INTRODUCTION
It has been estimated that more than 40 million people are infected with human immunodeficiency virus (HIV) worldwide. About 10 million (25%) of these individuals are also co-infected with hepatitis C virus (HCV) due to the same transmission routes [Reference Soriano1]. The survival time and quality of life of HIV patients has increased since the introduction of highly active antiretroviral therapy (HAART) in the late 1990s. HIV treatment resulted in the effective control of virus replication and improvement of the immune response (CD4+ lymphocyte increase), reducing the risk for opportunistic infections associated with AIDS. However, the morbidity and mortality rates are still significantly high in HCV co-infected patients. Health complications associated with HCV co-infection include severe liver dysfunction (e.g. hepatic impairment, cirrhosis and hepatocellular carcinoma), which is the main cause of death in HIV-infected patients [Reference Maier and Wu2–Reference Operskalski and Kovacs4].
HIV/HCV co-infection has a wide range of prevalence in different risk groups and geographical regions of the world. In subpopulations of HIV-positive persons, specifically those with a history of injecting drug use (IDU), the prevalence of co-infection is reported as high as 95% [Reference Aceijas and Rhodes5]. In addition, in other groups at risk for co-infection, these values are significantly lower; in HIV-positive persons who acquired their infection through sexual exposure, the prevalence of co-infection ranges from 9% to 27% in heterosexual men and 1–12% in homosexual men in North America and Europe [Reference Alter6]. This prevalence is also variable in Brazil: 18% in outpatient care centres [Reference Mendes-Corrêa, Barone and Guastini7], 42% in anonymous testing centres [Reference Pereira8], and 54% in adults infected with HIV in infectious diseases clinics [Reference Pavan9]. Variations are also observed according to gender, age and ethnic group [Reference Backus, Boothroyd and Deyton10, Reference Wolff11]. IDU has been the predominant transmission route in most studies [Reference Sulkowski12]. However, other transmission routes have been proposed, such as tattoos, body piercings, personal hygiene object sharing and non-injectable drug use [Reference Wolff11, Reference Howe13]. In the present study, we investigated the possible risk factors associated with HCV co-infection in HIV-positive patients in southern Brazil.
MATERIALS AND METHODS
Study population
A cross-sectional study was conducted in a reference outpatient treatment centre for HIV testing and AIDS treatment in Canoas (located in the metropolitan region of Porto Alegre, Rio Grande do Sul, Brazil). This centre is the only one in the city that specializes in STD/AIDS, and it is considered a referral service for patient care and antiretroviral drug supply. From July 2008 to January 2009, male and female patients with HIV/AIDS who attended the centre were eligible for the study if they were aged ⩾18 years. A total of 580 HIV-infected patients seeking medical treatment were consecutively enrolled, and 57 refused to participate. Socio-demographic and potential risk factors for HIV infection were obtained from a standardized individual questionnaire that was administered by a trained interviewer in a private room. Race was recorded as self-reported skin colour, and patients were classified as white or non-white. The Alcohol Use Disorders Identification Test (AUDIT), validated in Brazilian Portuguese, was used to screen for alcohol use disorders by a score ⩾8 [Reference Mendoza-Sassi and Béria14]. Illicit drug use was investigated using standardized questions. CD4+ counts and HIV viral loads were obtained from treatment centre medical records, using data from the most recent tests at the time of interview. HAART data were also obtained from medical records. The study was approved by the Research Ethics Committees of the Universidade Luterana do Brasil (ULBRA) (process 139H/2007). All participants signed an informed consent form.
Samples and HCV laboratory analysis
Blood samples were obtained via venepuncture in 5 ml tubes, using EDTA as an anticoagulant, and afterwards were centrifuged for plasma and cell separation. Plasma and buffy coat were divided into aliquots and stored at −20°C. All plasma samples were submitted for anti-HCV and HCV-RNA detection. Anti-HCV antibodies were determined by a third-generation immunoenzymatic assay (ELISA; Human Diagnostics, Germany). HCV-RNA was detected and quantified by a real-time PCR [Reference Martell15]. HCV genotypes were determined by restriction fragment analysis (RFLP), as described previously [Reference Krug16].
Statistical analysis
Double-entry data were performed with EpiData software, version 3.1 (EpiData Association, Denmark). All statistical analyses were performed with SPSS software, version 18·0 (SPSS Inc., USA). Distribution of variables stratified according to gender was analysed, due to behavioural differences. Data results were expressed as mean and standard deviation (±s.d.) or frequency percentage (%). Male–female variables were compared using Student's t test or the non-parametric Mann–Whitney test for categorical variables and the χ 2 test for qualitative variables. To measure the association between HCV infection and risk factors, the prevalence ratio (PR) and their 95% confidence interval (CI) were calculated. Multivariate models were conducted using a modified Poisson regression [Reference Barros and Hirakata17] to test the associations of HIV/HCV co-infection with participant demographic, socioeconomic, clinical, and behavioural characteristics. The PRs and their 95% CIs were computed, and the PR was adjusted to consider confounding factors such as age and education. Variables tested for inclusion in the multivariate models were race, sexual orientation, number of sexual partners in the past 12 months (⩽1 vs. ⩾2), illicit drug use (injected, smoked and/or snorted), blood transfusion and surgery history, tattoo and body piercing presence, and alcohol misuse. Variables that presented P values <0·20 in the bivariate analysis were included in the initial multivariate model. The final multivariate Poisson regression model was established through stepwise removal of covariates, starting with the variable with the highest P value; those covariates that altered the unadjusted PR by at least 10% were retained in the multivariable model. Covariates with borderline associations, for which clinical and/or biological relevance was assumed, were kept in the final model. All P values presented are two-tailed, and P < 0·05 values were considered statistically significant.
RESULTS
Socio-demographic factors and some risk factors of HIV patients included in this study are presented in Table 1. Out of 580 patients, the mean age was 40·6 (s.d. ± 10·8 years), and 319 (55%) patients were women. Men were significantly older than women (P = 0·018). Additionally, men had significantly higher household incomes than women (P < 0·001). Sexual practice was mainly heterosexual (96·6% in women, 74·7% in men; P < 0·001). Of men, 14·9% reported bisexual relations, and 10% were homosexual. Considering the number of sexual partners in the past 12 months, women had a significantly lower average (1·2) than men (6·4) (P < 0·001).
Table 1. Distribution of socio-demographic and clinical factors in HIV-1 patients

HAART, Highly active antiretroviral therapy.
* Totals do not coincide due to lack of data from certain study participants.
† Multiple response.
‡ Adjusted residual.
Sexual transmission (84·5%) was reported as the most probable HIV transmission route, followed by sharing needles (7·2%), blood transfusion (4·3%) and accidents with sharp objects (3·4%). A significant difference was observed in the proportion of women (89·3%) who reported infection by sexual transmission compared to men (78·5%, P = 0·021). Significant differences in HIV transmission routes between men and women were also found in blood transfusion (P = 0·041) and sharing needles (P < 0·001). IDU exposure was reported by 2·8% of women and 20·7% of men (P < 0·001). The presence of tattoos was more prevalent in males (33·0% vs. 22·6%, P = 0·006), while body piercing was more prevalent in females (10·3% vs. 5·4%, P = 0·032). A higher proportion of women (59·0%) than men (49·2%) had undergone surgery in the past (P = 0·032). Condom use during the last occurrence of sexual intercourse was higher in men than in women (75·8% vs. 64·8%, P = 0·008). Additionally, HIV data analysis showed that HAART use was more prevalent in women than men (P = 0·028).
A total of 138 (23·8%) patients had an HCV-positive result. Thirty-four (24·6%) patients had previous HCV infection (anti-HCV positive and HCV-RNA negative), and 104 (75·4%) were chronically co-infected (anti-HCV and HCV-RNA positive). No patients had results that suggested recent infection (anti-HCV negative and HCV-RNA positive). Of IDUs, 55 (87·3%) patients were co-infected. In the HIV/HCV group, 63 (60·6%) patients were infected with HCV genotype 1, five (4·8%) with genotype 2 and 36 (34·6%) with genotype 3. The mean HCV viral load was 6·8 ± 0·7, 6·9 ± 0·3, 6·6 ± 0·9 log10 IU/ml for genotypes 1, 2, and 3, respectively (P = 0·14). HCV genotypes were not significantly associated with probable HIV transmission.
Socio-demographic and clinical variables were comparatively analysed in HCV-positive and HCV-negative male patients (Table 2). Bivariate analysis for HIV/HCV co-infection in male patients showed that those with low educational levels (i.e. few years at school), who reported injecting, snorting or smoking drug use, who had tattoos, who reported alcohol misuse, and who reported their sexual debut before age 15 years were more likely to be HCV positive. Multivariate analysis was performed, and statistical significance was observed only for low educational levels (PR 1·9, 95% CI 1·2–3·0, P = 0·009), IDU (PR 2·9, 95% CI 2·0–4·3, P < 0·001) and smoking drug use (PR 2·3, 95% CI 1·4–3·9, P = 0·002). Borderline statistical significance was observed in blood transfusion history (P = 0·071) and sexual debut before age 15 years (P = 0·068) (Table 2).
Table 2. Crude and adjusted analysis between HCV infection and socio-demographic, clinical and behavioural variables in HIV-positive male patients (n = 261)
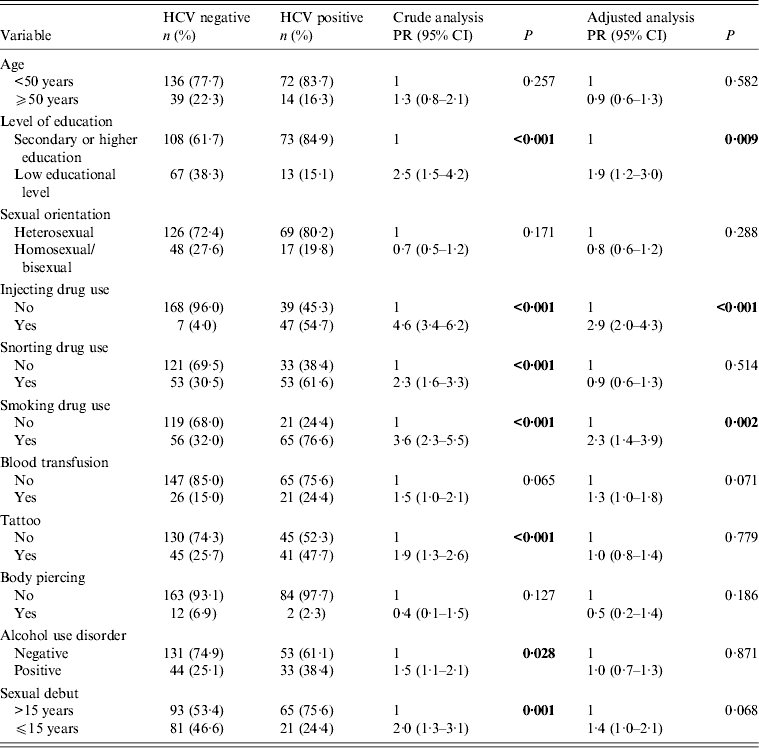
PR, Prevalence ratio; CI, confidence interval.
Bivariate analyses for HIV/HCV co-infection in female patients are shown in Table 3. Women who reported illicit (e.g. injecting, snorting or smoking drugs) or legal (e.g. alcohol) drug use were more likely to be HCV-positive. In multivariate analysis, statistical significance was observed for IDU (PR 3·8, 95% CI 2·0–7·3, P < 0·001) and alcohol misuse (PR 2·8, 95% CI 1·6–4·8, P < 0·001). Blood transfusion history showed borderline statistical significance (P = 0·088).
Table 3. Crude and adjusted analysis between HCV infection and socio-demographic and behavioural variables in HIV-positive female patients (n = 319)
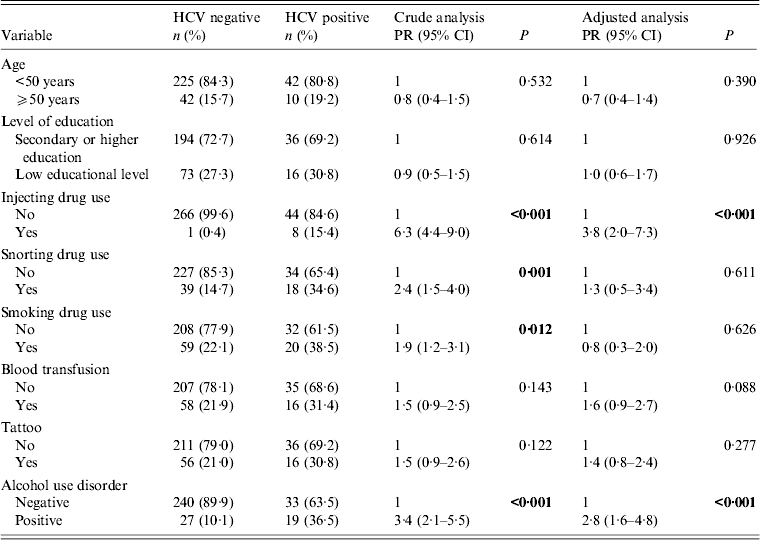
PR, Prevalence ratio; CI, confidence interval.
DISCUSSION
HIV epidemiology is complex and variable due to geographical differences and multiple population groups with different behaviours. This complexity is more pronounced in Brazil due to the continental country size and regional differences in population exposure factors. The highest incidence of HIV occurs in the southernmost state, Rio Grande do Sul [18]. A previous study demonstrated a HCV prevalence of about 31% in HIV-positive patients living in Porto Alegre, a city located in the same metropolitan area as neighbouring Canoas [Reference Wolff19]. In the present study, a slightly lower HCV prevalence (23·8%) was observed. Another study in the São Paulo metropolitan area demonstrated similar HCV prevalence data (18%) in the HIV population [Reference Mendes-Corrêa, Barone and Guastini7]. The present study's observed prevalence of HIV/HCV co-infection in IDUs (87·3%) was similar to that found in another Brazilian study (84·8%) [Reference Segurado20]. Several other studies have already shown that HIV/HCV co-infection prevalence is significantly higher in IDUs than in any other risk group [Reference Sulkowski12, Reference Bollepalli21].
In the multivariate analysis performed with gender, IDU was the only common risk factor for men and women. Although low educational levels and smoking drug use were also associated with HCV infection in men, alcohol misuse was the only other risk factor associated with women. IDU was a variable strongly associated with HIV/HCV co-infection, supporting previous findings that report it as a major risk factor for hepatitis C in the general population [Reference Wong and Lee22], for blood donors [Reference Kucirka23] and for HIV mono-infected individuals [Reference Bollepalli21, Reference Nurutdinova24]. Early onset of IDU is associated with a higher risk of HCV infection due to the possibility of repeated viral exposure episodes [Reference Van den Hoek25]. Sharing contaminated needles and syringes is the main mode of virus transmission in these situations, occurring at any time during drug preparation or administration [Reference Hagan26]. Smoking drug use was a risk factor for HIV/HCV co-infection in the male patient analysis. The use of non-injecting drugs, such as marijuana, snorted cocaine and crack cocaine, can contribute to HCV transmission and probably serves as a surrogate for other methods of transmission (e.g. IDU and high-risk sexual practices) [Reference Alter27]. Additional studies are needed to elucidate how this drug use potentiates other routes of transmission. On the other hand, low educational level was associated with the risk of HIV/HCV co-infection, similar to previous findings in other Brazilian populations [Reference Wolff19, Reference Navarro28]. Most patients included in this study had low educational levels, which is correlated to low socioeconomic situations, reduced hygiene standards, overcrowding and restricted health service access.
Interestingly, alcohol misuse was an important risk factor for HIV/HCV co-infection in women. Similarly, in a cohort study of HIV-infected women in the North America, baseline factors associated with harmful alcohol consumption included mainly HCV infection and illicit drug use [Reference Neblett29]. The prevalence of alcohol misuse in HIV-infected individuals is well known and may result in adverse consequences, such as social, physical and risky behaviours. Previous studies have already demonstrated that alcohol ingestion and drug use contribute to high-risk sexual behaviour, increasing HCV transmission in these groups [Reference Stein30, Reference Bonacini31]. On the other hand, alcohol abuse appears to reduce adherence to antiretroviral therapy, contributing to a high HIV load and subsequently immunodeficiency [Reference Neblett29, Reference Baum32]. In the current era of HAART, harmful drinking has a great impact on health outcomes because of its association with non-adherence to medication protocols.
HCV genotype is an important predictor of the likelihood of treatment failure. Patients with HCV genotype 1 have a lower rate of response to the standard treatment (interferon-α in combination with ribavirin), and genotypes 2 and 3 usually have good therapeutic responses, reaching a sustained virological response (SVR) with virus eradication [Reference Thomson33]. HCV mono-infected patients reach SVR in 50–80% of cases; conversely, the SVR rate is substantially lower in HIV/HCV co-infected subjects [Reference Payer34]. In the present study, we observed that most patients are co-infected with HCV genotype 1. Moreover, we observed that 25% of the patients (34 of 138 co-infected patients) had spontaneous resolution of HCV infection. These subjects were previously analysed and showed that spontaneous resolution was associated with genotypes of human polymorphism rs12 979 860 in the lL28B gene [Reference Lunge35]. Currently, HIV/HCV co-infection is gradually being recognized as a separate entity from HIV-1 or HCV mono-infections with an altered response to HAART that requires special care and treatment efforts [Reference Zhang36].
Some limitations must be considered in the interpretation of the present findings. First, it is difficult to establish factor causality because this is a cross-sectional study. Outcome and exposure data were collected simultaneously, and it was not possible to identify the time of infection for the two viruses (HIV and HCV). Finally, participants may have omitted or underreported their drug and alcohol use, thus underestimating the risk levels (although the survey questions were designed to avoid these potential limitations).
In conclusion, this study has contributed a better understanding of risk factors for HIV/HCV infection in an urban centre in southern Brazil. Some of these results could be useful to improve public policies that aim to educate and raise awareness regarding alcoholic beverages and illicit drug use. Further, the extremely high HCV rate of infection (87·3%) in IDUs is especially a cause for concern due to the increasing reports of viral hepatitis morbidity and mortality in HIV-positive patients. More research is needed to identify barriers to HIV-positive patient access to health services and information on prevention and safe practices; such research would help determine which supportive measures, guides and treatments are most useful for co-infected patients.
ACKNOWLEDGEMENTS
The authors thank the patients and staff of the Specialized Service (SAE) and Testing and Counselling Centre (CTA) of Canoas, RS for their collaboration in this study's development. This research project was funded by the Brazilian Ministry of Health, Secretaria de Vigilância em Saúde (Department of Health Surveillance), Programa Nacional de Doenças Sexualmente Transmissíveis e Aids (MS/SVS/PN-DST/AIDS – Brazilian Program of Sexually Transmitted Diseases and AIDS – Cooperation Term 282/07), through the International Technical Cooperation Project AD/BRA/03/H34 that was established between the Brazilian Government and the United Nations Office on Drugs and Crime (UNODC). The HCV molecular biology analysis procedures were determined by a project entitled ‘Development of Molecular Test Kits to Detect and Quantify Viral Agents – Hepatitis B and C and HIV – Based on the Real Time PCR Technology’, which was sponsored by the Financiadora de Estudos e Projetos (FINEP – Financial Support Organization for Research and Projects) and the Simbios Biotecnologia (FINEP/FULBRA/LDM – Simbios Biotechnology – Ação Transversal 2006 – convênio no. 0·1.07.0102·00).
DECLARATION OF INTEREST
None.






