) antibiotics have been widely used as empirical treatment for various infectious diseases including urinary tract infections (UTIs) for several decades [Reference Linder1]. Only a few years after the introduction of FQs, emergence of both chromosomally mediated and plasmid-mediated FQ resistance was reported [Reference Chin and Neu2–Reference Cullmann4]. FQ resistance is not only limited to Gram-negative organisms but has also disseminated to nearly all Gram-positive organisms [Reference Speciale5, Reference Goldstein and Acar6].
A number of studies have investigated risk factors for FQ resistance in UTIs in Gram-negative organisms. However, to our knowledge, there has never been a study focusing on enterococci, despite the fact that these organisms are the second most common cause of healthcare-acquired UTIs [Reference Hidron7, Reference Bouza8].
Although FQs are not the recommended antibiotics for treatment of enterococcal UTIs, their promising efficacy has been noted in a recent study of oral levofloxacin in the treatment of chronic prostatitis, which found a 65% eradication rate at 1-month post-treatment in the enterococcal subgroup [Reference Naber9]. Therefore, FQs may be a good alternative therapy for enterococcal UTIs especially chronic prostatitis. Furthermore, several studies have demonstrated potential benefits of using FQs as an adjunctive antibiotic [Reference Smith10–Reference Whitman12].
This study is, to our knowledge, the first specifically designed to identify risk factors for FQ resistance in healthcare-acquired UTIs caused by enterococci.
METHODS
The study was conducted at two medical centres within the University of Pennsylvania Health system (UPHS): (1) the Hospital of University of Pennsylvania (HUP), a 725-bed academic tertiary and quaternary medical centre and (2) Penn Presbyterian Medical Center (PPMC), a 324-bed urban community hospital centre. Both HUP and PPMC are located in Philadelphia.
Our main study question was, ‘Among all patients with healthcare-acquired UTI caused by enterococci, what are the risk factors for acquiring a FQ-resistant strain?’ To answer this question, we conducted a case-control study comparing patients with healthcare-acquired UTIs caused by FQ-resistant enterococci (cases) with patients with healthcare-acquired UTIs caused by FQ-susceptible enterococci (controls). All cases and controls were identified through records of the Clinical Microbiology Laboratory at HUP, which processes all specimens obtained from HUP and PPMC.
From 1 January 2003 to 31 March 2005, all patients in whom culture results were positive for enterococci and who met the Centers for Disease Control and Prevention (CDC) definition for healthcare-acquired UTI [Reference Garner13] were eligible for this study. Resistance to levofloxacin was considered an indicator of resistance to the FQ antibiotics. An isolate was considered resistant if it demonstrated a minimum inhibitory concentration (MIC) of ⩾8 μg/ml to levofloxacin. Susceptibilities to levofloxacin were determined according to existing criteria established by the Clinical and Laboratory Standards Institute [14]. The susceptibility test was performed by the semi-automated Vitek-2 identification and susceptibility system.
Of all patients with FQ-resistant enterococcal UTI, 139 patients were randomly selected as cases. Controls were defined as patients with FQ-susceptible enterococcal UTI and were frequency-matched to cases by month of isolation. Specifically, in every 1-month period, controls were randomly selected to equal the number of cases. Frequency-matching on the month of isolation was used to sample the controls to diminish the potential for selection bias. Because the percentage of FQ-resistant enterococci was likely to increase with time over the study period, failure to frequency-match on the calendar time of isolation time would probably result in a greater number of controls enrolled in the early study period and a greater number of cases enrolled in the later study period.
Each patient was included as a subject only once. If enterococci were isolated on multiple occasions in the same patient, only the first episode of infection was reviewed. Potential risk factors for FQ resistance were obtained by review of medical records. Data obtained included age, sex, race, hospital service, hospital location, number of hospital days both prior to and following the UTI, comorbid conditions, presence of a urinary catheter and use of in-patient antimicrobial therapy in the preceding 30 days.
The presence of the following comorbid conditions at the time of UTI was documented: diabetes mellitus, hepatic dysfunction (⩾2 of the following: bilirubin concentration 2·5 mg/dl, aspartate aminotransferase or alanine aminotransferase level >twice normal, or documented diagnosis of cirrhosis), cardiovascular diseases (documented diagnosis of severe congestive heart failure and/or inability to carry on any activity without chest pressure or pain), renal insufficiency (a creatinine clearance of <50 ml/min and/or requirement of haemodialysis or peritoneal dialysis), structural kidney diseases (hydronephrosis, chronic urinary retention, kidney or bladder stone, benign prostatic hypertrophy, interstitial cystitis), HIV infection, neutropenia (an absolute neutrophil count <500 cells/mm3), corticosteroid use [receipt of prednisone at a dosage of 20 mg/day (or equivalent) for at least 2 weeks in the preceding 30 days] and use of immunosuppressive agents (in the preceding 30 days).
We categorized antimicrobial use both by the individual agent and by the class [Reference MacAdam15, Reference Gasink16]. The specific antimicrobial agent and the class of antimicrobial agent to which it belongs were also documented as follows: (1) aminoglycosides (amikacin, gentamicin, tobramycin); (2) β-lactamase inhibitors (BLIs) (amoxicillin-clavulanic acid, ampicillin-sulbactam, piperacillin-tazobactam); (3) carbapenems (imipenem, meropenem); (4) first- and second-generation cephalosporins (cefadroxil, cefazolin cefuroxime, cephelexin); (5) third- and fourth-generation extended spectrum cephalosporins (cefepime, ceftazidime, ceftriaxone); (6) fluoroquinolones (ciprofloxacin, levofloxacin, gatifloxacin); (7) monobactam (aztreonam); (8) macrolides (erythromycin, azithromycin, clarithromycin); (9) penicillins (ampicillin, amoxicillin, dicloxacillin, nafcillin, penicillin, piperacillin); and (10) others (clindamycin, doxycycline, metronidazole, nitrofurantoin, linezolid, trimethoprim–sulfamethoxazole, vancomycin).
We also collected data on urinary devices including presence of a urinary catheter (Foley catheter, suprapubic catheter, condom catheter) and presence of invasive urinary devices (nephrostomy tube, ureteral stent).
Statistical analysis
Descriptive statistics were used to characterize cases and controls by all potential risk factors including demographic variables, comorbid conditions, presence of urinary devices and use of medications (particularly antibiotics) within the 30 days prior to the UTI. Categorical variables were expressed as proportions while continuous variables were expressed in the term of mean (±s.d.) or median (range), depending on the sample distribution.
Bivariable analysis was subsequently performed to determine the unadjusted association between FQ-resistant infection and potential risk factors. Categorical variables were compared by using χ2 test or Fisher's exact test. Continuous variables were compared by using Wilcoxon's Rank Sum test.
Finally, we performed multiple logistic regression analysis to estimate the association between FQ-resistant infection and potential risk factors. The multivariable model was built by the stepwise method. Variables were included in a multivariable model if they presented a P value ⩽0·20 in bivariate analysis and then removed from the multivariable model if they did not exhibit an adjusted P value ⩽0·05. In addition, we also included the variable denoting the month of isolation (on which controls were frequency-matched to cases) in the multivariable model. As suggested by Harris and colleagues, time at risk is an important confounding variable for case-control studies focusing on antimicrobial resistance and should be measured and controlled for in analyses [Reference Harris17]. We have included the number of hospital days prior to UTI in the final model as an estimate of time at risk. A two-tailed P value <0·05 was considered significant. All statistical calculations were performed using Stata version 10 (StataCorp, USA).
RESULTS
During the study period, there was a total of 595 episodes of enterococcal UTIs and 281 (55·6%) of these were caused by FQ-resistant strains. Of these, 139 patients with FQ-resistant enterococcal UTI were randomly selected as cases. As noted previously, controls were randomly selected to equal the number (i.e. 139) of the cases by frequency-matching. Only 136/139 (97·8%) cases had complete medical records available for abstraction. Therefore, the number of cases was slightly less than the number of controls (136 cases, 139 controls).
The antibiotic susceptibility profiles of urinary isolates from cases and controls are shown in Table 1. Susceptibility testing against quinupristin/dalfopristin and linezolid were performed only on urinary isolates obtained after 1 July 2004.
Table 1. Antibiotic susceptibility results of in cases and controls
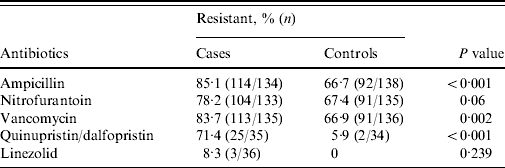
Fisher's exact test was used to compare percent of resistant strains between cases and controls (two-sided test).
Baseline characteristics and comorbid conditions of cases and controls are shown in Table 2. Age and sex were comparable between cases and controls. Moreover, cases were significantly more likely to have had longer hospitalizations prior to UTI. Cases had significantly greater overall antibiotic exposure as well as greater exposure to aminoglycosides, BLIs, carbapenems, cephalosporins, FQs, clindamycin, cotrimoxazole, metronidazole and vancomycin (Table 3).
Table 2. Baseline characteristics and comorbid conditions of cases and controls
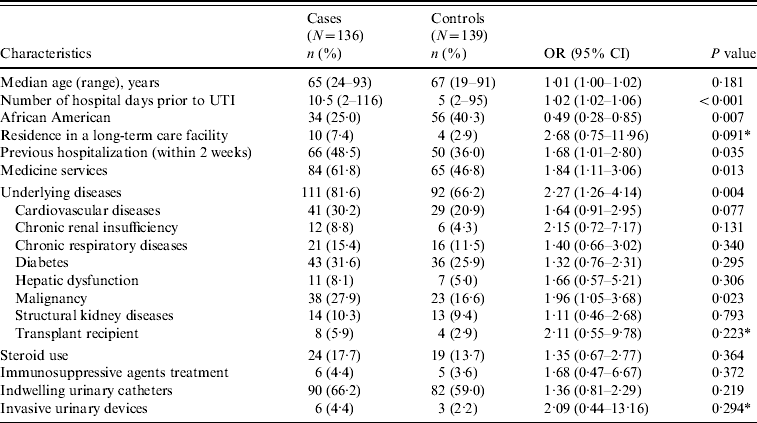
OR, Odds ratio; CI, confidence interval; UTI, urinary tract infection.
* Fisher's exact test was used to compare this variable.
Table 3. Recent antibiotic exposure of cases and controls
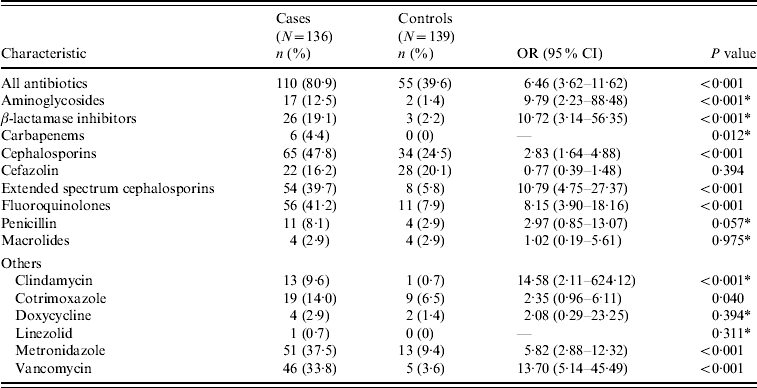
OR, Odds ratio; CI, confidence interval; UTI, urinary tract infection.
* Fisher's exact test was used to compare this variable.
The variables that remained independent risk factors for FQ resistance after multivariable analysis are shown in Table 4. Independent risk factors for FQ resistance included cardiovascular diseases, hospitalization within the past 2 weeks, hospitalization on a medicine service, recent exposure to BLIs, extended spectrum cephalosporins, FQs and clindamycin.
Table 4. Risk factors for fluoroquinolone resistance (multivariable analysis)
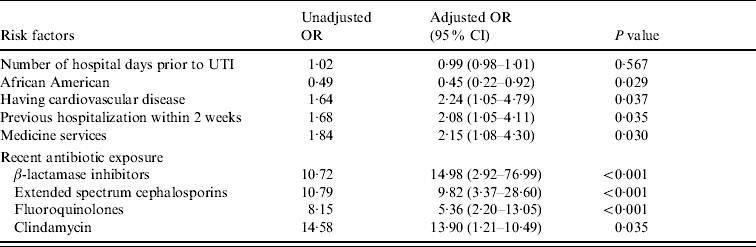
OR, Odds ratio; CI, confidence interval; UTI, urinary tract infection.
The month of isolation variable was included in this model but it was not noted as a risk factor.
DISCUSSION
Recent exposure to various antibiotics and antibiotic classes including BLIs, extended spectrum cephalosporins, FQs and clindamycin were noted as risk factors in our study. Furthermore, we also demonstrated the association between FQ resistance and cardiovascular diseases, hospitalization within the past 2 weeks and hospitalization on a medicine service.
Several studies demonstrated the association between infections caused by FQ-resistant Gram-negative organisms and previous exposure to various categories of antibiotics such as FQs [Reference Lautenbach18–Reference Killgore, March and Guglielmo20], aminoglycosides [Reference Lautenbach18] and metronidazole [Reference Rattanaumpawan21]. In our study, not only recent exposure to FQs but also recent exposure to BLIs, extended spectrum cephalosporins and clindamycin were identified as risk factors for acquiring resistant strains of enterococci. However, our study did not find the association between recent exposure to aminoglycosides or metronidazole and FQ resistance. Therefore, we should not assume that the risk factors for FQ resistance are similar between Gram-positive and Gram-negative organisms.
Our study found previous hospitalization and hospitalization on a medicine service were risk factors for FQ resistance. Both of these variables have previously been established as risk factors for infections caused by resistant pathogens [Reference Chen22, Reference Eagye, Kuti and Nicolau23]. This may be explained in part by higher antibiotic consumption and greater numbers of comorbidities in these populations. Moreover, previously hospitalized patients were more likely to acquire resistant organisms by horizontal transmission.
Having cardiovascular diseases was noted as an independent risk factor. This may be the result of higher antibiotic exposure in some specific subgroups such as patients with structural heart diseases who may require antibiotic prophylaxis for infective endocarditis prior to receiving an invasive intervention [Reference Wilson24].
This study is, to our knowledge, the first specifically designed to identify risk factors for FQ resistance in healthcare-acquired UTIs caused by enterococci. In addition, past studies that focused on Gram-negative uropathogens failed to distinguish between infection and colonization while our study included only patients who met the CDC definition for UTI [Reference Garner13].
Our study has several potential limitations. First, the lack of data on antibiotic exposure prior to hospitalization may result in information bias, although it is unlikely this would result in differential bias. Second, differences in the susceptibility profile across enterococcal species may affect the final results. Unfortunately, we could not adjust for this factor because we did not have data on the Enterococcus species. Third, generalizibility may be an issue. This study was conducted at HUP and PPMC; it may be inapplicable to smaller hospitals or community hospitals.
The last issue is a unique challenge in conducting a case-control study of antimicrobial resistance. Using patients with FQ-susceptible enterococcal UTIs as controls may bias the estimates of relative risk on antibiotic exposure. Treatment with FQs is more likely to eradicate FQ-susceptible enterococci in urine. Therefore, recent FQ exposure would appear to be more prevalent in cases more than controls. However, the optimal control group selection is dependent on the study question [Reference Harris17]. Our main study question was, ‘Among all patients with healthcare-acquired enterococcal UTI, what are the risk factors for acquiring a FQ-resistant strain?’ Based on this main study question, patients with healthcare-acquired FQ-resistant enterococcal UTI were designated as cases while controls were randomly selected from all patients with healthcare-acquired FQ-susceptible enterococcal UTIs.
In summary, recent exposure to BLIs, extended spectrum cephalosporins, FQs and clindamycin, were independent risk factors for FQ resistance in UTIs caused by enterococci. To reduce the emergence of FQ resistance, future strategies should concentrate on optimizing judicious use of these agents.
ACKNOWLEDGEMENTS
This study was primarily supported by National Institutes of Health (NIH) grants K23-DK02897 (E.L.) and K24-AI080942 (E.L.). Financial support also provided by an Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics cooperative agreement (U18-HS10399).
DECLARATION OF INTEREST
Dr Lautenbach has received research support from Merck, Ortho-McNeil, Cubist, and AstraZeneca.






