Metabolic syndrome is characterised by a low-grade chronic inflammation involving white adipose tissue( Reference Wisse 1 ). Db/db mice show a mutation in the leptin receptor gene and are a model of genetic obesity as well as metabolic syndrome( Reference Palmer, Aurrand-Lions and Contassot 2 ). Previous studies have shown an impairment of the lymphoproliferative response in these obese mice at two months of age, which seems to show a premature immunosenescence. It is well known that with ageing there is an imbalance in the secretion of cytokines( Reference Bruunsgaard, Pedersen and Pedersen 3 , Reference Arranz, Lord and De la Fuente4 ) with higher levels of pro-inflammatory (PI) (such as TNF-alpha and IL-1beta) compared to the anti-inflammatory (AI) (for example, IL-10) cytokines. For this reason, we have investigated the secretion of these cytokines by leucocytes in young obese mice. Female genetically obese mice (db/db), lean heterocygotic mice (db/+) and wild type mice (+/+) fed with a standard diet were used. The peritoneal leucocytes of these animals at two months of age were obtained and maintained in a culture supplied with lipopolysaccharide (LPS) of E.coli. After 24 hours the culture medium was collected and the secretions of IL-10, IL-1beta and TNF-alpha were measured by luminometry. The results showed that the secretions of IL-10 were similar in the three groups studied. However, the secretions of TNF-alpha and IL-1beta were higher (p<0.05 and p<0.01, respectively) in obese mice compared to wild control animals. In conclusion, young obese mice showed high levels of PI cytokines and this early PI/AI imbalance might contribute to the chronic inflammatory status associated with obesity and to the development of premature oxi-inflamm-ageing in these animals.
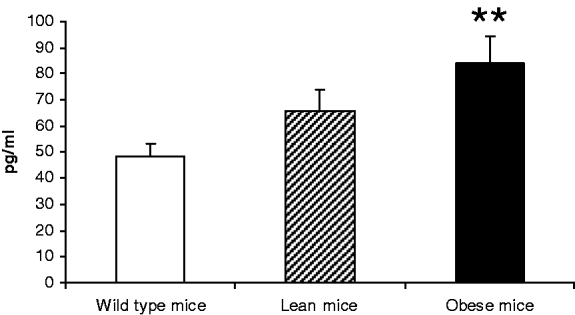
Figure 1. IL-1 BETA.
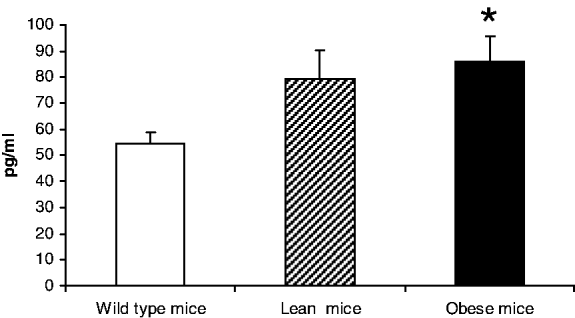
Figure 2. TNF-ALPHA.
Data are expressed as mean±se *p<0.05; **p<0.01 with respect to the Wild type group.
Financial support: MICINN (BFU2011–30336), UCM Research Group (910379ENEROINN) and RETICEF.
(RD06/0013/0003) (ISCIII) of Spain.




