Maternal nutrition is considered one of the most important and modifiable lifestyle factors influencing fetal growth and development during pregnancy, as it plays an important role in providing the necessary energy and nutrients for fetal growth(Reference Timmermans, Steegers-Theunissen and Vujkovic1–Reference Nnam3). Inadequate maternal nutrition could be a factor associated with preterm birth and low birth weight(Reference Triunfo and Lanzone4). Adequate maternal nutritional status is essential to the health of the mother and the child, as it influences fetal nutrition and endocrine status during pregnancy(Reference Mariscal-Arcas, Rivas and Monteagudo5).
Despite its popularity within the scientific community and the population at large, there is no single Mediterranean diet (MD) concept(Reference Serra-Majem, Ribas and Ngo6). MD is a dietary pattern characterised by increased consumption of mainly season-fresh unprocessed foods, including plant foods such as fruits and vegetables, pulses, nuts, whole-grain cereals and bread(Reference Willett, Sacks and Trichopoulou7–Reference Serra-Majem, Ribas, Serra-Majem, Aranceta and Mataix12). Meat consumption is limited to a few times a month, and there is a higher consumption of lamb, rabbit poultry and fish, while eggs are consumed few days a week(Reference Willett, Sacks and Trichopoulou7–Reference Serra-Majem, Ribas, Serra-Majem, Aranceta and Mataix12). Dairy (mainly derived from sheep or goat milk) consumption in the form of cheese and yoghurt is abundant, but milk consumption is lower than the present levels(Reference Willett, Sacks and Trichopoulou7–Reference Serra-Majem, Ribas, Serra-Majem, Aranceta and Mataix12). Low consumption of animal fats, sugars and salt and moderate wine consumption are also characteristics of the traditional MD pattern(Reference Willett, Sacks and Trichopoulou7–Reference Serra-Majem, Ribas, Serra-Majem, Aranceta and Mataix12). Olive oil remains a distinctive dietary element of MD, and it may be the only food common to all Mediterranean countries and peoples(Reference Serra-Majem, Ribas, Serra-Majem, Aranceta and Mataix12). The American Dietary Guidelines highlight the positive effect of MD on health and recommend it because it is a source of essential nutrients and useful in the prevention of diseases(13).
The effects of adherence to MD during the gestational period have been previously described(Reference Peraita-Costa, Llopis-González and Perales-Marín14) and associated with lower weight gain during pregnancy(Reference Mariscal-Arcas, Rivas and Monteagudo5,Reference Hillesund, Bere and Haugen15,Reference Silva-del Valle, Sanchez-Villegas and Serra-Majem16) and lower risk of miscarriage(Reference Gaskins, Rich-Edwards and Hauser17–Reference Xu, Wu and Yang19), preterm birth(Reference Mariscal-Arcas, Rivas and Monteagudo5,Reference Saunders, Guldner and Costet20–Reference Chatzi, Rifas-Shiman and Georgiou24) , hypertensive disorders(Reference Schoenaker, Soedamah-Muthu and Callaway25) or gestational diabetes(Reference Karamanos, Thanopoulou and Anastasiou26,Reference He, Yuan and Chen27) . Adherence to MD has also been associated with a lower risk of congenital malformations(Reference Vujkovic, Steegers and Looman28,Reference Botto, Krikov and Carmichael29) , intra-uterine growth restriction(Reference Hillesund, Bere and Haugen15,Reference Saunders, Guldner and Costet20,Reference Mikkelsen, Osterdal and Knudsen21,Reference Helmo, Alves and Moreira23,Reference Chatzi, Rifas-Shiman and Georgiou24,Reference Chatzi, Mendez and Garcia30–Reference Brantsaeter, Birgisdottir and Meltzer32) and long-term effects such as better bone quality(Reference Heppe, Medina-Gomez and Hofman33,Reference Yin, Dwyer and Riley34) , a lower risk of atopy(Reference de Batlle, Garcia-Aymerich and Barraza-Villarreal35,Reference Netting, Middleton and Makrides36) and/or abdominal obesity in children(Reference Saunders, Guldner and Costet20,Reference Mikkelsen, Osterdal and Knudsen21,Reference Helmo, Alves and Moreira23,Reference Chatzi, Rifas-Shiman and Georgiou24,Reference Fernandez-Barres, Romaguera and Valvi37) .
Preterm birth is one of the leading causes of neonatal mortality and morbidity, accounting for nearly 35 % of all neonatal deaths in the United States(Reference Mathews, MacDorman and Thoma38), and can have serious long-term consequences(Reference Allen39–Reference Sun, Mohay and O’Callaghan42). The incidence of preterm birth has remained close to 11 % and with an uncertain aetiology(Reference Martin, Hamilton and Osterman43). Maternal nutrition during pregnancy, as mentioned previously, has an important role in providing the necessary nutrients for fetal growth(Reference Blumfield, Hure and MacDonald-Wicks44). Maternal dietary nutrient intake has not been studied extensively in relation to preterm birth(Reference Carmichael, Yang and Shaw45,Reference Bobiński, Mikulska and Mojska46) . However, previous studies have suggested that certain maternal nutrient intakes may be associated with prematurity as well as low birth weight(Reference Imdad and Bhutta47,Reference Ota, Mori and Middleton48) .
Birth weight is considered the main determinant of perinatal morbidity and mortality(Reference Grisaru-Granovsky, Reichman and Lerner-Geva49), both in the short and long term(Reference Timmermans, Steegers-Theunissen and Vujkovic1,Reference Chatzi, Mendez and Garcia30) . The concept of small-for-gestational-age (SGA) infants takes into account birth weight, gestational age and sex(Reference Schlaudecker, Munoz and Bardají50). Maternal risk factors associated with SGA can be sociodemographic variables, chronic diseases, risk factors during pregnancy and maternal lifestyle risk factors(Reference McCowan and Horgan51). Some factors that are known to increase the risk of SGA are short stature, low weight, Indian or Asian ethnicity, nulliparity, mothers born SGA, cigarette smoking, cocaine use, maternal history of chronic hypertension, renal disease, anti-phospholipid syndrome and malaria(Reference McCowan and Horgan51). Maternal smoking is the single most important risk factor for SGA in developed countries(Reference McCowan and Horgan51). It is significantly correlated with SGA, and while it affects the fetus during all stages of pregnancy, the most significant effect on the birth weight of offspring occurs during late pregnancy, especially in the case of mothers who are heavy smokers (>8–10 cigarettes per d)(Reference Ko, Tsai and Chu52). An inverse correlation between birth weight and number of cigarettes smoked per day has also been found(Reference Ko, Tsai and Chu52). Maternal nutrition is recognised as one of the main determinants of fetal growth(Reference Timmermans, Steegers-Theunissen and Vujkovic1,Reference Chatzi, Mendez and Garcia30) and determines the metabolic patterns of both mother and child(Reference Timmermans, Steegers-Theunissen and Vujkovic1,Reference Chatzi, Mendez and Garcia30,Reference Delnord, Blondel and Zeitlin53–Reference Murphy, Stettler and Smith55) . Restricted fetal growth is associated with an increased risk of childhood morbidity and chronic diseases during adulthood such as respiratory infections, diabetes mellitus, obesity, CVD and psychiatric disorders(Reference Bruno, Faconti and Taddei56,Reference Werner, Savitz and Janevic57) .
Traditionally, nutritional assessment in pregnant women is performed by analysing caloric intake and intake of specific foods along with micro- and macronutrients(Reference Lee, Talegawkar and Merialdi58,Reference de Castro, Freitas Vilela and de Oliveira59) . Most studies published to date that evaluated diet during pregnancy have focused on the association between individual foods or nutrients and fetal growth(Reference Chong, Chia and Colega60). The foods or nutrients associated with a lower risk of SGA and prematurity are diverse depending on the study considered(Reference Grieger, Grzeskowiak and Clifton61–Reference Thompson, Wall and Becroft65). However, nutrition is a multidimensional exposure, and foods, micro- and macronutrients are not consumed in isolation(Reference Kourlaba and Panagiotakos66). For this reason, the effect of diet as a whole or as a dietary pattern should be evaluated. Interactions between foods and nutrients could be overlooked if maternal diet is not assessed as a whole(Reference Sanchez-Villegas, Brito and Doreste-Alonso67), hence there is a growing interest in the study of dietary patterns during pregnancy(Reference Timmermans, Steegers-Theunissen and Vujkovic1,Reference Saunders, Guldner and Costet20,Reference de Castro, Freitas Vilela and de Oliveira59) . Studies focusing on dietary patterns, unlike those focusing on single foods and nutrients, examined the effects of a combination of foods and allowed for interactions between nutrients(Reference Newby and Tucker68,Reference Hu69) . Given this, dietary pattern studies may provide more useful information than an isolated food or nutrient studies.
The main objective of the current study was to evaluate maternal MD adherence during pregnancy and its association with SGA and preterm birth outcomes and their impact. A secondary objective of the current study was to describe the sociodemographic, lifestyle and obstetric profiles of the mothers studied as well as the most relevant paternal and newborn characteristics according to diet adherence.
Materials and methods
Study design
The current study is a two-phase retrospective population-based study of maternal dietary habits during pregnancy and their effects on newborn size and prematurity. The descriptive first phase examined maternal dietary habits during pregnancy along with the maternal sociodemographic, lifestyle and obstetric profiles in a cross-sectional period study. In the second phase, newborn outcomes were evaluated in a nested case–control study.
In the descriptive phase, the characteristics of both parents, clinical and obstetric history of the mother, data related to gestation and delivery, and data on the state of the newborn were analysed. In the analytical phase, the data collected was assessed in relation to mothers’ adherence to MD and the possible association with prematurity and newborn weight.
Level of adherence to MD was the exposure variable, which was assessed using the sixteen-item Kidmed questionnaire(Reference Serra-Majem, Ribas and Ngo6). Women were classified into three groups, depending on Kidmex scores: ≥8 optimal (OA); 4–7 medium (MA) and ≤3 poor (PA) adherence(Reference Serra-Majem, Ribas and Ngo6). Information corresponding to these three groups was obtained before classification, ensuring the homogeneity of the sample and its representativeness as much as possible.
Study population
All mother–child pairs admitted after delivery at the obstetrics ward of the La Fe Hospital in Valencia during a 12-month period starting from January 2018 were assessed for eligibility (n 5208). Mother–child pairs were excluded in a first filter if they did not belong to the health coverage area of this hospital; access to complete clinical records of pregnancy and birth was not available; the mother had a diagnosis of a chronic disease and/or she was not available for interview. Other factors such as multiple births were no reason for exclusion, and the mothers presenting these factors were included. These and other possible confounding factors were taken into account during data analysis. A total of 1446 mothers were subsequently invited to participate. Signed consent was not given by 217 women, and a further 111 women were excluded due to the data corresponding to the newborn were not made available to be consulted and/or the mother’s responses were inconsistent or incomplete. At the end, 1118 provided complete outcome data after signing consent (a participation rate of 77·3 %). Figure 1 details the subject selection process.

Fig. 1 Selection of subjects
Information collected
Data were collected via a review of mothers’ and newborns’ clinical records and complemented by a later direct and personal interview with the mothers. Medical students trained by dietitians conducted the interviews, and the questionnaires were revised by these dietitians. Information on mother’s age, country of origin, education, marital status, employment status during pregnancy, maternal physical activity, maternal diseases, parity and mothers’ cigarette exposure, drug use, alcohol use, coffee intake, caffeine drinks, prenatal vitamins use, dairy products intake during breakfast and daily dairy intake was collected. Weight and height of both parents were taken from clinical records, and BMI was calculated for both parents. Obstetric and neonatal data were obtained from clinical records.
Adherence assessment
Nutritional assessment was carried out using the Spanish version of Kidmed index, which was developed to quickly and easily assess the degree of adherence to MD(Reference Serra-Majem, Ribas and Ngo6). The development of Kidmed index was based on the principles sustaining MD patterns as well as those that undermine it(Reference Serra-Majem, Ribas and Ngo6). The index ranged from 0 to 12 and was based on a sixteen-question test that could be self-administered or conducted during an interview(Reference Serra-Majem, Ribas and Ngo6). Foods positively associated with the MD pattern, such as vegetables, legumes, fruits, nuts, cereal, fish, dairy products and olive oil, were assigned a value of +1, whereas foods with a negative association such as sweets and fast foods were assigned a value of –1 (Fig. 2). The Kidmed test has been successfully used in numerous previous studies(Reference Serra-Majem, Ribas and Ngo6,Reference Peraita-Costa, Llopis-González and Perales-Marín14,Reference Navarro-González, López-Nicolás and Rodríguez-Tadeo70–Reference Dura Trave and Castroviejo Gandarias75) and is a simple and quick way to assess MD adherence insituations where the use of several day-long food journals or extensive FFQ may not be appropriate or practical, as is the present case.

Fig. 2 Kidmed test to assess the Mediterranean diet quality(Reference Newby and Tucker68)
Parental data
Data on lifestyle, dietary habits, sociodemographic and anthropometric characteristics of both parents were collected. The value used for pre-pregnancy weight was the last record available before pregnancy confirmation, and all were dated within the 1 year previous to pregnancy. Total maternal weight gain was taken from clinical records. The interview included a section dedicated to the level of physical activity in leisure time, and another to determine the level of activity during working hours. The first section considered four possible levels of activity, to each of which a score was attributed, from 0 to 3 as follows:
-
0 – no exercise
-
1 – light physical activity (e.g. walking or gentle gymnastics at least one time per week)
-
2 – moderate physical activity (e.g. gymnastics, athletics, swimming, cycling at least two times per week)
-
3 – sports training (at least three times per week)
In relation to physical activity during working hours, similar levels were considered with the following scores:
-
0 – sitting most of the day
-
1 – standing most of the day, without making large displacements or efforts
-
2 – walking, carrying some weight, making frequent trips
-
3 – performing tasks that require a great physical effort
Total physical activity was calculated as the sum of the halves of the scores awarded in both sections. However, for those women who did not work during pregnancy, the free-time exercise accounted for the total of the exercise performed.
Clinical and obstetric historical data of the mother, including data differentiating between primigravida and multigravida women and primiparous and multiparous women, were collected. Information on the number of previous miscarriages was also collected.
Newborn’s data
Information relating to newborns was collected both via interviews and from a review of clinical records. Data included sex and anthropometric values such as weight, height and cephalic perimeter. Newborns were classified as SGA when birth weight was below the 10th percentile(Reference Schlaudecker, Munoz and Bardají50,Reference Sellen76) compared with that expected for the same sex and gestational age according to the Spanish standards(Reference Carrascosa Lezcano, Ferrández Longás and Yeste Fernández77,Reference Carrascosa Lezcano, Fernández García and Fernández Ramos78) , or as LGA when birth weight was above the 90th percentile(Reference Sellen76). Percentiles were calculated using the Gestational Calculator v2017.4 developed by BCNatal (Centre Medicina Maternofetal i Neonatal de Barcelona of the Hospital Clínic i Provincial de Barcelona), which takes into consideration birth weight, gestational age, sex and if the pregnancy single or multiple. Information on birth complications and admission to a neonatal care unit or a neonatal intensive care unit was collected. Apgar scores, heart rate, muscle tone, reflexes and skin colour were recorded(Reference Apgar79–Reference Casey, McIntire and Leveno81) along with the results of a gasometric analysis (pH, PO2, PCO2) on arterial and venous blood of the umbilical cord, which was systematically performed by the participating hospital.
Statistical analysis
Adherence to MD was the primary exposure of interest. For the descriptive part of the study, the outcome of interest was the sociodemographic, lifestyle and obstetric profiles of the mothers and the characteristics of the newborn with special attention being paid to newborn size or prematurity. Normality of distribution was assessed using the Kolmogrov–Smirnov and Shapiro–Wilk tests for the sample as a whole and within each adherence group individually. For quantitative variables, an ANOVA was used for the comparison of different variables in the levels of adherence to maternal MD, while the χ 2 test was used for qualitative variables. With adherence to MD as the independent variable, an analysis to determine the presence of statistically significant differences among the study variables was performed.
With the results obtained in the descriptive phase, a nested case–control study was carried out in a second phase to evaluate SGA v. normal for gestational age and preterm v. term birth depending on the level of adherence to MD (OA v. MA and OA v. PA). The association between adherence to MD and the outcome of interest taking confounders into account was evaluated using multivariate logistic regression models. Apart from crude OR, four adjusted models – one that includes adjustments for all the variables identified as statistically significantly different between the adherence groups and others with different combinations of these variables identified as potential confounding factors – were created. OR, adjusted OR (aOR) and 95 % CI were calculated assuming a 0·05 significance level.
To determine the relationship between adherence to MD and the risk of giving birth to an SGA or preterm newborn, OR was calculated using binary logistic regression models. A conditional multiple logistic regression model was used to obtain aOR in order to account for the effects of several potential confounders simultaneously, using different models in relation with the characteristics identified as significantly different between the adherence groups. The CI applied in all cases was 95 %.
All analyses were performed using IBM SPSS Statistics 22 software, considering P < 0·05 as significance level.
Results
Lifestyle and sociodemographic characteristics of the participants are shown in Table 1. Table 1 presents the degree of adherence to MD among the 1118 pregnant women included in the current study: 14·5 % (n 162) met the criteria of PA, 34·8 % (n 389) met the criteria of MA and 50·7 % (n 567) met the criteria of OA. The overall mean Kidmed score was 7·25 ± 2·40 with a median of 8 and a non-normal distribution displaced to higher values. The mean score of the OA group was 9·18 ± 0·98 with a median of 9 and a range between 8 and 12 and a non-normal distribution displaced to lower values. The mean score of the MA group was 5·81 ± 0·81 with a median of 6 and a range between 4 and 7 and a non-normal distribution displaced to higher values. The mean score of the PA group was 2·13 ± 1·19 with a median of 3 and a range between –2 and 3 and a non-normal distribution displaced to lower values.
Table 1 Maternal sociodemographic data and dietary habits (n 1118 pregnant women)

* P-value obtained by ANOVA (P < 0·05) for quantitative variables, by χ 2 test (P < 0·05) for qualitative variables.
† Caffeinated drinks NOT including coffee such as soft drinks/soda/pop/sugary drinks/fizzy drinks and energy drinks.
‡ (2S)-2-((4-(((2-amino-4-hydroxypteridin-6-yl)methyl)amino)phenyl)formamido)pentanedioic acid.
§ Ferrous fumarate, ferrous gluconate, ferrous succinate and ferrous sulphate.
In the PA group, the women were significantly younger with 46·3 % being under 30 years compared with 28·2 % in the MA group and 18·3 % in the OA group. There were significant differences in marital status and nationality. Those with better adherence were more likely to be married. In the OA group, women of African origin were overrepresented compared with the other two groups. Participants with PA were significantly less educated, 38·2 % of PA had no or primary studies v. 19·5 % in the MA group and 11·8 % in the OA group. Pregnant women with PA were significantly less physically active, less employed and more likely to smoke before and during pregnancy. No differences were observed in maternal drug and alcohol use during pregnancy.
Table 1 also shows the maternal dietary habits according to their adherence to MD. No significant differences were observed for the intake of coffee, tea or chocolate. Caffeinated drinks were consumed more by the PA group. Significant differences were found in folic acid supplement use and Fe supplement use, observing a lower intake in the PA group. No significant differences appeared in the use of prenatal vitamins.
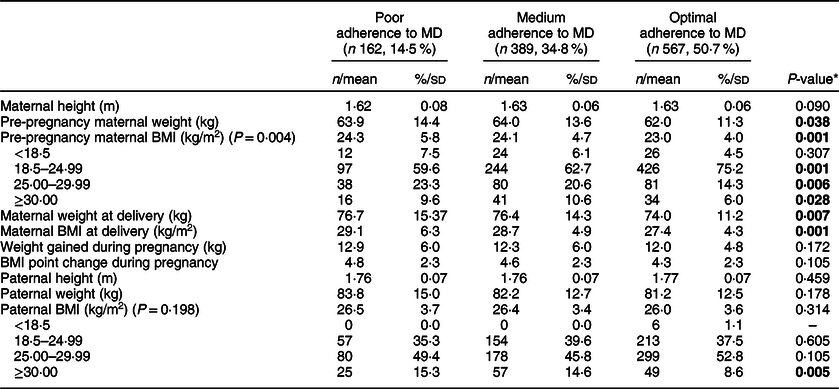
In the current study, statistically significant differences were found for the following maternal anthropometric values: pre-pregnancy weight, pre-pregnancy BMI, weight at delivery, BMI at delivery, but not for weight gained during pregnancy. No differences were found in paternal anthropometric values (Table 2).
Table 2 Parental anthropometric data
* P-value obtained by ANOVA (P < 0·05) for quantitative variables, by χ 2 test (P < 0·05) for qualitative variables.
There were no statistically significant differences in maternal obstetric factors or most characteristics of newborns (Table 3). Differences were found for newborn sex with proportionally more male babies in the MA group and large for gestational age (LGA). 45·1 % of births within the PA group corresponded to male children, while 56·7 % of newborns were male in the MA group and 50·9 % in the OA group. In regard to LGA newborns, the highest percentage was seen in the MA group with 10·3 %, followed by the OA group with 9·2 % and finally the PA group with 6·2 %.
Table 3 Obstetric and neonatal data

SGA = birth weight < 10th percentile; AGA = 10th percentile ≤ birth weight ≤ 90th percentile; LGA = birth weight > 90th percentile.
* P-value obtained by ANOVA (P < 0·05) for quantitative variables, by χ 2 test (P < 0·05) for qualitative variables.
As seen in Table 4, the risk of having SGA newborns for mothers with poor or medium adherence to MD compared with those with OA was non-significant in any of the adjusted models.
Table 4 Risk of small-for-gestational-age or preterm infants associated with the level of adherence to Mediterranean diet

ORc, crude odds ratio; ORa, adjusted odds ratio.
* Adjusted for maternal age, education, physical activity, employment, smoking before and during pregnancy, and BMI before and at the end of pregnancy.
† Adjusted for maternal age, education, physical activity, employment, smoking before and during pregnancy, BMI before and at the end of pregnancy, dietary supplements, folic acid supplements and Fe supplements.
‡ Adjusted for newborn sex, maternal age, education, physical activity, employment, smoking before and during pregnancy, and BMI before and at the end of pregnancy.
§ Adjusted for newborn sex, maternal age, education, physical activity, employment, smoking before and during pregnancy, BMI before and at the end of pregnancy, dietary supplements, folic acid supplements and Fe supplements.
‖ Reference category.
Meanwhile, the risk of having a preterm newborn in mothers with medium adherence to MD compared with those with OA was significant in both adjusted models. It had an aOR of 2·28 (95 % CI 1·10, 4·72) when adjusted for newborn sex, maternal age, education, physical activity, employment, smoking before and during pregnancy, and BMI before and at the end of pregnancy and an aOR of 3·04 (95 % CI 1·35, 6·87) when adjusted for newborn sex, maternal age, education, physical activity, employment, smoking before and during pregnancy, BMI before and at the end of pregnancy, dietary supplements, folic acid supplementation and Fe supplementation.
Discussion
Diet during pregnancy or foetal nutrition is an important health determinant for the newborn, and inadequate maternal nutrition has been linked to adverse pregnancy outcomes and chronic childhood and adult diseases(Reference Shapiro, Kaar and Crume82,Reference Godfrey and Barker83) . Therefore, a varied and balanced diet is essential to ensure infant wellbeing. In the current study, 49·3 % of pregnant women in our area followed an unbalanced diet, specifically a low adherence to MD, which is the traditional and reference dietary pattern in the geographic area studied. The loss of a traditional MD pattern is associated with increased risks of childhood and adulthood diseases(Reference Trichopoulou, Costacou and Bamia9,Reference Kastorini, Milionis and Esposito84,Reference Estruch, Martínez-González and Corella85) . This lack of adequate adherence to MD has been identified to increase the risk of preterm birth. Therefore, optimal adherence to the MD pattern could be considered a potential modifiable protective factor against preterm birth. The current study suggests that perhaps a nutritional intervention programme to improve adherence to MD might reduce the risk of preterm birth associated with poor adherence to MD. Given that it is one of the few modifiable factors in prematurity, further research on the benefits of improving maternal diet should be carried out.
A study reported on the level of adherence among pregnant women in Spain and Greece using another method of classification(Reference Chatzi, Mendez and Garcia30) with results (~44 % PA) similar to the current study. Another study carried out in Spain showed that 36·1 %(Reference Chatzi, Torrent and Romieu86) of mothers followed a low-quality MD during pregnancy when assessed using the MD score(Reference Trichopoulou, Kouris-Blazos and Wahlqvist87). Similarly, another study found that 43·2 % of women from southern Spain did not follow an MD(Reference Mariscal-Arcas, Lopez-Martinez and Granada88).
The finding that younger single women with a lower level of education show worse adherence to MD ratifies the results of numerous previous studies(Reference Timmermans, Steegers-Theunissen and Vujkovic1,Reference Peraita-Costa, Llopis-González and Perales-Marín14,Reference Hillesund, Bere and Haugen15,Reference de Castro, Freitas Vilela and de Oliveira59,Reference Rodriguez-Bernal, Rebagliato and Iniguez64,Reference Leon-Munoz, Guallar-Castillon and Graciani89–Reference Zazpe, Estruch and Toledo91) . A possible explanation to this consistent finding is that women who fall under this profile are less aware of the effect of their diet on their own health and that of their child.
Educational level and employment status can both be indicators of the socioeconomic status, which has been found to be associated with dietary pattern adherence(Reference de Castro, Freitas Vilela and de Oliveira59,Reference Olmedo-Requena, Fernández and Prieto90,Reference Alvarez Alvarez, Aguinaga Ontoso and Marin Fernandez92) . Given that higher-quality products tend to be more expensive, it seems normal that women of lower socioeconomic status would resort to cheaper, lower-quality products, and this could explain the difference found in the current study regarding employment status(Reference Alvarez Alvarez, Aguinaga Ontoso and Marin Fernandez92).
An unhealthy diet has been associated with unhealthy habits such as smoking and a sedentary lifestyle(Reference Timmermans, Steegers-Theunissen and Vujkovic1,Reference Hillesund, Bere and Haugen15,Reference Leon-Munoz, Guallar-Castillon and Graciani89,Reference Olmedo-Requena, Fernández and Prieto90,Reference Hu, Toledo and Diez-Espino93) . In the current study, the PA group had a lower level of physical activity and a higher proportion of smokers both before and during pregnancy, corroborating the findings of previous studies(Reference Timmermans, Steegers-Theunissen and Vujkovic1,Reference Hillesund, Bere and Haugen15,Reference Leon-Munoz, Guallar-Castillon and Graciani89,Reference Hu, Toledo and Diez-Espino93) .
During pregnancy, certain dietary changes are recommended(Reference Okubo, Miyake and Tanaka63,Reference Lawson, LeMasters and Wilson94–Reference Chen, Bell and Browne98) . The WHO guideline on caffeine intake during pregnancy recommends an intake <300 mg/d(99), which was followed by the majority of women in the current study and is in agreement with previous findings(Reference Hillesund, Bere and Haugen15,Reference Chen, Bell and Browne98) . However, there is a significant difference in the intake of caffeinated drinks among the sample, and therefore it is important to monitor this trend due to the high caffeine and sugar content of these drinks(Reference Vartanian, Schwartz and Brownell100).
Micronutrient deficiencies are increasingly common, especially among the women of childbearing age(Reference Mariscal-Arcas, Rivas and Monteagudo5,Reference Black, Victora and Walker101) , and may be exacerbated during gestation by increased nutritional requirements(Reference Haider and Bhutta102,Reference Rodríguez, Méndez and Martínez103) . Some studies have reported a greater contribution of some of these micronutrients (vitamins C, E, B, folate, Mg, Ca, Fe, vitamin D or Zn) associated with MD(Reference Mikkelsen, Osterdal and Knudsen21,Reference Rodriguez-Bernal, Rebagliato and Iniguez64,Reference Feart, Alles and Merle104,Reference Castro-Quezada, Roman-Vinas and Serra-Majem105) .
In a recent review, Haider & Bhutta(Reference Haider and Bhutta102) found the possible benefits of using multivitamins, including folic acid and Fe, as supplementation could perhaps compensate dietary deficiencies. Notably in the current study, women with non-OA consumed significantly more general dietary supplements than women with OA. This seems to suggest that women with a non-OA to MD tend to compensate their poor diet quality with the use of dietary supplements. However, the results showed that the use of supplements had no beneficial effect in this case. When it comes to other types of supplements, women with non-OA were significantly less likely to consume specific Fe supplements and even less likely to consume folic acid supplements. An increase in the use of folic acid and Fe supplements within these groups might positively affect the mothers and newborns.
The only significant differences in parental anthropometric data were found for maternal weight and BMI both before pregnancy and at delivery. The PA group had higher weights and BMI before pregnancy and at delivery. While no differences were observed, it must be noted that the average paternal BMI values would fall into the overweight range in all groups.
The association between parity and adherence to a healthy dietary pattern is unclear as some studies have found worse adherence among primiparous women(Reference Hillesund, Bere and Haugen15,Reference Abreu, Santos and Moreira106) , while other studies associate greater parity to a lower adherence to a healthy dietary pattern(Reference de Castro, Freitas Vilela and de Oliveira59,Reference Northstone, Emmett and Rogers107) . In the sample studied, there was no significant difference in adherence in relation to gravidity or parity. It would be logical, however, to expect multiparous women to follow healthier diets during pregnancy due to the knowledge gained from previous pregnancies. Also, previous childbirths could contribute to a greater adherence to the Mediterranean pattern since more attention might be given to the diet(Reference Alvarez Alvarez, Aguinaga Ontoso and Marin Fernandez92).
As regards the characteristics of newborns, significant differences were found in the sex of newborns and for LGA. Sex of the newborn did not follow a set pattern according to the adherence level. Women with better adherence were noticeably more likely to have an LGA baby. This association between diet quality and size of newborn corroborates the results of previous studies(Reference Timmermans, Steegers-Theunissen and Vujkovic1,Reference Rodriguez-Bernal, Rebagliato and Iniguez64,Reference Ferland and O’Brien108) . The mean scores for 1- and 5-min Apgar test were adequate in the sample studied. In this sense, there are no studies with consistent results relating the Apgar test and the maternal diet during gestation(Reference Gresham, Byles and Bisquera109). The results of gasometric cord blood analysis, which provides an objective measure of foetal condition prior to birth(Reference Alegria and Cerda110), of all adherence groups were within normal ranges, and no studies have been found with which to compare our results.
Previous studies have found that adherence to MD protects against SGA(Reference Peraita-Costa, Llopis-González and Perales-Marín14,Reference Mendez, Plana and Guxens111) , and Mediterranean dietary patterns have been associated with higher birth weights and a lower risk of SGA offspring(Reference Poon, Yeung and Boghossian112). However, one study could not establish a significant relation between maternal diet and the risk of SGA, low birth weight and inadequate infant growth(Reference Schenker, Yang and Perez113).
In the current study, the adjusted models, taking into account the variables identified as statistically significantly different among the adherence groups, found no association between MA or PA and SGA. This lack of association between non-OA and SGA is nuanced and, therefore, must be interpreted carefully. The use of a model adjusted for all variables identified as statistically significantly different among the adherence groups for a sample like ours might introduce errors in results. Dietary patterns are usually accompanied by many other factors that may be important sources of confounding and even more so due to possible synergistic or antagonistic effects(Reference Timmermans, Steegers-Theunissen and Vujkovic1,Reference Hillesund, Bere and Haugen15,Reference Leon-Munoz, Guallar-Castillon and Graciani89,Reference Hu, Toledo and Diez-Espino93,Reference Martinez-Carrasco, Brugarolas and Martinez-Poveda114) . It may be advisable to avoid stating that there is no association between non-OA to MD and SGA newborns given the sample size and evidence from previous studies to the contrary.
Preterm birth is an underlying factor in about half of all deaths among normally formed infants, and survivors often suffer from permanent handicaps(Reference Pless115). Further research is needed as very few causal factors have been identified. In the current study, there were no significant differences in the duration of pregnancy or prematurity, which contrasts with the results of other studies in which adherence to MD was a protective factor against preterm birth(Reference Mariscal-Arcas, Rivas and Monteagudo5,Reference Mikkelsen, Osterdal and Knudsen21) . For the population studied, mothers with OA curiously had a higher percentage of preterm newborns. This could be explained, in part, by older child-bearing age, resorting more to assisted reproduction techniques and having a higher proportion of twins. In the current study, the risk of having a preterm newborn in mothers with medium adherence to MD was significant in both the adjusted models. Meanwhile, none of the models showed a risk for preterm birth within the PA group. Previous studies examining the MD have reached contradictory conclusions regarding prematurity.
A prospective cohort study carried out in Denmark found a decrease in the risk of early preterm birth related to adherence to MD during pregnancy(Reference Mikkelsen, Osterdal and Knudsen21). In the TIMOUN study, adherence to MD was associated with a lower risk of preterm birth only in overweight and obese women(Reference Saunders, Guldner and Costet20). Another study found that preterm risk tended to be lower by around 29 % in the MD group, while the risk of early preterm birth was 72 % lower and statistically significant(Reference Mikkelsen, Osterdal and Knudsen21). An intervention study showed that MD reduced the incidence of preterm birth(Reference Khoury, Henriksen and Christophersen22). Another study supports recent evidence that increasing regular consumption of healthy food is more important than reducing the consumption of unhealthy food(Reference Englund-Ogge, Brantsaeter and Sengpiel116).In a Norwegian cohort study, no association was found between adherence to MD and preterm birth(Reference Haugen, Meltzer and Brantsæter117). In the National Birth Defects Prevention Study, no association with preterm birth was found(Reference Carmichael, Yang and Shaw45). The conflicting study results may be a consequence of variations across studies in the definitions of MD(Reference Martinez-Gonzalez, Holgado and Gibney118,Reference Noah and Truswell119) . Also, the times of diet assessment and data collection vary, which might contribute to the inconsistency of findings. While a comparison with the current study may not be perfect given the differences in the definition of MD and other methodological differences among the studies, it is important to highlight that a loss of healthy eating pattern and its possible effects on newborns is a widespread issue.
Strengths and limitations
A homogeneous selection method within a single hospital is one of the strengths of the current study. All the data were collected by identically trained professionals using the same questionnaire and later contrasted and/or completed with clinical records. Another strength is the evaluation of a diet as a whole by the degree of adherence, which will provide a greater ease in managing dietary patterns in clinical practice(Reference Hillesund, Bere and Haugen15,Reference Sanchez-Villegas, Brito and Doreste-Alonso67) .
It should be noted that the sample size hindered appropriate control for confounders. It is advisable to increase the sample size in future studies to obtain more consistent and reliable results. Maternal and paternal SGA is an important possible confounder when studying SGA outcomes of newborns, which was not considered in the current study as a majority of parents were unable to provide this information. Collection of data after birth could ignore variations in dietary habits during gestation(Reference Saunders, Guldner and Costet20). However, a previous study has found no significant differences in dietary patterns across different periods of pregnancy(Reference Cuco, Fernandez-Ballart and Sala120). Also, data were obtained retrospectively and might have been affected by recall bias.
Previous studies have shown that frequency-of-use questionnaires are appropriate to estimate intake during pregnancy(Reference Silva-del Valle, Sanchez-Villegas and Serra-Majem16,Reference Chatzi, Mendez and Garcia30) . Several studies on pregnant women have adapted the general population indices for the exclusion of alcohol use(Reference de Castro, Freitas Vilela and de Oliveira59,Reference Rodriguez-Bernal, Rebagliato and Iniguez64,Reference Abreu, Santos and Moreira106) . The Kidmed index used here in pregnant women was validated for a population between 2 and 24 years, and meat (red/white) and alcohol are not included(Reference Serra-Majem, Ribas and Ngo6,Reference Štefan, Prosoli and Juranko74) . It may not be as effective as other diet questionnaires in obtaining an accurate picture of adherence to MD for adults given these exclusions. The Kidmed index does not assess intake quantitatively, which could be a problem since previous studies have found that it may be more important to increase the variety of healthy foods than to reduce the regular consumption of unhealthy foods(Reference Michels and Wolk121,Reference Zazpe, Sánchez-Tainta and Toledo122) . However, it was used in an attempt to make dietary assessment as simple as possible for the persons being assessed.
Conclusion
In the population studied, optimal adherence to the MD pattern among pregnant women was about 50 %. Non-OA to MD was associated with a higher risk of a preterm newborn. Optimal adherence to the MD pattern might be a protective factor against preterm newborns. The use of dietary supplements during pregnancy does not combat the negative effects of a poor-quality diet, and their use cannot replace a balanced and diverse diet. Early intervention programmes geared towards pregnant women to raise awareness of a healthy lifestyle and balanced diet would be beneficial. An appropriate nutritional intervention to aid women in reaching OA to MD could reduce the risk attributable to preterm newborn. As other factors responsible for prematurity are not modifiable, it is important to act on those that are modifiable, such as diet, to reduce the risks of preterm birth as much as possible. Further studies are needed to better understand the mechanisms of the effects of diet on SGA newborns, prematurity and the most relevant window of exposure. A further follow-up of this cohort will allow more accurate determination of the effects of adherence to MD and whether these effects persist in older children.
Acknowledgements
Acknowledgements: The authors would like to thank all the mothers who participated in the current study. Financial support: This research received no specific grant from any funding agency, commercial or not-for-profit sectors. Conflict of interest statement: None. Authorship: conceptualisation: I.P.-C., A.L.-G., A.P.M. and M.M.-S.-V.; data curation: A.P.M., J.M.S. and A.L.-M.; formal analysis: I.P.-C., A.L.-G., A.P.M. and M.M.-S.-V.; investigation: A.P.M., J.M.S. and A.L.-M.; methodology: I.P.-C., A.L.-G., A.L.-M. and M.M.-S.-V.; project administration: M.M.-S.-V.; resources: A.P.M. and V.D.; supervision: M.M.-S.-V.; writing – original draft: I.P.-C., A.L.-G., V.D. and M.M.-S.-V.; writing – review and editing: I.P.-C., A.L.-G., A.P.M., V.D., J.M.S., A.L.-M. and M.M.-S.-V. Ethical standards disclosure: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving study participants were approved by the Ethics Committee of La Fe Hospital (CEIC 2014/0116). All participants gave their informed consent, which included a confidentiality agreement according to the Protection of Data of Official Nature Organic Law 15/1999 of December 13.









