Introduction
Conservationists need to know the distribution of species if they are to monitor populations and initiate management to reverse declines, yet the distributions of rare species are often poorly known. The Slender-billed Curlew Numenius tenuirostris illustrates this latter situation. It is ‘Critically Endangered’, with an estimated population of < 50 individuals (BirdLife International 2015a). The last irrefutable sighting came from Morocco in 1995, in the birds wintering range (Buchanan et al. Reference Buchanan, Crockford and Gretton2010), whilst intensive surveys of passage and wintering areas between 2009 and 2014 have failed to find any birds (Crockford and Buchanan in press). Historical records show that the species formerly wintered across the Mediterranean basin, especially in Italy, and along the Atlantic coast of Morocco (e.g. summaries in Cramp and Simmons Reference Cramp and Simmons1983, Gretton Reference Gretton1991). It appears to have passed through the Caspian and Black Sea areas in spring and autumn (Cramp and Simmons Reference Cramp and Simmons1983, Gretton Reference Gretton1991). The only definite breeding records concern a small number of nests in raised bogs near the village of Krasnoperova c.10 km south of Tara, Omsk province, Russia (55.00°N, 73.35°E), during the first quarter of the 20th century (Ushakov Reference Ushakov1916, Reference Ushakov1925). The habitat in this area was a mix of raised bogs and forest (Ushakov Reference Ushakov1912, Reference Ushakov1916, Reference Ushakov1925), typical of the forest-steppe and hemi-boreal forest ecoregions of Western Siberia (Olson et al. Reference Olson, Dinerstein, Wikramanayake, Burgess, Powell, Underwood, D’Amico, Itoua, Strand, Morrison, Loucks, Allnutt, Ricketts, Kura, Lamoreux, Wettengel, Hedao and Kassem2001). Others (e.g. Belik Reference Belik1994) have suggested the species might breed on the steppe of southern Russia and northern Kazakhstan (the Kazakh steppe ecoregion of Olson et al. Reference Olson, Dinerstein, Wikramanayake, Burgess, Powell, Underwood, D’Amico, Itoua, Strand, Morrison, Loucks, Allnutt, Ricketts, Kura, Lamoreux, Wettengel, Hedao and Kassem2001) or in a broad band encompassing aspects of both habitats (e.g. Kozlova Reference Kozlova1962 in Cramp and Simmons Reference Cramp and Simmons1983). None of the breeding reports, whether putative or confirmed, come from west of the Ural mountains (Gretton Reference Gretton1991). This supports the suggestion of Bannikov (1978, in Cramp and Simmons Reference Cramp and Simmons1983), that the breeding range lay east of the Urals.
The poor knowledge of the breeding areas was identified as a hindrance to the conservation of the species by Gretton (Reference Gretton1991). Even if the species is extinct, the identification of the former breeding range could still help understand the factors that might have contributed to its extinction. Such information could be particularly valuable to the conservation of other Numeniini, a tribe that appears to be under considerable pressure. Of eight other species in the genus Numenius, the Eskimo Curlew Numenius borealis is ‘Critically Endangered, Probably Extinct’ (BirdLife International 2015b), one subspecies of the Whimbrel N. phaeopis alboaxilaris is ‘Critically Endangered’, the Far-eastern Curlew N. madagascariensis is ‘Endangered’, the Bristle-thighed Curlew N. tahitiensis is ‘Vulnerable’ and the Eurasian Curlew N. arquata is ‘Near Threatened’ (BirdLife International 2015b).
The identification of the ranges of poorly known species is clearly difficult. However, the natural geographic variation in stable isotope values, and the subsequent fixing of these isotopes into body tissues, particularly feathers, provides a tool to narrow the search. Stable isotopes are established tools for the geographical assignments of tissues to the locations at which they were synthesised (e.g. Hobson Reference Hobson1999, Hobson and Wassenaar Reference Hobson and Wassenaar2008, Bowen Reference Bowen2010). Isotopes are transferred from the environment (food or water) into body tissues of consumers. These isotopes are fixed into feathers during the discrete period in which they were synthesised (the moult site, or in the case of juvenile plumage, the natal site) (Hobson et al. Reference Hobson, Van Wilgenburg, Wassenaar and Larson2012, Ofukany et al. Reference Ofukany, Hobson and Wassenaar2012). Comparison of the composition of isotopes in feathers with gradients in the environment means geographic assignments can be made (Hobson and Wassenaar Reference Hobson and Wassenaar2008, Bowen et al. Reference Bowen, Liu, Vander Zanden, Zhao and Takahashi2014).
Geographic assignment using stable isotopes requires maps of geographic variation in isotopes across the study areas, or rigorous sampling of potential source populations, which can produce isoscapes by interpolation from tissues collected within an area. While it is possible to produce such detailed isoscapes for well-studied areas (Hobson et al. Reference Hobson, Lormée, Van Wilgenburg, Wassenaar and Boutin2009), it may be less feasible for remote areas from which few animal tissue samples are available. In these cases, large-scale models of stable isotopes values can be derived from models of climate, vegetation type and ‘surrogate’ samples (e.g. West et al. Reference West, Bowen, Dawson and Tu2010). Stable-hydrogen isotope values (δ 2H) are one of the most widely used tools for geographic assignment, and isoscapes of the δ 2H in water are available for the globe through www.waterisotopes.org. It is necessary to calibrate these isoscapes for the tissue samples under consideration, because of fractionation during incorporation into living tissues. This calibration is best done with tissues of the study species (Wunder and Norris Reference Wunder and Norris2008). However, if the study species is rare it may not be possible to use samples of known origin. In these cases, the use of surrogate species might be necessary; this is the case with Slender-billed Curlew, a species for which no feather samples from a known breeding location are available.
Here, we aim to identify the potential breeding area of the Slender-billed Curlew, using stable hydrogen isotope values. We focus on an area between 45°–65°N and 55°–90°E, based on existing literature (Aksakov Reference Aksakov1852, Kozlova Reference Kozlova1962 in Cramp and Simmons Reference Cramp and Simmons1983, Bannikov Reference Bannikov1978 in Cramp and Simmons Reference Cramp and Simmons1983, Gretton Reference Gretton1991, Belik Reference Belik1994). We measured δ 2H in juvenile Slender-billed Curlew feathers and in feather samples from > 200 juveniles of other wader species collected from known locations across the putative breeding range. The δ 2H in the surrogate species were compared to an isoscape to produce a calibration relationship (e.g. Wunder and Norris Reference Wunder and Norris2008). This relationship was then applied to samples from 35 first-year Slender-billed Curlew primary feathers taken from museum skins, to allow us to identify the areas from which feather samples, and hence birds, might have come. As only feathers of juvenile birds were used, these would represent the candidate breeding areas for the species, and represent key areas for future survey effort.
Methods
Feather sampling
Feather samples were obtained from 35 Slender-billed Curlew museum skins that were aged as first-year birds based upon plumage criteria described by Zenatello and Serra (Reference Zenatello and Serra2002). These skins were collected between 1859 and 1944, although not all exact dates were known, and came mainly from countries around the Mediterranean, including North Africa. Samples were taken from the inner part of the sixth primary feather, which is grown on the breeding sites prior to migration. Tissue samples for surrogate species (216 juveniles of the sub-orders Charadrii and Scolopaci) were collected from late May to early July between 2003 and 2005 in Kazakhstan and Russia, between 45°–63°N and 55°–95°E (Figure 1, Table 1).
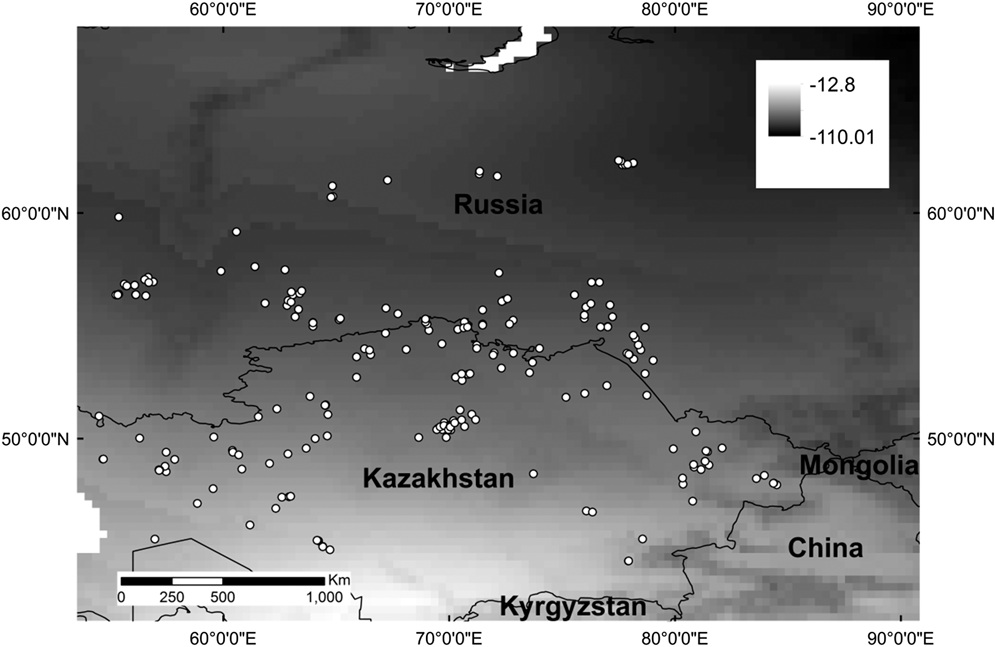
Figure 1. Locations from which the surrogate wader tissue samples used in this study were collected, with δ 2H isoscape from www.isoscapes.net as background.
Table 1. Sample size, mean, minimum and maximum δ 2H values for the samples used in this study (field samples from 14 ‘surrogate’ wader species and museum samples from Slender-billed Curlew).

Stable isotope analysis
Analysis was undertaken by Iso-Analytical Limited, UK. Each feather sample was washed in 0.25M sodium hydroxide solution followed by two separate washes in de-ionised water. The washed feathers were placed in clean screw-top vials in a drying oven at 50°C overnight. After drying, the feathers were clipped into fine sections in the sample vials using surgical scissors. The clipped feather contained sections of 0–2 mm in length.
Prepared feather samples were allowed to equilibrate with laboratory air for three weeks prior to δ 2H analysis. The technique used for this analysis was EA-Pyr-IRMS (elemental analyser pyrolysis isotope ratio mass spectrometry). Samples and references (approximately 1.0 mg) were placed in silver capsules and loaded into an autosampler from which they dropped into a furnace at 1,080 °C and thermally decomposed to H2 and CO over glassy carbon (Kelly et al. Reference Kelly, Parker, Sharman and Dennis1998). Any traces of water produced were removed by magnesium perchlorate, while any traces of CO2 formed were removed via a Carbosorb™ trap. The reference material used was IA-R002 (Mineral Oil “B”) with a δ 2HV-SMOW value of -111.2 ‰, and IAEA-CH-7 (polyethylene foil, δ 2HV-SMOW = +100.3 ‰). IA-R002 is calibrated against NBS-22 (mineral oil) distributed as an isotope reference standard by the International Atomic Energy Agency. NBS-22 has a δ 2HV-SMOW value of -118.5 ‰. Feather samples were analysed with 20% duplication, where possible. Samples of IA-R002, and NBS-22 were analysed along with the feather samples as quality control checks.
Data analysis
Feathers contain both exchangeable and non-exchangeable fractions of H (Wassenaar and Hobson Reference Wassenaar and Hobson2003), but only the non-exchangeable portion is of interest. We attempted to adjust values for the proportion of exchangeable H by analysing BWB-II standard reference material (exchangeable δ 2H = –108 ± 4 ‰). But, as this was a single-point calibration, we used surrogate wader species of known origin to determine the δ 2H feather-precipitation relationship (Hobson et al. Reference Hobson, Van Wilgenburg, Wassenaar and Larson2012, Meier-Augenstein et al. Reference Meier-Augenstein, Hobson and Wassenaar2013).
δ 2H values from the www.waterisotopes.org isoscape map (Bowen et al. Reference Bowen, Liu, Vander Zanden, Zhao and Takahashi2014) were extracted at the spatial locations from which surrogate species samples were collected (using ArcMap 10.1). We used the growing season precipitation δ 2H map, as the growing season will coincide with the bird breeding season. A general linear model (with normal error structure), in which growing season precipitation δ 2H was the dependent variable, and the surrogate feather samples values the explanatory variable, was fitted (Hobson et al. Reference Hobson, Van Wilgenburg, Wassenaar and Larson2012). Analysis was conducted using PROC GENMOD in SAS 9.1 (SAS Institute 2003). The output parameter estimates were used to convert all juvenile Slender-billed Curlew feather δ2H to “mapped” δ 2H values, with the 95% confidence intervals around these, using the “predict” command. These 95% confidence intervals were compared to those in the δ2H from the isoscape map. Values of δ2H from the isoscape map that fell within the 95% confidence intervals were identified in turn for each of the sampled 35 Slender-billed Curlews, producing a binary map (1 for within the 95% CI, 0 for outside) for each individual. These were overlaid in ArcMap 10.1 to summarise the number of birds that were assigned to each location.
Results
Growing season precipitation δ 2H values explained a significant amount of variance in the surrogate wader feather δ 2H values (χ21 = 14.82, P < 0.0001). In other words, the resultant equation linking the two sets of values (δ2Hprecipitation = 0.34 ± 0.08 δ2Hfeather -37.56 ± 8.44) can be used to predict the probability of each juvenile Slender-billed Curlew originating in different parts of the study area. Doing so placed the majority of the 35 juvenile Slender-billed Curlews in a band that spans the Kazakh forest-steppe and Kazakh steppe ecoregions (Figure 2). The area involved is longitudinally extensive, but narrow in terms of latitude. Over 1 million km2 were suggested as the possible source of two or more juveniles. The area identified as the source of 10 or more was around 200,000 km2. The confirmed breeding site is situated within the predicted potential breeding range, but the potential breeding area is centred much further south in the Kazakh steppe ecoregion, and overlaps with the locations of almost all May to July records of Slender-billed Curlew (Figure 2). It also extends eastwards into the Altai steppe and semi-desert (Figure 2). Another area, at the north of the Ural Mountains was also identified as the potential source of a some of the sampled birds.
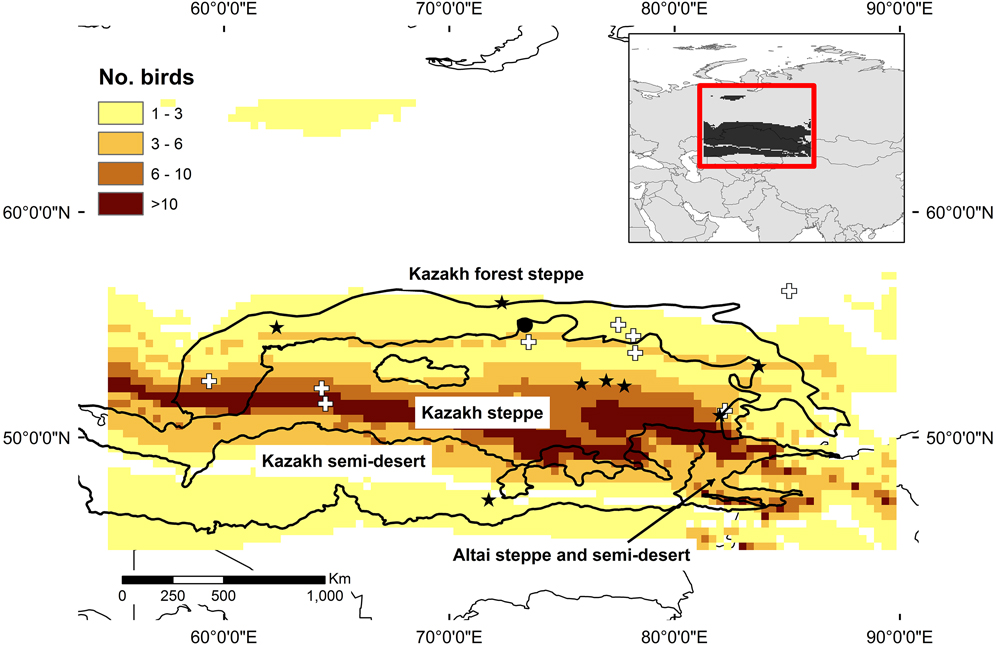
Figure 2. Potential breeding areas of Slender-billed Curlew, based on the number of juvenile birds assigned to cells based on δ2H values from feather samples in comparison to an isoscape. Stars represent locations of sightings of birds in the study region between May and July, while crosses indicate birds shot in these months. Broken lines indicate boundaries of ecoregions from Olsen et al. (Reference Olson, Dinerstein, Wikramanayake, Burgess, Powell, Underwood, D’Amico, Itoua, Strand, Morrison, Loucks, Allnutt, Ricketts, Kura, Lamoreux, Wettengel, Hedao and Kassem2001). A filled circle indicates the only known nesting site.
Discussion
Stable isotope values in feather samples have been used previously with success to identify putative ranges of species (e.g. Hobson and Wassenaar Reference Hobson and Wassenaar2008). Here, through the application of this approach to samples from Slender-billed Curlew museum specimens, we identified a rather narrow band stretching across southern and northern Kazakhstan as the putative breeding area for the species. The latitudinal narrowness of this area might indicate that the Slender-billed Curlew was restricted by some factor that covaries with latitude, perhaps indicating some degree of specialisation in the species. The identified area overlapped the southern edge of the Kazakh forest-steppe ecoregion, but the majority fell within the Kazakh steppe ecoregion (following the Olson et al. Reference Olson, Dinerstein, Wikramanayake, Burgess, Powell, Underwood, D’Amico, Itoua, Strand, Morrison, Loucks, Allnutt, Ricketts, Kura, Lamoreux, Wettengel, Hedao and Kassem2001 categorisation). The area we identified extends northwards into the area around Tara, from which the only confirmed records of nesting come (Ushakov Reference Ushakov1925). However, Tara falls at the northern edge of the area (and in an area of low predicted occurrence probability), suggesting a breeding range was not centred on the Omsk area. The area to the south of Omsk province in Kazakhstan, which is identified as the potential breeding area for most birds, coincides with the area that has previously been suggested as the main breeding zone for the species by other authors (Kozlova Reference Kozlova1962 in Cramp and Simmons Reference Cramp and Simmons1983, Belik Reference Belik1994). It reaches as far east as Zmeinogorsk in Russia (51.2°N, 82.2°E), from which there are possible nesting records (Gretton Reference Gretton1991).
The analysis suggests that the species was more of a steppe species than a forest-steppe species. The band of potential breeding could continue to the west of the Ural Mountains, but we used 55°E to define our study area, based on previous analysis and opinion on the breeding range of the species (e.g. Aksakov Reference Aksakov1852, Kozlova Reference Kozlova1962 in Cramp and Simmons Reference Cramp and Simmons1983, Bannikov Reference Bannikov1978 in Cramp and Simmons Reference Cramp and Simmons1983, Gretton Reference Gretton1991, Belik Reference Belik1994). Areas to the east of Kazakhstan into the Russian Altai were also identified as candidate breeding areas. There are breeding claims from this area, including on the edge of the Altai Mountains, which Gretton (Reference Gretton1991) placed under the heading of “Doubtful nesting records”.
Given the likely imminent extinction of the Slender-billed Curlew, there is a pressing need to locate actual breeding sites of the Slender-billed Curlew, and ground-truth our predictions. The area around Tara has been searched without success in 1989, 1990 and 1994 (Boere and Yurlov Reference Boere and Yurlov1998, Gretton et al. Reference Gretton, Yurlov and Boere2002). These field surveys did note that much of this area immediately around the breeding site at Krasnoperova has remained largely untouched although much of the surroundings has been converted to agriculture. This might indicate that there are pockets of suitable habitat remaining, albeit within a matrix of agriculture. The impact that this conversion would have had on the Slender-billed Curlew is unknown, but some have suggested that it is not loss of this habitat that has caused the decline of the species (Boere and Yurlov Reference Boere and Yurlov1998). Such conversion might have opened up the breeding areas to predatory species that are associated with agricultural landscapes. Problem native species were identified as having a major impact on other species in the Numeniini tribe (Pearce-Higgins et al. Reference Pearce-Higgins, Brown, Douglas, Alves, Belliograzia, Bocher, Buchanan, Clay, Conklin, Crockford, Dann, Alts, Friis, Fuller, Gill, Gosbell, Johnson, Marquez-Ferrando, Masero, Melville, Millington, Minton, Mundkur, Nol, Pehlak, Piersma, Robin, Rodgers, Ruthrauff, Senner, Shah, Sheldon, Solovjev, Tomkovich and Verkuil2017). However, the majority of Slender-billed Curlew records are from an area further south. The potential breeding area is extensive, making the task of finding what is likely to be only a small number of breeding individuals at best extremely difficult. Conversely, previous surveys could have easily overlooked a breeding population.
The example of another species breeding on the Eurasian steppes, the Sociable Lapwing Vanellus gregarius might indicate that remnant Slender-billed Curlews remain undiscovered. Around the year 2000, the world population of Sociable Lapwings was thought to be around 200–600 birds (Kamp et al. Reference Kamp, Sheldon, Koshkin, Donald and Biedermann2009). Following extensive field surveys across the steppe belt of Kazakhstan both during the breeding season and autumn migration (Figure S1 in the online supplementary material), a reassessment indicated that the global population of the species might stand perhaps two orders order of magnitude larger (Sheldon et al. Reference Sheldon, Grishina, Kamp, Khrokov, Knight and Koshkin2006). Unfortunately, observers on neither the mentioned surveys for Sociable Lapwings, nor those to collect surrogate tissue samples for this study encountered a Slender-billed Curlew.
Extensive areas across the Eurasian steppes region from Ukraine through to the Altai Mountains were converted to arable land during the 18th and 19th centuries (Wesche et al. Reference Wesche, Ambarli, Török, Kamp, Treiber and Dengler2016). In Kazakhstan, 25.4 million ha were ploughed in the steppe belt when large-scale wheat farming was introduced during Khrushchev’s ‘Virgin Land Campaign’ between 1953 and 1960 (Durgin Reference Durgin1962). Changes in agricultural practice in this area have been linked to declines in many steppe bird species (Kamp et al. Reference Kamp, Urazaliev, Donald and Hölzel2011). These could have been responsible for declines in Slender-billed Curlews, if the Eurasian steppes were the core breeding areas. The areas identified in this study as having the highest probability of being within the breeding range of the species are largely situated within the northern steppe areas of Kazakhstan, the most productive steppe type that was nearly entirely ploughed for arable farming (Wesche et al. Reference Wesche, Ambarli, Török, Kamp, Treiber and Dengler2016). The natural steppes that remain are nearly entirely situated on poorer soils, and therefore show different vegetation to those areas that were lost during the 1950s (J. Kamp unpubl. data). The loss of natural grasslands, albeit on passage rather than breeding areas, has been implicated as one of the potential drivers in the (probable) extinction of the Eskimo Curlew (Gollop et al. Reference Gollop, Barry and Iversen1986, Gill et al. Reference Gill, Canevari, Iversen, Poole and Gill1998), perhaps through the extinction of the orthopteran Melanopus spretus (Rocky Mountain locust), the eggs and young of which were an important prey item for the curlews (Gill et al. Reference Gill, Canevari, Iversen, Poole and Gill1998).
However, so little is known about Slender-billed Curlew populations that it is difficult to determine whether agricultural expansion in Kazakhstan contributed to its decline. Ushakov (Reference Ushakov1925) suggested the species was already in decline around the 1920s, based on nest searches in the area around Tara. By the 1930s other authors had noticed that the species was declining based on records during passage and in winter, raising the first concerns over the long-term future of the species (Stresemann and Grote Reference Stresemann and Grote1943). A decline at this time would be prior to the major cropland expansion further south in the potential breeding range of the species.
Five of the eight species of Numenius are of conservation concern on the IUCN Red List (BirdLife International 2015b). The loss of natural habitats has been identified as the major threat to the Numeniini tribe (BirdLife International 2015b), especially in the East Atlantic flyway (Pearce-Higgins et al. Reference Pearce-Higgins, Brown, Douglas, Alves, Belliograzia, Bocher, Buchanan, Clay, Conklin, Crockford, Dann, Alts, Friis, Fuller, Gill, Gosbell, Johnson, Marquez-Ferrando, Masero, Melville, Millington, Minton, Mundkur, Nol, Pehlak, Piersma, Robin, Rodgers, Ruthrauff, Senner, Shah, Sheldon, Solovjev, Tomkovich and Verkuil2017). It remains unknown what the contribution of habitat loss was to the decline of Eskimo Curlew and Slender-billed Curlew populations. Hunting may well have played a part in the decline of Eskimo Curlew (Gill et al. Reference Gill, Canevari, Iversen, Poole and Gill1998), and sufficiently large number of Slender-billed Curlew were shot for hunting to be cited as a major cause of decline (Gretton Reference Gretton1991, Donald et al. Reference Donald, Collar, Marsden and Pain2010). If, as suggested here, the main breeding areas were the steppes of Kazakhstan, rather than the taiga and forest steppe bogs of southern Russia, then it is possible that agricultural development played a part in the decline of the species as well. A combination of pressures from habitat loss or changes in predation pressure associated with land cover change on the breeding areas and hunting on non-breeding areas could have had a major impact on the species. Irrespective of the historical drivers of population declines, the priority action must be to identify undisturbed areas within the predicted breeding range (e.g. by comparison with high resolution satellite images) and search these in the field to determine if the species still occurs in this area.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270916000551
Acknowledgements
We are indebted to the following museums and collections for kindly allowing us to take samples from their skins: University Museum of Zoology, Cambridge, UK, Zoological Museum, University of Copenhagen, Denmark, National Museums of Scotland, Edinburgh, UK Museum of Natural History of the University of Florence, Italy, Ornithological Museum “F. Foschi”, Forli, Italy, Museum of the National Wildlife Institute (INFS), Bologna, Italy, World Museum, Liverpool, UK, Manchester Museum, Manchester, UK, Great North Museum: Hancock, Newcastle, UK, State Museum for Nature and Man, Oldenburg, Germany, National Museum of Natural History, Paris, France, Natural History Museum, Pisa, Italy, Civic Museum of Zoology, Rome, Italy, Natural History Museum of the Siena Academy of Sciences, Siena, Italy, Ulster Museum, National Museums Northern Ireland, and Natural History Museum, Vienna, Austria. The Association for the Conservation of Biodiversity of Kazakhstan (ACBK) organised the Kazakhstan surveys which were undertaken by V. Khrokov, E. Bragin, A. Koschkin and V. Vilkov. Surveys in Russia were organised by the Russian Bird Conservation Union (RBCU) and we thank A. Kuzmitch, G. Boiko, V. Tarasov, Riabitsev, S.V. Kornev, A.I. Shepel, V. Belik and A.A. Yemtsev. We thank G. Tyler and M. Brombacher for fieldwork support, advice and coordination and also U. Gallo-Orsi, K. Smith, N. Baccetti, and M. Zenatello for advice and comment. We thank A. Gretton and an anonymous referee for comments on an earlier version of this manuscript.





