The public health and nutrition policy in Europe is focused largely on addressing problems of over-consumption. Yet, even in the midst of an abundant dietary supply, important questions remain regarding the prevalence of suboptimal micronutrient intakes across the region. A number of national dietary surveys indicate widespread prevalence of suboptimal intakes for several micronutrients, notably Fe, Ca, Zn, vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B6, vitamin D and folate(Reference Bernier1–Reference van der Wielen, Lowik and van den10). A recent review of published data, performed as part of the EURRECA project (http://www.eurreca.org), also indicated a relatively high prevalence of inadequate micronutrient intakes for adults in Europe, ranging from 11 to 30 % for Cu, folate, Se, I, vitamin B12 and vitamin C(Reference Roman-Viñas, Barba and Ngo11). However, efforts to make meaningful comparisons of micronutrient adequacy across European countries have been hampered not only by major differences in methods used in data collection, but also by disparities in data analysis. These include diverse population age ranges and different yardsticks of adequate intake such as the proportion of people with intakes below the recommended nutrient intake, estimated average requirement (EAR) or dietary reference value(Reference Doets, de Wit and Dhonukshe-Rutten12). Therefore, further analysis, repartition and harmonisation of the data are necessary to enable a more valid comparison of intakes across Europe. The main objective of the present study was to provide a clearer and more accurate map of the extent of inadequate micronutrient intakes in eight selected European countries. To our knowledge, this is the first analysis of the adequacy of micronutrient intakes in multiple countries using national survey data, with reanalysis of raw data to obtain uniformity in age groups and using a single set of recommendations and a uniform method to determine adequacy. As such, it provides an overview of the European situation and an invaluable resource for assessing the status of European populations in terms of micronutrient intake.
Materials and methods
Dietary survey experts of several European countries were invited to participate, including those who contributed to a previous publication(Reference Flynn, Hirvonen and Mensink13). The prerequisites for participating countries were as follows: (1) recent nationally representative food consumption data obtained from individuals (i.e. not household budget data) and (2) the capability and resources to reanalyse the data to obtain the required estimates. A total of eight countries (Belgium, Denmark, France, Germany, The Netherlands, Poland, Spain and the UK) met these criteria. For Serbia, dietary intake data were derived from a 7 d household food consumption survey and therefore not included in the main part of the present study. However, as they may be of relevance in providing the perspective of a region with different dietary patterns, the Serbian data are presented in Supplementary Tables B (available online)(Reference Gurinovic, Kadvan, Vukotic and Nedeljkovic14).
The national surveys were conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the following committees. The Belgium survey was approved by the Ethical Committee of the Ghent University Hospital, the Danish survey was approved by the Danish Data Protection Agency, the French INCA2 survey was approved by the French data protection authority and the French national council for statistical information, the German surveys were part of national health surveys and were approved by the Federal Office for the Protection of Data and for children and adolescents additionally by the Charité Universitätsmedizin Berlin ethics committee, the Spanish surveys were approved by the National School of Health, the Spanish Ministry of Health and by the Catalan Department of Health and the UK surveys were approved by the Oxfordshire A Research Ethics Committee. The Dutch surveys were conducted in existing consumer panels, where no separate approval and informed consent were necessary. Similarly, the Polish study was conducted as part of a household budget survey and was approved by the Ministry of Health and Social Welfare. In Belgium, Germany, Spain and the UK written informed consent was obtained from all participants aged 18 years and above and from parents of younger participants (and also from participants aged 14–17 years in Germany and 16–17 years in the UK). In France, verbal informed consent was obtained from all participants and parents of participants up to 18 years of age, this was witnessed and recorded. Written consent was considered unnecessary because there was no invasive measurement.
A number of different design and dietary assessment methods were used in the surveys, including single or repeated 24 h recalls; 2, 3, 4 or 7 d prospective food records; estimated or weighed amounts consumed; and modified dietary histories with a reference period of 4 weeks (Table 1). In Germany, The Netherlands and Spain, different methods were used for children and adults. Except for the Dutch young adult survey, the Belgian dietary survey in children and the Polish survey, the surveys covered all seasons of the year. The Danish survey was based on a total random sample. In all other countries, the samples were drawn using different ways of clustering (regions, communities, schools or households) and stratification (e.g. region, community size, sex, age, social class). This was done for practical, logistical and statistical efficiency reasons depending on organisational or geographical structures in the countries.
Table 1 Dietary survey methods used for the present study

YUSAD, Yugoslav Study of precursors of Atherosclerosis in School Children in Serbia.
Data analysis
The nutrients were selected based on availability of reference values, well-founded food composition data, known or suspected problems of nutrition adequacy status in the particular countries and related to this, supplementation and fortification practices. Based on these criteria, intake information was collected for Ca, Cu, I, Fe, Mg, K, Se, Zn and the vitamins A, B1, B2, B6, B12, C, D, E and folate. In order to evaluate the proportions of low intakes, the percentages of persons whose micronutrient intakes were below the lower reference nutrient intake (LRNI) and below the EAR were estimated. To evaluate whether intakes are adequate on a population level, it is generally considered appropriate to use the EAR, as has been communicated previously(Reference Beaton, Shils, Olson and Shike15, Reference Carriquiry16). The EAR is defined as the intake adequate for 50 % of the population. The LRNI is an intake value below which it is unlikely that normal health can be maintained over longer periods, and it was used to evaluate the proportion with very low intakes in each country. In order to enable comparisons between different countries in a comparable way, a standard set of reference values for all countries was used. The reference values for the specific requirements were obtained primarily from the dietary reference values for Food Energy and Nutrients for the UK 1991(17). Although these values were set in 1991, there is little evidence to suggest that they have changed from that time to the present. Where reference values were not available from the UK tables, they were drawn from the Nordic Nutrient Recommendations 2004(Reference Alexander, Andersen and Aro18). Where neither source could provide a reference, the EAR was calculated as 75 % of the reference nutrient intake (recommended nutrient intake from the UK or recommended daily intakes (RI) from the Nordic reference intake) and subsequently the LRNI as 75 % of this calculated EAR (both indicated in Table 2). Individual daily micronutrient intakes were estimated from nationally representative food consumption surveys in combination with the specific national food composition databases for the participating countries. Information on dietary supplement use was included for most countries (except Belgium, Spain and the Dutch 1997–1998 survey). For most surveys, information on supplement intake was collected within the dietary assessment method and the reference period was the same as for the dietary assessment. Exceptions were Denmark (separate questions referring to usual intake), German adults and France (both referring to 12 months). There were also some differences in the level of detail on the consumption of dietary supplements. Almost all countries collected information on brand names and doses of consumed supplements, with the exception of Denmark and Poland, which asked for type and doses of supplements. National or study-specific supplement composition databases were used to calculate vitamin and mineral intake from dietary supplements as amounts per d. Composition information in these databases was obtained from the packaging, websites or manufacturers. Denmark used generic supplement information, where nutrient content was calculated from household purchase data. An adjustment for within-person variation in supplement intake was performed for the Netherlands with a first add then shrink approach, where intake from supplements is added to intake from foods and then corrected for within-person variation with the method described later. In France, a first shrink than add approach was used, where usual nutrient intake from foods was estimated per individual with the method described later and then the individual mean supplement intake of the past 12 months (considered as a long-term intake) was added to obtain total usual nutrient intake. For the other countries, nutrient intakes were calculated straightforward with and without supplements included.
Table 2 Cut-off reference values for recommended nutrient intake (RNI), estimated average requirement (EAR) and lower reference nutrient intake (LRNI) for the evaluated vitamins and minerals according to age and sex

RE, retinol equivalent; TE, tocopherol equivalent.
* Calculated as 75 % of calculated EAR.
† Calculated as 75 % RNI.
‡ Nordic nutrient recommendations.
The arithmetic mean and 5th percentile of the intake of distribution, as well as the percentages of the population with intake below the EAR and the LRNI were calculated for defined age and sex groups. Except for Belgium, Denmark and Poland, the samples were analysed using a calculated weighting factor to correct for differences between the structure of the survey samples and the actual population (e.g. age and community size) in order to improve representativeness. Given that estimates of low intakes should preferably be based on habitual intake, intake distributions from dietary assessments with short reference periods (Belgium, France, The Netherlands and Spain) were statistically corrected for within-person (intra-individual) variation using SPADE (Statistical Program to Assess Dietary Exposure)(Reference Souverein, Dekkers and Geelen19) for The Netherlands, the multiple source method(Reference Harttig, Haubrock and Knüppel20) for France and Belgium and the Liu method(Reference Liu, Stamler and Dyer21) for Spain. Application of these methods needs transformation to a normal distribution, which is especially difficult for supplemental intake. In addition, differences in within-person variation between intake from food and intake from supplements are not taken into account. Together, this may lead sometimes to somewhat higher estimates of the percentage below the LRNI and EAR for diet including supplements than without. New methods that account for this could not yet be applied. Surveys with multiple days of assessment estimated the usual intake directly, by taking the mean intake over the observed days per individual (see Table 1).
Dietary assessment is generally affected by under-reporting, which may have a particular impact on the estimates at low percentiles of the intake distribution. Therefore, low energy reporters were identified by calculating the ratio of total energy intake and an estimate of BMR. BMR were estimated using the equations proposed by Schofield(Reference Schofield22). Persons with a ratio value below 1·0 were considered to be low energy reporters in the following referred to as under-reporters. The percentages of under-reporters are presented for each subgroup, for all countries except Poland and the UK. Analyses were additionally performed while excluding under-reporters for Belgium, Denmark, France, Germany and Spain to evaluate the impact on the results (data not presented).
Intakes were calculated for the base diet, namely from food, including the contributions of mandatory fortified foods, and also the base diet plus dietary supplements. Mandatory fortification was included in the definition of base diet because in most countries this fortification is either required by statute or officially encouraged in order to maintain availability of the specific nutrients. The contribution from voluntary fortified foods was explicitly assessed for children and adolescents in Germany, young adults and young children in The Netherlands or for particular foods for which fortification information was available in the food composition databases in Denmark, Spain and the UK. Base diet values include any contributions from voluntary fortification in countries where this is normal.
Presentation of results
Intakes of the selected micronutrients in each country are provided in Supplementary Tables A (available online). Vitamin A intakes were determined by combining values for retinol and β-carotene intakes expressed as retinol equivalents (RE). A conversion factor of 6 μg of β-carotene equals 1 μg of retinol. Countries varied in the manner of calculation of folate from synthetic sources in supplements or as fortificants added to food products. The UK made no adjustment for source of folate, whereas in Denmark, France and Germany synthetic folic acid added or in supplements was multiplied by 1·7 to give folate equivalents. In The Netherlands, folate added to food was multiplied by 1·7 and in supplements by 2 because these were assumed to be taken separately from meals. Poland and Spain did not include synthetic sources of folate and Belgium did not provide folate intake values.
The age ranges used are based on those used by the Scientific Committee on Food recommendations on nutrient intakes(23). If the defined age ranges were not fully covered in a particular survey, intakes were estimated for the available age range. This is indicated in the relevant results tables. Intakes are given separately for children aged 1–3 years (exception The Netherlands 2–3 years), boys and girls aged 4–10 years (exceptions Germany 6–10 years, The Netherlands separately for 4–6 and 7–10 years), boys and girls aged 11–17 years (exception Germany 12–17 years), men and women aged 18–60 years (exception The Netherlands 19–30 and 31–60 years) and above 60 years. Additionally, women aged 14–50 years (exception Germany 17–50 years) were listed separately, as there are specific concerns for women of child-bearing age, e.g. for Fe status. Only Belgium, Poland, The Netherlands and the UK reported micronutrient intakes for children aged 1–3 years. The impact of excluding under-reporters was evaluated as proportional differences in means and 5th percentiles and as absolute differences in percentages of intakes below the EAR and LRNI (not presented in tables).
Results
Under-reporting
The estimated percentage of possible under-reporters was 0·6 % in Belgium and 1·7 % in The Netherlands for children aged 1–3 years. For those aged 4–10 years, it ranged from 0·5 % of the Danish boys to about 5 % of the German girls and for the 11- to 17-year-olds, it ranged from 0·6 % of the Dutch boys to 34 % of the Danish boys. For women aged 14–50 years, it ranged from 1·1 % in The Netherlands to 14 % in Germany. For those aged 18–60 years, it ranged from about zero in the Dutch men to 26 % of the French women. For those aged over 60 years, it ranged from 0·4 % of the Dutch women to 28 % of the Spanish women.
Calcium
Calcium intake – children
For children aged 1–3 years, mean Ca intakes ranged from 595 mg/d in Poland to 857 mg/d in Belgium. Few children had intakes below the LRNI, except for Poland, at 8 %. For those aged 4–10 years, intakes ranged from 563 mg/d in the Polish girls to 1106 mg/d in the Danish boys. In this age group, few were identified as having intakes lower than the LRNI and the EAR (Table A1, available online), except in Poland, where 20 % of the girls and 15 % of the boys had intakes below the LRNI and 32 % of the girls and 28 % of the boys had intakes below the EAR. For adolescents aged 11–17 years, intakes ranged from 651 mg/d in the Polish girls to 1487 mg/d in the German boys. In several countries, the percentage with intakes below the EAR was substantial (5–57 %), and in France, Poland and the UK, the proportion of intakes below the LRNI was higher than 5 % among the boys and higher than 10 % among the girls. There was little additional intake from dietary supplements.
Calcium intake – adults
For adults aged 18–60 years, mean intakes ranged from 512 mg/d for the Polish women to 1329 mg/d for the German men (Table A1, available online). The percentage with intakes below the LRNI was low in most countries, except in the UK (7 % of women) and France (5 % of women), and was highest in Poland (45 % of women, 29 % of men). For adults aged over 60 years, intakes ranged from 529 mg/d in the Polish women to 1031 mg/d in the Dutch men and the prevalence of intakes below the LRNI was less than 5 %, except in Poland and Denmark. In Poland, the percentage with intakes below the EAR was exceptionally high (55 % of women, 48 % of men). The additional intake from the dietary supplements was generally not substantial, except in Denmark (about 5–12 %).
Calcium intake – under-reporting
Excluding under-reporters from the calculations increased the estimated mean slightly, but increased the 5th percentile substantially in the age groups above 11 years. For those aged 11–17 years and above 60 years, excluding under-reporters reduced the proportion of intakes below the LRNI and EAR substantially, especially in Denmark and France.
Copper
Copper intake – children
In children aged 1–3 years, mean Cu intake ranged from 0·6 mg/d in the UK to 0·8 mg/d in Belgium (Table A2, available online). The prevalence of intakes below the LRNI and EAR was low, less than 5 % for the LRNI and less than 9 % for the EAR. Mean Cu intake in those aged 4–10 years ranged from 0·7 mg/d for girls aged 4–6 years in The Netherlands to 1·6 mg/d for boys in Germany. Prevalence of intakes below the LRNI and below the EAR was low (the highest was 9 % below the EAR among girls in Poland and the UK). Among adolescents aged 11–17 years, the intakes were in the range 1–3 mg/d, with lower values for girls than for boys. The lowest intake was seen for girls in the UK and the highest for German boys. Prevalence of intakes below the LRNI was less than 1 % for all countries. The proportion of intakes below the EAR was low in most countries, the highest being for girls from the UK (24 %). Inclusion of supplements in the analysis did not change the mean intakes.
Copper intake – adults
Mean Cu intakes in adults ranged between 1 and 2 mg/d in most countries (Table A2, available online). The lowest intake (1 mg/d) was observed in the UK women aged 14–50 years and in the Dutch women aged above 60 years, whereas the highest levels were seen among males in Germany (almost 3 mg/d). Intakes below the LRNI were less than 1 % in most countries, with the UK and Poland somewhat higher at 1–2 %. The proportion with intakes below the EAR ranged from 0 % in Germany to 19 % among the Polish women aged above 60 years. Where information on supplementation was included in the assessment, it did not contribute substantially to the mean and the 5th percentile intake, and had almost no impact on the proportions below the EAR and LRNI.
Copper – under-reporting
Excluding under-reporters increased the mean intake in France up to 9 % but only slightly in other countries, whereas the 5th percentiles increased by about 5–10 % in Germany and up to 30 % in France, but it made little difference to the proportions below the EAR and LRNI.
Iodine
Iodine intake – children
For children aged 1–3 years, mean iodine intake was 71 μg/d in Poland and 146 μg/d in the UK (Table A3, available online). The proportion with intakes below the LRNI was 10 % in Poland and 1 % in the UK. The proportion with intakes below the EAR was 33 % in Poland and 6 % in the UK. For girls aged 4–10 years, mean intakes were the lowest in Germany (78 μg/d) and the highest in Denmark (163 μg/d). The proportion with intakes below the LRNI was about 20 % in Poland and Germany, and much less in Denmark (0 %), France (1·9 %) and the UK (2·9 %). The percentage of intakes below the EAR was highest in Germany (61 %) and the lowest in Denmark (0 %).
The mean intake for boys aged 4–10 years was the lowest in Germany (84 μg/d) and the highest in Denmark (186 μg/d). The proportion with intakes below the LRNI was about 14 % in Poland and Germany. The lowest proportion of intakes below the EAR was seen in Denmark (0 %) and the highest in Germany (55 %).
For girls aged 11–17 years, mean intakes were close to 100 μg/d in France, Germany, Poland and the UK, with the highest intakes in Denmark (170 μg/d). Intakes below the LRNI were observed from 0·3 % in Denmark to 21 % in Poland. More than 50 % of girls in Germany (59 %), France (58 %) and the UK (50 %) had intakes below the EAR.
For boys aged 11–17 years, the lowest mean intake was seen in France (115 μg/d) and the highest in Denmark (211 μg/d). The proportion with intakes below the LRNI ranged between 0·4 % in Denmark and 13 % in Poland. Proportions of intakes below the EAR ranged from 1 % in Denmark to 40 % in Germany. The additional intake from dietary supplements was low for all countries except in Denmark, where it resulted in an increase of about 20 % in the mean intake for those aged 4–17 years, of both sexes.
Iodine intake – adults
For women aged 18–60 years, the lowest mean intake was observed in Germany (105 μg/d) and the highest in Denmark (173 μg/d) (Table A3, available online). The percentage of intake below the LRNI was highest in Poland (18 %) and lowest in Denmark (1 %). The proportion of intake below the EAR ranged between 7 % in Denmark and 52 % in Germany.
For men aged 18–60 years, the lowest mean intake was observed in Germany (121 μg/d) and the highest in Denmark (210 μg/d). The percentage of intakes below the LRNI was highest in France (7·1 %) and lowest in Denmark ( < 1 %). The highest proportion of intake below the EAR was seen in Germany (33 %) and the lowest in Denmark (3·5 %).
For women aged over 60 years, mean intake was lowest in Germany (98 μg/d) and highest in Denmark (164 μg/d). The highest proportion of intake below the LRNI was in Poland (17 %). The proportions of intakes below the EAR were highest in Germany (61 %) and lowest in Denmark (13 %). For men aged over 60 years, the highest proportion of intakes below the LRNI was seen in Germany (10 %) and the lowest in the UK (0 %). Similarly, the proportion of intakes below the EAR was highest in Germany (47 %) and the lowest in the UK (3·6 %). Where data on supplements were included, there was no substantial change in mean intake and in the proportions of intakes below the LRNI and EAR, except in Denmark.
Iodine intake – under-reporting
Excluding the under-reporters increased the mean intake for French adults by 8 %, but only slightly in other countries. After excluding under-reporters, the 5th percentiles increased by up to 25 % in Germany and Denmark and up to 34 % in France. In France, the proportions below the EAR were about 4–12 % and the proportions below the LRNI were about 2–8 % lower, but they did not change much in Germany and Denmark.
Iron
Iron intake – children
For children aged 1–3 years, mean intakes from the base diet varied from 5·2 mg/d for Poland to 7·1 mg/d for Belgium (Table A4, available online). The proportion below the LRNI ranged between 3·1 % (Belgium) and 27 % (Poland). Proportions below the EAR were considerable, ranging from 23 % (Belgium) to 55 % (Poland).
For children aged 4–10 years, mean intakes ranged from 6·7 mg/d for the Dutch girls (aged 4–6 years) to 12·5 mg/d for the Spanish boys. In most countries, very few children ( < 1·5 %) had intakes below the LRNI. The highest proportions of intakes below the LRNI and EAR were seen in the Polish girls, with 5·6 and 28 %, respectively. Among 11- to 17-year-olds, mean intakes ranged from 7·7 mg/d for the Danish girls to 18·6 mg/d for the German boys. These were below the LRNI for girls from Denmark. The percentages of intakes below the LRNI and EAR were high for girls, the highest being in Denmark, with 55 % below the LRNI and 94 % below the EAR. The contribution of supplements to the mean intake was only substantial in Poland and high in Denmark.
Iron intake – adults
For those aged 18–60 years, mean intakes ranged from 8·9 mg/d for the Danish women to 17 mg/d for the German and the Polish men (Table A4, available online). The percentages of intakes below the LRNI and EAR were low for men (less than 2·2 and 11 %, respectively, in all countries) and very high for women (more than 25 % for intakes lower than the LRNI in four countries and more than 50 % for intakes lower than EAR in six countries), especially for women aged 14–50 years. For those aged over 60 years, mean intakes ranged from 8·5 mg/d for the Danish women to 15 mg/d for the German men. The percentages of intakes below the LRNI and EAR were low in most countries, except in Denmark and Poland, where about 10 % of men and 20 % of women had intakes below the EAR.
Supplements substantially increased mean Fe intake in Denmark for all age groups and for those aged over 60 years in Poland, with a corresponding decrease in the proportion of people with low intakes. In the other countries, this contribution was considerably smaller.
Iron intake – under-reporting
Excluding under-reporters increased mean intakes by up to 8 % in some age groups. After excluding under-reporters, the 5th percentiles increased up to about 27 %, with the highest increases in France. The proportions below the EAR and below the LRNI reduced by about 5–10 % in several age groups.
Magnesium
Magnesium intake – children
For children aged 1–3 years, the lowest mean intake was observed in the UK (154 mg/d) and the highest in Belgium (191 mg/d) (Table A5, available online). About 1 % or less had intakes below the LRNI and 2·5 % or less below the EAR.
For children aged 4–10 years, mean intakes ranged from 185 mg/d for girls from the UK to 290 for German boys. The percentages of intakes below the LRNI were low; the highest were 3 % for girls and 4 % for boys from Poland. In most countries, less than 5 % had intakes below the EAR, except in the UK and Poland, where it ranged from 5 % (UK boys) to 11 % (Polish girls).
Among those aged 11–17 years, mean intakes ranged from 190 mg/d for girls from the UK to 531 mg/d for the German boys. Intakes below the LRNI exceeded 5 % in most countries and ranged from 0·4 % (German and Spanish boys) to 47 % (UK girls). Percentages of intakes below the EAR were high in most countries and ranged from 2 % (German boys) to 86 % (UK girls). The additional intake from dietary supplements was generally not substantial in this age group, and the greatest differences in means and percentages of low intakes were observed among those aged 11–17 years in Denmark.
Magnesium intake – adults
For women aged 14–50 years as well as 18–60 years, the lowest mean intakes were found in the UK (209 mg/d, 14–50 years) and the highest in Germany (423 mg/d, 14–50 years) (Table A5, available online). The women aged 14–50 years in the UK had the highest proportion of intakes below the LRNI (26 %) and below the EAR (59 %). Among those aged 18–60 years, the proportion of intakes below the EAR was about 25–27 % for the Polish and the French women and 40 % for the UK women. In the other countries, these proportions were much lower.
For men aged 18–60 years, mean intakes ranged from 294 mg/d in the UK to 522 mg/d in Germany. The highest percentage of intake below the LRNI was seen in the UK (15 %). The highest proportions of intakes below the EAR were seen in France (32 %) and in the UK (36 %).
Mean intakes of those aged over 60 years ranged from 227 mg/d for women in the UK to 421 mg/d for German men. The percentage of intakes below the LRNI was highest in Poland for women (12 %) and in the UK for men (19 %). The highest proportions of intakes below the EAR were seen in the UK (36 % in women, 44 % in men). Intake of supplements increased the mean intake substantially in most countries, but did not alter the proportion of intakes below the LRNI and EAR meaningfully.
Magnesium – under-reporting
Excluding under-reporters increased the mean intake substantially, only in France, by 5–8 % (but not among those aged 4–10 years). The 5th percentiles of intakes increased by about 6–10 % in Germany and Spain and by 6–28 % in Denmark and France. The only country with a substantial effect on the proportions of intakes below the EAR and the LRNI was France (5–16 %) and additionally for those aged 11–17 years and men aged over 60 years it was Denmark (6–20 %).
Potassium
Potassium intake – children
For children aged 1–3 years, mean intake from the base diet ranged from 1807 mg/d (UK) to 2322 mg/d (Belgium) (Table A6, available online). The proportions with intakes below the LRNI and the EAR were small (about 1 % in Poland and the UK).
For those aged 4–10 years, mean intake from the base diet ranged from 2077 mg/d (Dutch girls aged 4–6 years) to 3004 mg/d (Dutch boys aged 7–10 years). In all countries, less than 1 % had intakes below the LRNI. The proportion with intakes below the EAR ranged from 0 to 11 %, the highest proportion in girls being in Germany (11 %) and for boys in Poland (8 %). Mean intake among those aged 11–17 years ranged from 2148 mg/d (UK girls) to 3899 mg/d (German boys). The proportions with intakes below the LRNI exceeded 5 % in most countries and ranged from 0 to 30 %, with the highest proportions in the UK for both girls (30 %) and boys (15 %). The proportions with intakes below the EAR were generally high for girls (24–75 %) and somewhat lower for boys (10–50 %). The additional intake from dietary supplements was generally very low.
Potassium intake – adults
For adults aged 18–60 years, mean intake ranged from 2485 mg/d (UK women) to 4447 mg/d (Polish men) (Table A6, available online). Prevalence of intakes below the LRNI exceeded 5 % in most countries and ranged from 0 to 31 %, with highest proportions for Poland, France and the UK. The proportions with intakes below the EAR were higher for women (11–63 %) than for men (3–37 %), and were highest in Spain, France, the UK and Poland and lowest in The Netherlands. Intakes including dietary supplements were not appreciably different than from diet alone.
Potassium – under-reporting
Excluding under-reporters increased the mean intake substantially in France by 5–8 % (but not among those aged 4–10 years). The 5th percentile of intakes increased by 5–15 % in Germany and by 10–28 % in France. The proportion of people with intakes below the EAR reduced by about 5–10 % in Germany and France and the proportion with intakes below the LRNI reduced substantially only in France (up to 13 %) when under-reporters were excluded.
Selenium
Selenium intake – children
For children aged 1–3 years, mean intakes ranged from 16 μg/d in Belgium to 25 μg/d in the UK (Table A7, available online). The proportion with intakes below the LRNI was 6 % in Belgium and 1 % in the UK; intakes below the EAR were 3 % in the UK and 25 % in Belgium. For children aged 4–10 years, the highest mean intakes were observed for boys in France (38 μg/d) and the lowest for girls in Belgium (16 μg/d). Correspondingly, the highest percentages with intake below the LRNI and EAR were observed for Belgium (19 and 50 %, respectively, for girls). In the other countries, intakes below the LRNI were generally low ( < 1·5 %). The lowest percentages of intakes below the EAR for this age group were for France and the UK at less than 2 %.
For those aged 11–17 years, mean intakes ranged from 29 μg/d for the Danish girls to 46 μg/d for the French boys. The overall percentage with intake below the LRNI was high for girls, ranging from 32 to 60 %, the highest being in Denmark. For boys, this proportion was generally lower, ranging between 18 and 27 %. The percentage with intake below the EAR for girls ranged from 54 % in France to 80 % in Denmark. For boys, this ranged from 20 % in the UK to 57 % in Denmark. Only in Denmark were intakes, including supplements, considerably higher than from the base, diet alone.
Selenium intake – adults
Mean intakes ranged from 33 μg/d for women in Denmark to 54 μg/d in men from France, The Netherlands and the UK (Table A7, available online). Among women, the proportion of intakes below the LRNI ranged from 36 % in France to 76 % in Denmark, and among men, from 8 % for the Netherlands (19–30 years) to 47 % in Denmark. In all countries, the proportion of intakes below the EAR was high, for women between 55 % in France and 87 % in Denmark and for men between 59 % in France and 86 % in Denmark.
For adults aged over 60 years, mean intakes were close to or below the EAR. In Denmark, mean intakes were particularly low, and lower than the LRNI. However, the mean intake including supplements was above the EAR. The percentages of intake below the LRNI ranged from 31 % in France to 76 % in Denmark among women and from 14 % in France to 54 % in Denmark among men. Among women, the proportions of intake below the EAR ranged from 50 % in France to 85 % in Denmark; among men, they were even higher, from 59 % in France to 90 % in Denmark. In Denmark, the mean intakes increased considerably when supplements were included, in all age groups.
Selenium – under-reporting
Excluding under-reporters increased the mean intakes by 6–7 % in most groups. The 5th percentile intakes increased by about 6–20 % in groups of children aged above 11 years, with the highest difference observed in Danish men aged over 60 years (28 %). The percentages below the LRNI and the EAR were reduced substantially (6–10 % in most groups).
Zinc
Zinc intake – children
For children aged 1–3 years, mean intakes ranged from 5·2 mg/d for The Netherlands and the UK to 7·9 mg/d for Belgium (Table A8, available online). The percentages of intakes below the LRNI and EAR were low for most countries; only in Poland was the proportion with intakes below the LRNI greater than 10 %.
For girls aged 4–10 years, mean intakes ranged from 5·4 mg/d for The Netherlands (4–6 years) to 8·4 mg/d for Denmark. For boys aged 4–10 years, mean intake ranged from 5·9 mg/d for The Netherlands (4–6 years) to 9·6 mg/d for Denmark. The percentages of intakes below the LRNI were low ( < 10 %), while the percentages below the EAR were higher for some countries (between 1·5 % for boys in Denmark and 39 % for girls aged 4–6 years in The Netherlands).
For girls aged 11–17 years, mean intakes ranged from 6·6 mg/d for the UK to 11·2 mg/d for Germany. For boys aged 11–17 years, mean intake ranged from 8·4 mg/d for the UK to 14·7 mg/d for Germany. The percentages of intakes below the LRNI ranged from 1 to 19 % and the percentages below the EAR between 3 and 48 %, with the highest proportions for the UK boys and girls. Except for Denmark, the contribution of dietary supplements was low.
Zinc intake – adults
For women aged 18–60 years, mean intakes ranged from 7·6 mg/d for the UK to 11·2 mg/d for Germany (Table A8, available online). For men aged 18–60 years, mean intake ranged from 9·5 mg/d for Spain to 15·1 mg/d for Germany. Consequently, the percentages of intakes below the LRNI and EAR were rather low (below 10 and 21 %, respectively). For women aged over 60 years, mean intakes ranged from 6·9 mg/d for Spain to 10·0 mg/d for Germany. For men aged over 60 years, mean intakes ranged from 7·8 mg/d for Spain to 12·4 mg/d for Germany. The percentages of intakes below the LRNI were low for most countries ( < 10 %). The proportions below the EAR were higher, up to 39 %, for Spanish men aged over 60 years. There was a substantial contribution from supplementation in Denmark.
Zinc – under-reporting
Excluding under-reporters increased the mean intakes by 5–7 % in some groups. The 5th percentile intakes increased by about 8–25 % in groups aged above 11 years. The percentages below the LRNI and the EAR did not change substantially, except for girls aged 11–17 years in Denmark and France (where the percentage below the EAR was about halved).
Vitamin A
Vitamin A intake – children
For children aged 1–3 years, Belgium, The Netherlands and the UK had similar mean values of about 545–600 μg RE/d, whereas the values for Poland were almost twice as high (Table A9, available online). The proportion of intakes below the LRNI ranged from 2 % for The Netherlands to 11 % for Belgium, and the proportion of intake below the EAR ranged from 12 % for The Netherlands to 22 % for the UK.
For girls aged 4–10 years, mean intakes ranged from about 450 μg RE/d in Spain to over 900 μg RE/d in Denmark and Poland. The proportion of intakes below the LRNI ranged from less than 1 % for Spain, Denmark and France to 11 % for Belgium. The proportions of intakes below the LRNI were little affected by addition of intake of supplements. The proportion of intakes below the EAR ranged from 1 % for France to 30 % for Belgium.
For boys aged 4–10 years, mean intakes showed a similar variation by country as for girls, ranging from just over 500 μg RE/d for Spain to over 1000 μg RE/d for Poland and Denmark. The proportion of boys with intakes below the LRNI ranged from less than 1 % for Denmark, Spain, France and Germany to 5 % for Belgium. The proportion of intakes below the EAR ranged from 1·2 % for France to 20 % for Belgium.
Intakes for children aged 11–17 years showed similar patterns as for younger children. For girls aged 11–17 years, mean intakes ranged from about 420 μg RE/d for Spain to over 1500 μg RE/d for Germany. The proportion of intake below the LRNI was less than 1 % for France and Germany and 12 % for the UK. The proportion of intakes below the EAR ranged from 1 % for Germany to 39 % for Spain. For boys, mean intakes ranged from 528 μg RE/d for Spain to over 1800 μg RE/d for Poland. The proportion of intakes below the LRNI ranged from less than 1 % for Germany to 11 % for the UK. The proportion of intakes below the EAR ranged from 2 % for Germany to 30 % for the UK. Supplements made little difference to mean intakes and proportions below cut-offs.
Vitamin A intake – adults
Women showed the lowest mean intakes for Spain, at about 500 μg RE/d, and much higher values for the other countries, all over 800 μg RE/d, with Poland and Germany having the highest at 1215 and 1756 μg RE/d, respectively (Table A9, available online). The proportion of intakes below the LRNI ranged from less than 1 % for France, Spain, Germany and The Netherlands to over 7 % for the UK. The proportion of intakes below the EAR was the highest in the UK (22 %) and Poland (18 %). Mean intakes for men for most countries were about or over 1000 μg RE/d, but for Spain, mean intake was 547 μg RE/d. Mean values for Poland and Germany were considerably higher than that for the other countries at 1846 and 1991 μg RE/d, respectively. The proportion of intakes below the LRNI ranged from less than 1 % for Germany, France, The Netherlands and Spain to 10 % for the UK. The proportion of intakes below the EAR was low in most countries, except for the UK (29 %) and Spain (36 %).
All countries except Spain had mean intakes over 1000 μg RE/d for both men and women aged over 60 years. For Spain, mean intakes were 482 μg RE/d for men and 413 μg RE/d for women, which were both below the EAR. The proportion of intakes below the LRNI ranged from less than 1 % for France, Germany and The Netherlands to over 8 % for Poland. The proportion of intakes below the EAR was 62 % for men and 50 % for women in Spain, about 18 % for both men and women in Poland and 17 % for UK women; for the others, the proportions were low. Supplements made little difference to values, except for mean intakes for women in Denmark and Poland, which increased by some 400–500 μg RE/d with supplements.
Vitamin A – under-reporting
Excluding under-reporters made little difference to mean values and the proportion of intakes below the LRNI and EAR.
Vitamin B1
Vitamin B1 intake – children
For children aged 1–3 years, mean vitamin B1 intakes from the base diet varied from 0·45 mg/4184 kJ (1000 kcal) per d in Poland to 0·85 mg/4184 kJ (1000 kcal) per d in the UK (Table A10, available online). However, the proportions with intake below the LRNI were zero, except in Poland, where 2·5 % of children had intakes below the LRNI. The proportions of children with intakes below the EAR were also very small; the highest proportion was observed in Poland (7 %). Taking into account dietary supplements increased the mean intake, but did not change the proportions of children with low intakes.
For children aged 4–17 years, the lowest mean intakes were observed in Poland for those aged 4–10 years (0·47 mg/4184 kJ (1000 kcal) per d in girls and 0·48 mg/4184 kJ (1000 kcal) per d in boys). In almost all countries, there were no intakes below the LRNI. The proportions with intakes below the EAR were low: the highest value, 8 %, was observed for girls aged 4–10 years from Poland. Adding vitamin B1 from supplements increased mean intakes slightly, but had no appreciable impact on the proportions of children with low intakes.
Vitamin B1 intake – adults
For adults, intakes showed the same patterns for all age and sex groups as for children (Table A10, available online). Mean intakes from the base diet varied between 0·52 mg/4184 kJ (1000 kcal) per d in Polish women aged over 60 years and 0·88 mg/4184 kJ (1000 kcal) per d in UK women aged over 60 years. The proportion with intakes below the LRNI was less than 1 % for all countries. The proportion of adults with intakes lower than the EAR varied from 0 % to 6 % for Polish women aged over 60 years. As in children, the highest proportions were observed in Poland. Dietary supplement intake did not affect these values substantially.
Vitamin B1 – under-reporting
Excluding under-reporters increased the mean for some German adult age groups up to 8 %, but only slightly for other countries. Under-reporting did not change the proportions of subjects with intakes lower than the LRNI and the EAR.
Vitamin B2
Vitamin B2 intake – children
In children aged 1–3 years, mean intakes of vitamin B2 from the base diet varied between 1·2 mg/d in The Netherlands and Poland and 1·5 mg/d in Belgium (Table A11, available online). Less than 1 % had intakes below the LRNI. The proportion with intakes below the EAR varied between 1 % in The Netherlands and 5 % in Poland. Taking into account dietary supplements increased the mean intake, especially in Poland, without changing the proportions of low consumers substantially.
In children aged over 3 years, mean intakes of vitamin B2 from the base diet varied between 1·2 mg/d in the Dutch girls aged 4–6 years and 2·6 mg/d in the German boys aged 11–17 years. The proportion with intakes below the LRNI was low in the 4- to 10-year age group ( ≤ 3 %), but varied considerably for those aged 11–17 years, from 0 % in the Spanish boys and the Belgian girls to 17 % in the UK girls. The highest proportion of intakes below the EAR was observed in girls aged 11–17 years from the UK (26 %). Taking into account dietary supplements decreased the proportion with intakes below the LRNI from 13 % to 9 % in the Danish girls aged 11–17 years, but very little in other countries.
Vitamin B2 intake – adults
In adults, mean intakes from the base diet ranged between 1·3 mg/d in the Spanish women aged older than 60 years and 2·6 mg/d in the Belgian men aged 18–60 years (Table A11, available online). The proportion of adults with intakes lower than the LRNI was always lower than 15 %. High proportions were generally observed in women (15 % in Polish women aged 18–60 years and 12 % in UK women aged 18–60 years), whereas in men, the proportion was always less than 7 % (highest for Polish men aged over 60 years). The proportion with intakes below the EAR was also higher in women than in men, but did not exceed 22 %. Taking into account dietary supplements increased the mean value, but did not modify the proportion of low intakes substantially.
Vitamin B2 intake – under-reporting
After exclusion of under-reporters, mean intakes increased only slightly, but the 5th percentiles increased substantially in several groups (about 10–30 %). The proportions with intakes below the LRNI were slightly reduced in most groups, but substantially in the Danish girls aged 11–17 years.
Vitamin B6
Vitamin B6 intake – children
For children aged 1–3 years, mean vitamin B6 intakes ranged from 23·3 μg/g protein in Poland to 33·9 μg/g protein in the UK (Table A12, available online). Few children had intakes below the LRNI and the EAR, the highest proportion being in Poland at 8 %. For those aged 4–10 years, mean intakes from the base diet varied between 17·7 μg/g protein in the Danish girls and 33·2 μg/g protein in the UK girls. For those aged 4–10 years, the prevalence of intakes below the LRNI was almost zero in all countries, except in Poland (1 %). The proportions with intakes below the EAR were higher, although less than 5 % in all countries.
For those aged 11–17 years, mean intake from the base diet varied between 17·3 μg/g protein in the Danish boys and 36·0 μg/g protein in the UK girls. The prevalence of intakes below the LRNI and EAR was low in most countries. About 5 % of adolescents from Poland had intakes below the EAR. The addition of supplements did improve the mean intake substantially in Poland and Denmark, but had little impact on the prevalence of intakes below the EAR.
Vitamin B6 intake – adults
In adults aged 18–60 years, mean intake from the base diet varied from 18·8 μg/g protein in the Danish men to 33·2 μg/g protein in the UK men (Table A12, available online). The prevalence of intakes below the LRNI and the EAR was low ( ≤ 1·5 and ≤ 4·0 %, respectively). The proportion below the EAR was highest in Poland. For adults aged over 60 years, mean intake from the base diet was lowest in Denmark (20·0 μg/g protein for men and 20·2 μg/g protein for women) and highest in the UK (31·4 μg/g protein in men and 30·1 μg/g protein in women). The proportion of intakes below the LRNI was low ( < 4 %). The proportion of intakes below the EAR was zero in several countries, the highest proportion being for women in Poland (7 %). Inclusion of dietary supplements did increase mean intakes, especially in Denmark, the UK and Poland.
Vitamin B6 – under-reporting
Exclusion of under-reporters increased the mean of some German adult age groups up to 8 % but did not change other values substantially.
Vitamin B12
Vitamin B12 intake – children
Among those aged 1–3 years, mean vitamin B12 intake from the base diet was between 2·3 μg/d (Poland) and 3·9 μg/d (UK) (Table A13, available online). The proportion of intakes below the LRNI and the EAR was very low in all countries ( < 1 and < 2 %, respectively). Among those aged 4–10 years, mean intakes ranged from 2·6 μg/d in the Dutch girls aged 4–6 years to 6·9 μg/d in the Spanish boys. Poland had the highest prevalence of intake below the LRNI and the EAR, both for boys (5 % below the EAR and 2·3 % below the LRNI) and girls (6·4 % below the EAR and 2·6 % below the LRNI). In the other countries, the prevalence was almost zero. For those aged 11–17 years, the lowest mean intake was found in Polish girls (3·5 μg/d) and the highest in Spanish boys (8·6 μg/d). The prevalence of intakes below the LRNI and the EAR was low, except for Poland, where 7 % of girls and 6 % of boys had intakes below the LRNI. The additional intake from dietary supplements was generally not substantial, except in Denmark (10–15 %), and for young children in The Netherlands (5–10 %).
Vitamin B12 intake – adults
For men aged 18–60 years, mean intake ranged between 4·9 μg/d in The Netherlands (31–60 years) and 7·9 μg/d in Germany (Table A13, available online). The prevalence of intakes below the LRNI and the EAR was highest in Poland (7 % below the EAR and 4 % below the LRNI) and < 1 % in most other countries. For women aged 18–60 years, the lowest mean intakes were observed in Poland and The Netherlands (19–30 years; 3·5 μg/d in both countries) and the highest in Germany (5·2 μg/d). About 22 % of women in Poland had intakes below the EAR and 15 % had intakes below the LRNI. The prevalence was low ( < 5 %) in the other countries. The addition of supplements to the base diet did not change the prevalence substantially.
For adults aged over 60 years, the UK showed the highest mean intake in both men (7·1 μg/d) and women (6·0 μg/d). Spain showed the lowest mean intake in men (4·1 μg/d) and Poland in women (3·3 μg/d). The prevalence of intakes below the LRNI and the EAR was generally low ( ≤ 2 %), except in Poland, where 12 % of women and 6 % of men had intakes below the LRNI and 17 and 12 %, respectively, had intakes below the EAR.
Vitamin B12 intake – under-reporting
Excluding under-reporters increased the mean intakes by 5–8 % in most groups. The 5th percentile intakes increased by about 8–25 % in groups aged above 11 years. The percentages below the LRNI and the EAR, however, did not change substantially.
Folate
As indicated in the Methods, countries varied in the manner in which folate was calculated for supplements and when added to foods for fortification. All results are described as ‘folate’, irrespective of the manner of calculation.
Folate intake – children
Mean intakes ranged from 105 μg/d (Dutch children aged 2–3 years) to 381 μg/d (German boys aged 11–17 years) (Table A14, available online). The proportion of intakes below the LRNI for children aged 1–3 years was close to zero and among those aged 4–10 years, it was less than 2 %. For those aged 11–17 years, the proportion with intakes below the LRNI ranged from less than 1 % (Danish boys) to 4 % (Spanish boys). The proportion with intakes below the EAR generally was greater with increasing age and ranged from less than 1 % (2–3 years in The Netherlands, 4–10 years in Denmark and France) to 63 % (Spanish girls aged 11–17 years). Including dietary supplements resulted in a higher mean intake of about 2–75 μg/d, but did not change the proportions of intakes below the EAR and LRNI substantially.
Folate intake – adults
Mean intake from the base diet ranged from 156 μg/d (The Netherlands, 19–30 years) to 299 μg/d (Denmark, 18–60 years) for women and from 199 μg/d (The Netherlands, over 60 years) to 333 μg/d (Poland, 18–60 years) for men (Table A14, available online). For women, the proportion with intakes below the EAR ranged from 0 % (Spain, older than 60 years) to 46 % (The Netherlands, 19–30 years) and for men from less than 2 % (France and Spain, over 60 years) to 16 % (The Netherlands, over 60 years). Intakes below the LRNI were less than 6 % in all countries. Including dietary supplements resulted in a higher mean intake of 10–100 μg/d approximately. The highest increase was observed for women from Denmark (18–60 years) and The Netherlands (19–30 years).
Folate – under-reporting
Excluding under-reporters increased the mean intake up to 8 % in several groups. This difference was greater for adults compared with children. At the 5th percentile, intakes were generally 10–25 % higher and up to 39 % higher in Danish men aged over 60 years. The proportions with intakes below the EAR were substantially lower in some age groups in France and Denmark (6–8 %), while the percentages below the LRNI did not change substantially.
Vitamin C
Vitamin C intake – children
For children aged 1–3 years, the mean intake ranged from 50 mg/d in Poland to 81 mg/d in Belgium (Table A15, available online). The prevalence with intakes below the LRNI was less than 4 % for the four countries reporting intakes in this age group. The prevalence of intakes below the EAR was highest in Poland (21 %) and below 4 % for the other countries. For Poland, dietary supplements doubled the mean intake and decreased the proportion with intakes below the EAR to 14 %. For girls aged 4–10 years, intake ranged from 65 mg/d for The Netherlands (4–6 years) to 106 mg/d for Germany. For boys aged 4–10 years, intakes ranged from 67 mg/d for The Netherlands (4–6 years) to 108 mg/d for Germany. The proportion with intakes below the LRNI was zero for both girls and boys in most countries; the proportion below the EAR ranged from less than 1 % for Denmark, France and The Netherlands (4–6 years) to 9 % for Poland. Again, the largest increase in intake through dietary supplements was observed for Poland. For girls aged 11–17 years, the intake ranged between 69 mg/d for Spain and 201 mg/d for Germany and for boys from 71 mg/d for The Netherlands to 203 mg/d in Germany. Intakes in Germany were double those in any other country. The proportion with intakes below the LRNI was low, at less than 3 % in all countries; the proportion below the EAR ranged from 0·2 to 8·0 % and was the highest for Poland. There was a substantial increase in mean intake in some countries when use of dietary supplements was included, but this did not change the proportions with intakes below the EAR and LRNI very much.
Vitamin C intake – adults
In Denmark, France, Spain and The Netherlands (18–60 years), mean intake was higher for women compared with men (Table A15, available online). In women, mean intake ranged from 81 mg/d in Poland to 152 mg/d in Germany; for men, the range was from 81 mg/d for France and The Netherlands (31–60 years) to 152 mg/d for Germany. The proportion of intakes below the LRNI was very low, at less than 3 % for all countries. The proportion below the EAR was highest for Poland (8 % for men and 13 % for women). For women aged over 60 years, intake ranged from 69 mg/d for Poland to 132 mg/d for Germany and for men aged over 60 years from 77 mg/d for Poland to 142 mg/d for Germany. Again, the proportion with intakes below the LRNI was low. The proportion below the EAR was also low, except for the UK (7 % for women and men) and Poland (15 % for men and 13 % for women). Dietary supplements resulted in a higher mean intake of about 20–30 mg/d, but only a slightly lower proportion of intakes below the LRNI or the EAR.
Vitamin C – under-reporting
After excluding under-reporters, the mean intake increased from 5 to 9 % in some adult groups, but only about 1 % in children. At the 5th percentile, intakes were about 6–20 % higher, especially in Denmark, where increases as large as 29 % were observed in some age groups. The proportions with intakes below the LRNI and the EAR were generally low, and excluding under-reporters did not change this substantially.
Vitamin D
Vitamin D intake – children
For children aged 1–3 years, mean intakes from the base diet ranged from 1·3 μg/d for Poland to 2·3 μg/d for Belgium and were all below the LRNI (Table A16, available online). The percentages of intakes below the LRNI were all greater than 75 %. Supplements reduced this proportion markedly for The Netherlands, from 84 to 26 %. For girls aged 4–10 years, mean intake ranged from 1·6 μg/d for Spain and Germany to 2·9 μg/d for The Netherlands (age 7–10 years). For boys aged 4–10 years, mean intake ranged from 1·9 μg/d for Spain, France, Germany and the UK to 3·5 μg/d for The Netherlands (age 7–10 years). The percentages of intakes below the LRNI (28–94 %) were high, all values being over 65 %, except for The Netherlands (7–10 years). The proportions below the EAR were also high (91–100 %). For girls aged 11–17 years, mean intakes ranged from 1·5 μg/d for Spain to 3·2 μg/d for Poland. For boys aged 11–17 years, mean intake ranged from 1·9 μg/d for France to 4·8 μg/d for Poland. The percentages of intakes below the LRNI ranged from 17 % for boys from The Netherlands (7–10 years) to 98 % for girls from Spain. The percentages below the EAR were high (72–100 %). The additional intake from dietary supplements was generally high in Denmark and substantial for young children in The Netherlands.
Vitamin D intake – adults
For women aged 18–60 years, mean intake ranged from 1·3 μg/d for Spain to 3·4–3·5 μg/d for Poland and The Netherlands (31–60 years) (Table A16, available online). For women age 14–50 years, the figures were similar as for those aged 18–60 years. For men aged 18–60 years, mean intake ranged from 1·7 μg/d for Spain to 6 μg/d for Poland. The percentages with intakes below the LRNI ranged from 7 % for men from The Netherlands (31–60 years) to 95 % for women from Spain. The percentages below the EAR were high (63–100 %). For women aged over 60 years, mean intakes ranged from 0·7 μg/d for Spain to 4·1 μg/d for The Netherlands. For men aged over 60 years, mean intakes ranged from 0·8 μg/d for Spain to 5·2 μg/d for The Netherlands. The percentages of intakes below the LRNI ranged from 6 % for men from The Netherlands to 100 % for Spain. The percentages below the EAR were high (87–100 %). There was, in general, a high contribution of supplementation in Denmark and a substantial contribution among women aged over 60 years in Germany.
Vitamin D – under-reporting
Excluding the under-reporters increased the estimated mean by about 6–8 % in some age groups and the 5th percentile by 5–20 % in most groups, but up to 34 % in some groups. The percentages below the LRNI and the EAR did not change substantially, however.
Vitamin E
Vitamin E intake – children
For children aged 1–3 years, mean intakes ranged from 4·9 mg/d for the UK and Poland to 7·1 mg/d for The Netherlands (2–3 years) (Table A17, available online). The proportion with intakes below the LRNI ranged from 1 % for The Netherlands to over 20 % for Poland and Belgium. The proportion of children with intakes below the EAR was low in The Netherlands (6 %) but 30–40 % in the other countries with data for this age range.
For girls aged 4–10 years, intake ranged from 5·8 mg/d for Spain to 9·4 mg/d for Germany and 10·8 mg/d for girls aged 7–10 years in The Netherlands. The prevalence of intakes below the LRNI was 5 % or lower for all countries except Belgium, which had over 20 % of girls below the LRNI. The proportion with intakes below the EAR was also less than 5 % in most countries but 13 % in Denmark, 14 % in Poland, 21 % in Spain and 35 % in Belgium. For boys aged 4–10 years, mean intakes ranged from 5·9 mg/d for Spain to 10·5 mg/d for Germany and 11·9 mg/d for The Netherlands (7–10 years). The proportion with intakes below the LRNI was below 3 % for all the countries except for Belgium, where it was over 20 %. The proportion with intakes below the EAR was low in most countries but 41 % in Belgium.
For girls aged 11–17 years, intakes ranged from 6·4 mg/d for Spain to 16·4 mg/d for Germany. The proportion with intakes below the LRNI was very low for all countries ( < 2 %), except for Denmark at 9 %. The proportion with intakes below the EAR was also low, with the highest values for the UK (25 %) and Denmark (37 %). For boys aged 11–17 years, intakes ranged from 7·6 mg/d for Spain to 18·6 mg/d for Germany. There were low proportions below the LRNI, all being less than 4 %, except for Denmark at 10 %. The proportions with intakes below the EAR ranged from less than 2 % for Germany to 38 % for Denmark. The additional intake from dietary supplements was substantial for children aged 1–3 years in Poland and older children in Denmark.
Vitamin E intake – adults
For women aged 18–60 years, mean intakes ranged from 6·7 mg/d for Denmark to over 11 mg/d for Germany, Poland and The Netherlands (31–60 years) (Table A17, available online). The proportion below the LRNI was low, the highest being for Poland at 3·7 %. The proportion with intakes below the EAR was below 20 %, except for Denmark (25 %) and the UK (30 %). For men, mean intakes ranged from 7·7 mg/d for Denmark to 17·1 mg/d for Poland. The prevalence of intake below the LRNI was very low, with all countries having less than 3 %, except for Denmark at 8 %. The proportion with intakes below the EAR was below 31 %.
For women aged over 60 years, mean intakes ranged from 6·5 mg/d for Denmark to 11·9 mg/d for The Netherlands. The proportion of intakes below the LRNI was below 1 % for all countries, except for Poland and Denmark, both about 4 %. The proportion of intakes below the EAR was below 20 %, except for Denmark (29 %) and the UK (28 %). For men, the mean intakes ranged from 7·2 mg/d for Denmark to 13·7 mg/d for The Netherlands. The proportion with intakes below the LRNI was below 2 %, except for Poland at 7 %, the UK at 8 % and Denmark at 13 %. The proportion of intakes below the EAR was below 20 %, except for Denmark (39 %) and the UK (22 %). The contribution to intake from dietary supplements among adults, particularly women, was substantial in Denmark, Germany, Poland and the UK.
Vitamin E intake – under-reporting
Excluding the under-reporters increased the mean intake among French adults up to 10 % but only slightly in other countries. After excluding the under-reporters, the 5th percentiles increased by about 5–20 % in Germany and Denmark and up to 37 % in France, but it made little difference to the proportions below the EAR and the LRNI.
Discussion
To our knowledge, the present study is the first analysis of the adequacy of micronutrient intakes in multiple countries using national survey data, with reanalysis of raw data to obtain uniformity in age groups and using a single set of recommendations and a uniform method to determine adequacy. As such, it provides an overview of the European situation and an invaluable resource for assessing the status of European populations in terms of low intakes of micronutrients. There is a substantial literature of adequacy of nutrient intakes for individual countries(Reference Sebastian, Cleveland and Goldman24–Reference Serra-Majem, Ribas-Barba and Pérez-Rodrigo26), in studies of specific age groups(Reference Sebastian, Cleveland and Goldman24, Reference Bucholz, Desai and Rosenthal27) and of vulnerable populations(Reference Arab, Carriquiry and Steck-Scott28, Reference Rees, Doyle and Srivastava29), such as pregnant women(Reference Haugen, Brantsaeter and Alexander30), infants, vegetarians(Reference Thane and Bates31), those of low socio-economic position(Reference Tedstone25) or those living in challenging physical environments(Reference Hopping, Mead and Erber32). Efforts to compare dietary adequacy of micronutrients across countries have been hampered by the different dietary intake methodologies used and in the variation in nutritional recommendations from country to country and in the methods used to derive these(Reference Roman-Viñas, Barba and Ngo11, Reference Cavelaars, Kadvan and Doets33–Reference Tabacchi, Wijnhoven and Branca36). Pooling of different surveys or cohorts has generally been limited to specific nutrients and investigations of relationships with diseases where diet is thought to play a role(Reference Knekt, Ritz and Pereira37, Reference Larsson, Orsini and Wolk38). A comparison has recently been published of the prevalence of nutrient intake inadequacy in adults for a number of European countries(Reference Roman-Viñas, Barba and Ngo11). That review concluded that vitamin C, vitamin D, folate, Ca, Se and I showed a high prevalence of inadequate intakes (above 20 %) in Europe. The evaluation of inadequate intake was, however, based on the percentages below the EAR, which were estimated from the published mean and standard deviations of intake, assuming a normal distribution. The authors also used EAR values different from ours, which were in particular much higher for vitamin C (men 60 mg/d and women 50 mg/d) and Ca (adults 19–64 years 800 mg/d and elderly 1000 mg/d) and lower for Fe, particularly for girls and women of childbearing age (6 mg/d). This may partly explain the differences in concerns (higher for vitamin C and Ca, less for Fe) about these nutrients compared with the present study(Reference Roman-Viñas, Barba and Ngo11). That review was also limited to adults, and there are concerns about adequacy of certain micronutrients in children. Hence, the present study provides a more uniform analysis, covering all age groups, and uses set lower cut-offs as the major determinant of low intake for each of the micronutrients reported.
The main rationale for the present study was to compare the prevalence of low intakes in different European countries. The present study complemented that carried out in similar countries, which reported on the likelihood of high intakes of micronutrients(Reference Flynn, Hirvonen and Mensink13), and like that initiative, attempted to examine the impact of supplementation and fortification by assessing the effects of these practices on average intakes and the prevalence of intakes above the upper levels. This project attempted to harmonise the age groups studied, requiring investigators to reanalyse their raw data to obtain uniform age groups so that more direct comparisons could be made. In addition, the project assessing upper levels did not investigate the influence of under-reporting, as it was assumed that this would be unlikely to affect intakes at the upper end of the spectrum. With low intakes, however, under-reporting might well result in overestimates of inadequacy, as those with low intakes of energy would be more likely to have low intakes of micronutrients than those who are adequate reporters. All countries, apart from Poland, The Netherlands and the UK, reported their values with and without individuals expected to have under-reported. For Poland, where only one 24 h recall was used, estimation of under-reporting would be inappropriate as it is plausible that energy intake for a single day is lower than the BMR, and for the UK, the data were from the new National and Diet and Nutrition Survey rolling programme, and the extent of under-reporting has not yet been released. The Netherlands did estimate the percentage of under-reporters, but did not estimate intakes excluding under-reporters.
The contribution of supplements to micronutrient intake has been investigated in the past works in both individual studies(Reference Briefel, Hanson and Fox39–Reference Schwarzpaul, Strassburg and Luhrmann41) and the national survey data(Reference Sebastian, Cleveland and Goldman24, Reference Bates, Prentice and van der Pols42, Reference Tetens, Biltoft-Jensen and Spagner43), but this is the first time, to our knowledge, where attempts have been made to bring together data from a number of countries to compare the contribution of supplements with the mean and lower percentiles of the intake distributions. A recent investigation in the USA of the contributions from food, supplements and fortified foods to intakes in the National Health and Nutrition Examination Survey is the most comprehensive analysis to date(Reference Fulgoni, Keast and Bailey44). Investigating the impact of fortification is a difficult task, and only few data in our project could be obtained, as most food composition databases in Europe do not differentiate voluntary from mandatory fortification or the contribution from fortification to the total content of micronutrients in food products. This remains an area worthy of further investigation. European countries vary considerably in their fortification practices, both voluntary and mandatory, and the impact of these may vary with the degree of wealth or social structure and may affect particular subgroups of the population more than others. Efforts should be made to assess the impact of these practices to inform policies elsewhere.
The comparison of data from different sources is not without challenges. Dietary assessments are conducted differently in different locations, not only in terms of the methods used, but also for the same method in the manner in which the assessment is carried out. There are few large studies comparing 24 h recall with estimated diaries, the main two methods used here; both Belgium and the UK have compared these two methods prior to national nutrition surveys, with differing results. In the UK, energy intakes and percentage mis-reporting were similar in both methods(45), while in Belgium 24 h recalls gave higher intakes(Reference De Keyzer, Huybrechts and De Vriendt46). This variation may depend on the precise way the assessments are conducted. For example, 24 h recall can be conducted in a short period of time with little detail or can take an extended period with multiple passes through the preceding day to obtain as complete a record as possible. Furthermore, 24 h recalls can be repeated, giving greater precision to estimates; in the two studies mentioned earlier, Belgium used two computerised recalls, the UK administered four interviews and the diary methods varied as well. The main methods used in the surveys reported in this compilation were also carried out differently, and some used different methods for adults v. children. The surveys also varied in terms of recruitment methods, individual and household representativeness, numbers of subjects per household and weighting factors used.
Another important factor in determining intakes consistently is in the alignment of food composition databases. There is a need for composition data to be collected and presented in a standardised way to enable comparison between countries and across Europe. One nutrient where methods were different was folate. Some countries account for the greater bioavailability of synthetic folic acid as added to foods or as supplements, while other countries do not. This difference in methods could not be harmonised completely for this project, and since the majority of folate is food folate, and the included countries do not have a mandatory folic acid fortification, all countries were considered together. The problem of uniformity in food composition databases has been addressed by several European projects, most recently by the European Food Information Resource project, which developed draft standards for food data.
In spite of some differences in methods, the various investigators involved created uniform intake data in terms of age groups included, and carried out new calculations on the adequacy of nutrients using common cut-offs. These were determined to be the EAR and LRNI, as described in the dietary recommendations for the UK. Since the UK has the largest number of recommended intake levels in Europe, their values were used(17); where the UK does not have a recommendation, the Nordic Nutrition Recommendations were used as a second resource(Reference Alexander, Andersen and Aro18). By recalculating the national data to these common age groups and cut-offs, comparisons could be made for recent intake data from eight European countries.
When examining the micronutrients in detail, differences in proportions below the EAR and LRNI appeared, which may be due in part to varying methods; the most notable example of this was the higher proportions below the EAR and LRNI for Poland. This might be explained by a poorer diet in Poland as a result of more difficult socio-economic conditions at the time of data collection. However, the reason may instead be due to the use of a single 24 h recall to estimate intake, since the mean intakes were similar to those of the other countries. Since with a single 24 h recall no correction for within-person variation is possible, and as a consequence the intake distribution is wider, an overestimation of the proportion with inadequate intakes is likely.
In terms of the minerals reported, there were concerns about the proportion of individuals below the EAR and LRNI for several, with few concerns for others. For Ca, for example, mean intakes were sufficient and the proportions of intakes below the EAR were low for all countries, except for some age groups in Poland, where intakes were generally low. Similarly, the proportion of individuals from all age groups below the LRNI was very low for all countries except for Poland. For Cu, the proportions of intakes below the EAR and the LRNI were low in all age groups. For both Ca and Cu with low proportions below the LRNI, under-reporting had little impact on results.
There were concerns about iodine, where the proportion of children with intakes below the EAR was high in several countries, and for older adults in France and Germany, with 40–60 % below the EAR. Intakes below the LRNI ranged from 1 to 15 %, the highest proportion being in Polish women aged 18–60 years at 18 %. About 20 % of those aged 4–10 years and more than 10 % of those aged 11–17 years in Poland had intakes below the LRNI. In many countries, voluntary iodine fortification of salt is a common practice, but the contributions of iodine from this source are not reflected in the national food composition databases, except in Denmark. True iodine intake will, therefore, be underestimated in these countries.
There were also high proportions of intakes below the LRNI for Mg among the adolescent girls in Denmark, France, Poland and the UK, and mean intakes of Se were below the EAR in almost all countries, with high proportions with intakes below the EAR and LRNI. For adolescents, for example, the proportion below the LRNI ranged from 31 to 60 % for girls and from 38 to 52 % for boys. With such high proportions, there was a marked effect of taking under-reporting into account, with the proportion below the LRNI being reduced by some 6–10 % when under-reporters were excluded. Even with this consideration, the proportion below the LRNI remained much higher than for many other minerals. For Fe, the sex difference in adequacy was clearly seen, with few concerns for men, while for girls aged 11–18 years and women aged 14–50 years, high proportions were below the LRNI. For girls aged 11–17 years, mean intakes were below the EAR for Denmark, France, Poland, The Netherlands and the UK, indicating the universal nature of dietary inadequacy of Fe intake in teenage girls. It was noted by Carriquiry(Reference Carriquiry16), however, that the use of the EAR as a cut-point is justified by the assumption that the distribution of requirements is symmetrical. For Fe, it is known that this distribution, especially for women of childbearing age, is skewed to the right. As a consequence, the proportions with intakes below EAR underestimate the true prevalence of inadequate intakes(Reference Carriquiry16).
Intakes of vitamins were generally less of a concern than those of minerals. Proportions of individuals below the LRNI for vitamins A, B1 and B2 were generally low (see Table 3). Intakes of vitamins B6 and B12 seemed to be generally adequate, although the proportions below the LRNI for vitamin B12 were higher for Poland than other countries. For vitamin E, the prevalence of low intakes was low, with only Belgium reporting high proportions below the LRNI. Intakes of vitamin C were generally adequate.
Table 3 Overview of age groups with more than 5 % of intakes below the LRNI
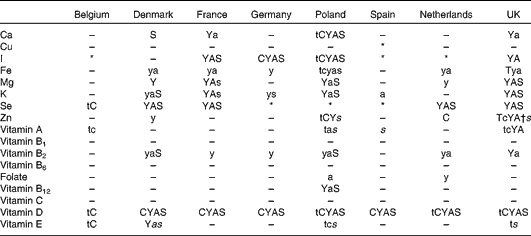
T, toddlers (1–3 years (both sexes)); C, children (4–10 years); Y, youth (11–17 years); A, adults (18–60 years); S, seniors (>60 years); capitals, both sexes; lower case, women only; lower case italic, men only.
* No data available for any age group in country.
† Only for women 14–50 years.
The one vitamin where there was universal concern in terms of intake was vitamin D. The proportions of the population with vitamin D intakes below the EAR and LRNI were exceptionally high, and in many cases, the mean intakes were below the LRNI and the proportion below the LRNI was over 90 % in many age and sex groups. Exclusion of under-reporters increased the mean values for vitamin D, but the proportion of those below the LRNI remained very high. Clearly, the concern about vitamin D intakes varies by country, as those at more northern latitudes need to obtain more vitamin D from their food than those countries on the Mediterranean, where vitamin D can more readily be obtained from conversion through the skin stimulated by UV radiation. Thus, vitamin D is one nutrient where the importance of a specific proportion of low intakes varies from country to country. A high proportion of low intakes is less serious for Spain, for example, than for the Nordic countries. It may then be advantageous to have country-specific recommendations for this vitamin; however, it remains difficult to set these and to know the exact proportion that should be obtained from the diet v. UV exposure for each country.
The impact of supplements on mean intakes was variable. In some cases, such as for Se in Denmark, supplements appeared to increase intakes markedly, and for vitamin C, for which there is substantial supplement use, mean intakes were again increased by supplement use. However, in most cases, supplements made very little difference to the proportion of individuals below the EAR or LRNI. There are several reasons for this; in some cases, the supplements provided very little additional intake, as in the case of Cu. Where the proportion of individuals below the LRNI was very low, it was not possible for supplements to have a large impact on adequacy. Finally, the lack of effect may also have been due to the common finding that those who take supplements are not those with low micronutrient intakes from food(Reference Tetens, Biltoft-Jensen and Spagner43, Reference Archer, Stamler and Moag-Stahlberg47–Reference Vatanparast, Adolphe and Whiting53). However, it is not always the case that supplements make no difference to the prevalence of adequacy. In a number of studies from the USA, multivitamins have been found to improve the measure of adequacy(Reference Murphy, White and Park50, Reference Weeden, Remig and Holcomb54), although the measure of adequacy used was not as low as the LRNI cut-off used in this analysis. Moreover, there was also an increased risk of excessive intakes in these studies, and it has been suggested that multivitamins should be formulated with greater consideration for the intakes of micronutrients from foods(Reference Murphy, White and Park50, Reference Weeden, Remig and Holcomb54). In Europe, supplement use is common in some countries, such as Germany and Denmark(Reference Tetens, Biltoft-Jensen and Spagner43, Reference Beitz, Mensink and Rams55), but less common in others such as the UK(56) and Belgium(Reference Mullie, Clarys and Hulens57). The impact of supplements is dependent on the supplement formulation, the frequency of use and the micronutrient intakes of those taking supplements, and these factors vary from country to country and between Europe and North America. There is an additional methodological point in the estimation of habitual micronutrient intake from foods and dietary supplements. Simply adding the amounts taken from dietary supplements to the intake from foods prior to statistical correction for within-person variation may result in lower intake estimates at the low percentiles of the intake distribution for the intake from food and dietary supplements compared with the intake from food only. This is caused by differences in variances between intake from food and dietary supplements and potential multimodality. Recently, a new so-called ‘first shrink then add’ approach was proposed to solve this methodological problem. In this method, habitual intake is first estimated separately for each source (food and dietary supplements) and thereafter combined to get the habitual total intake(Reference Verkaik-Kloosterman, Dodd and Dekkers58), but this method could not be applied in the present analyses.
The one vitamin where supplementation reduced the prevalence of inadequacy was vitamin D. This has been shown not only in the present analysis, but also in other studies in Europe(Reference Haugen, Brantsaeter and Alexander30, Reference Zgaga, Theodoratou and Farrington59) and North America(Reference Briefel, Hanson and Fox39, Reference Weeden, Remig and Holcomb54). While vitamin D status is clearly dependent on exposure to UV light as well as intake, low sun exposure at the latitude of northern Europe has led to a recommendation to supplement certain age groups, for instance in the UK(60) and The Netherlands(61), and the potential impact of this on adequacy is demonstrated by the present results. However, vitamin D and the case for supplementation remains an area of considerable debate, and further work is still needed to determine both the requirements for health and the relative contributions from diet and from UV exposure to assess whether supplementation would be of benefit.
This analysis of intakes and adequacy of micronutrients for all ages and both sexes presents valuable information about dietary intakes across Europe. It also confirms previous assertions that for many micronutrients and life stages, high-quality comparable data are still lacking. While the present study is as consistent in method as currently possible, it emphasises the need for harmonised European data acquired, for example, using the European Food Safety Authority guidelines on methodologies and procedures to be used in pan-European dietary surveys(62). One of the health goals of the WHO Second Action Plan is to reduce the prevalence of micronutrient deficiencies in Europe(63). To achieve this goal, the WHO has proposed the harmonisation and establishment of national and regional surveillance systems on nutritional status, food availability and consumption, dietary intake of micronutrients with adequacy monitoring in different age and socio-economic groups(63).
There is also a need to obtain specific information on the consumption of fortified foods. This additional information was available for some surveys and was partly included in the base diet, but only few countries were able to separate this contribution to intake. We therefore decided to not include this separately in the tables.
Except for vitamin D, the present study suggests that the current intakes of vitamins from foods lead to low risk of low intakes in all age and sex groups. For minerals, the study suggests that the risk of low intakes is likely to appear more often in specific age groups. In spite of the limitations of the data, the present study provides valuable new information about micronutrient intakes across Europe and the likelihood of inadequacy country by country. It contributes to the identification of subgroups at risk for nutritional deficiency and provides a useful resource for policy making in relation to dietary recommendations as well as for supplementation and fortification advice and regulation.
Supplementary material
To view supplementary material (i.e. micronutrient intake tables) for this article, please visit http://dx.doi.org/10.1017/S000711451200565X
Acknowledgements
The present work was commissioned by the Addition of Nutrients to Foods Task Force of the European branch of ILSI (ILSI Europe). Industry members of this task force are BASF SE, Bunge Europe, Coca-Cola Europe, Danone, DSM, FrieslandCampina, Kellogg Europe, Mars and Red Bull. This publication was coordinated by Athanasia Baka and Dr Christophe Matthys, Scientific Project Managers at ILSI Europe. For further information about ILSI Europe, please email [email protected] or call +32 2 771 00 14. The opinions expressed herein and the conclusions of this publication are those of the authors and do not necessarily represent the views of ILSI Europe, nor those of its member companies. The ILSI Europe Expert Group on ‘Mapping Low Intakes of Micronutrients Across Europe’ wishes to thank Belinda Antonio and Toula Aslanidis for their administrative support. Furthermore, a great thanks goes to Sergi Migallon for his invaluable contribution in preparing the numerous tables of this publication. We have particular appreciation of the excellent work of Dr Celia Greenberg of the MRC Human Nutrition Research in Cambridge and the Department of Health and the Food Standards Agency for providing data on the National Diet and Nutrition Survey (NDNS); Romana Novakovic, Marina Nikolic, of the University of Belgrade, Serbia; Dr Katarzyna Stos and Maciej Ołtarzewski of the National Food and Nutrition Institute, Poland; Tue Christensen and Dr Vibeke Kildegaard Knudsen of the National Food Institute, DTU, Denmark for their help in calculating and interpreting the data. The authors declare that they have no conflicts of interest. The Belgian child nutrition survey was funded by the Belgian Nutrition Information Center (NICE). The French INCA2 survey was funded by the French Food Safety Agency (formerly AFSSA, now ANSES). The German surveys were funded by the German Federal Ministry of Health, the Robert Koch Institute and partly by the Federal Ministry of Education and Research and the German Federal Ministry of Food, Agriculture, and Consumer Protection. The Dutch surveys were funded by the Dutch Ministry of Health, Welfare and Sports. The Polish survey was funded by the FAO of the UN. The Spanish childhood survey was partially financed by a Kelloggs Spain Grant to the Nutrition Research Foundation and the Catalan Nutrition Survey was financed by the Catalan Ministry of Health. Funding for the UK surveys was provided by the Department of Health and the Food Standards Agency. All authors contributed to the manuscript concept, the interpretation of results and wrote parts of the draft version. G. B. M. M., M. G., I. H., L. L., L. S.-M., L. S., I. T., J. V.-K. and A. M. S. provided calculations of country-specific intake data. G. B. M. M., R. F. and A. B. coordinated the compilation of all information. R. F. chaired all meetings. G. B. M. M., A. M. S. and A. B. had the primary responsibility for the final content of the manuscript. All authors critically revised the manuscript and read and approved the final version. Athanasia Baka is employed by ILSI Europe. M. G., I. H., L. L., L. S.-M., L. S. and A. M. S. received an honorarium from ILSI Europe for their participation in this publication and reimbursement of their travel and accommodation costs for attending the related meetings. G. B. M. M., I. T. and J. V.-K. received reimbursement of their travel and accommodation costs for attending the related meetings.





