Introduction
HIV pre-exposure prophylaxis provides a new opportunity to address the HIV epidemic in the United States. There were over 227,000 persons using PrEP in 2019,Reference Sullivan, Woodyatt, Koski, Pembleton, McGuinness, Taussig, Ricca, Luisi, Mokotoff, Benbow, Castel, Do, Valdiserri, Bradley, Jaggi, O’Farrell, Filipowicz, Siegler, Curran and Sanchez 1 yet there remains substantial opportunity for improvement with CDC estimating 1.1 million persons indicated for PrEP.Reference Smith, Van Handel and Grey 2 Moreover, PrEP scale-up has not been in accordance with population need for HIV protection, with lower PrEP uptake among Black and Latino gay/bisexual men, women, and Black transgender women than their epidemic burden.Reference Siegler, Mehta and Mouhanna 3
Developing structural support for individuals to practice healthy behaviors is a foundation of public health practice. Clinically-based prevention interventions, such as preventive medications or disease screenings, are often underutilized. This is understandable, especially when people do not have symptoms, when the benefits of prevention behaviors are dependent on an unknown future probability of disease acquisition, and when there are substantial financial costs of clinical prevention services.
The need to optimize promotion of clinical prevention interventions drove the creation of the US Preventive Services Task Force in 1984, an independent panel that reviews and grades evidence for clinical prevention services.Reference Bauchner, Fontanarosa and Golub 4 PrEP received the highest grade (A) from this panel, and the Affordable Care Act requires prevention services with this grade be covered with no cost-sharing (e.g., fees) by group health plans and health insurance issuers. This same guidance on coverage is also implemented for Medicaid expansion programs. Critically, guidance issued by the Departments of Health and Human Services (HHS) issued in 2021 clarified that coverage of PrEP services not only include the cost of medication, but also the cost of ongoing services requisite to care including quarterly clinician visits, required laboratory testing including for HIV and STD, and medication adherence counseling. 5
Coverage of laboratory and clinician costs removes a central barrier to PrEP care, because laboratory costs of PrEP are high. Using the laboratory fee reimbursement schedule from CMS 6 and CDC guidance 7 regarding requisite labs for PrEP care among gay, bisexual, and other men who have sex with men, we found that a standard first-year set of PrEP laboratory tests was $1,013.10 (Appendix 1). This does not include the cost of four annual clinical visits, which adds substantial additional costs.
PrEP Laboratory Services Coverage Gap
Despite the supportive USPSTF recommendation for PrEP, the no cost-sharing benefit does not apply to all persons in the United States. There remain substantial gaps that leave some of the persons hoping to use PrEP with substantial out-of-pocket costs, discouraging PrEP seeking and maintenance in care.
There are many ways to cover PrEP laboratory service gaps faced by many people in need of prevention services. We suggest developing a two-track system — in-person and telehealth care — that would allow the most users to initiate and remain on PrEP. Moreover, we do not anticipate that covering both options would increase the per user cost of the system; instead, telehealth services may potentially be cost-saving given that both lab and facility costs may be lower for telemedicine. Implementation science frameworks and change strategies should be incorporated into a national system to cover PrEP laboratory services.
For up to 48.9% of the US population, the ACA’s preventive services coverage and cost-sharing requirements are not federally required to be followed. Plans not federally required to follow CMS guidance include grandfathered healthcare plans (23.7 million persons),Reference Keit 8 traditional, non-expansion Medicaid (59.8 million), 9 Medicare Part D (48.0 million),Reference Cubanski and Damico 10 and uninsured persons (31.2 million)Reference Cohen, Terlizzi, Cha and Martinez 11 (Appendix 2).
State Medicaid plans cover the cost of PrEP medication, but states may cover ancillary laboratory and clinician services at varying levels, 12 resulting in a panoply of coverage rules. To our knowledge, there is no systematic source of data regarding which state Medicaid programs cover which services for PrEP care. Moreover, there is no systematic source of data regarding no-cost coverage for persons in grandfathered plans and for uninsured persons. In sum, no-cost sharing coverage is sufficiently fragmented and complex across this 48.9% of the US population that no systematic documentation of coverage has been made available to date.
An array of programs have been developed to fill gaps in PrEP ancillary services and drug coverage, including drug manufacturer assistance programs, state PrEP assistance programs, and usage of 340b drug program returns for service subsidy. There may be differential access to these services by place, program eligibility, and over time (e.g., as generics become more common, manufacturer programs may change). This changing landscape of reimbursement makes navigating these different coverage rules incredibly challenging. In fact, most large PrEP prescribers have dedicated “navigators” to help patients seek coverage for their PrEP care across the various sources of funding.
This patchwork system leads to considerable uncertainty for healthcare consumers and providers, likely deterring consumers from seeking PrEP and providers from prescribing it. In fact, PrEP usage is 99% greater in states that have expanded Medicaid and provide PrEP services coverage through state-level fee coverage programs that frequently feature PrEP navigation. 13 Moreover, some of these sources of coverage might not be sustainable over time, potentially leading to future unexpected disruptions in PrEP care and lapses in protection from HIV infection.
The complexity of service coverage is even higher with the recent FDA approval of injectable cabotegravir. 14 CDC’s updated clinician guidance on laboratory testing and eligibility assessment includes injectable cabotegravir, 15 but how the medication and surrounding laboratory services will be covered by insurers is currently unclear. USPSTF has released a draft of systematic review questions that includes assessment of the benefits of injectable cabotegravir and other new PrEP regimens.
A national PrEP program, such as that proposed by Killelea and colleagues, could fill in PrEP laboratory coverage gaps, ensuring costs do not prevent those at risk from seeking PrEP care. Moreover, such a system could incorporate considerations of new PrEP regimens as they come to market. A recent cost-effectiveness analysis found that when PrEP drug costs are low, PrEP is a cost-saving intervention.Reference McKenney, Chen and Hoover 16 This makes a national program a particularly attractive idea, because a likely outcome of such a program would be use of generic PrEP formulations that have low costs. Below, we detail a number of options for covering laboratory services nationally, using an implementation science framework to consider how the program might be developed and evaluated.
Laboratory PrEP Program
A national PrEP services program, such as that proposed by Killelea and colleagues, will require laboratory support services that accommodate an array of patients and providers. We detail a patient-centered national service to cover PrEP laboratory services, proposing complementary models of in-person and telemedicine services to facilitate numerous PrEP access avenues. Using the Expert Recommendations for Implementing Change (ERIC) strategies,Reference Powell, Waltz and Chinman 17 structures needed to support a national program are explored in Table 1.
Table 1 National PrEP Coverage Plan and ERIC Implementation Science Strategies to Facilitate Program Success

Standard care laboratory services could be covered with direct contracts with major laboratory services providers, or with a standard series of reimbursement payments for eligible laboratories. ERIC strategies to enhance provider prescription practices should include arrangements to ease billing by using existing channels and monitoring of service provision quality through ongoing surveys of patients and stakeholder interviews with providers to ensure services minimize provider burden. Audits should use PrEP utilization metrics and targets that feature not only assessments of overall use, but use according to equity. The PrEP-to-need ratioReference Siegler, Mouhanna and Giler 18 and other similar metrics allow for assessment PrEP equity relative to HIV epidemic burden. Such metrics have previously been used to demonstrate disparities in PrEP receipt among women,Reference Siegler, Bratcher, Weiss, Mouhanna, Ahlschlager and Sullivan 19 and geographic areas with lower income or higher concentrations of Black residents.Reference Siegler, Bratcher and Weiss 20 Educational outreach to patients, providers, and PrEP navigators will facilitate increased use of the national program.
Telehealth laboratory services to promote PrEP are critical to provide primarily due to the high patient burden of four in-person clinic/lab visits per year, and to optimally serve those in need of PrEP who may be distant from PrEP providers such as those in rural areas. 21 In ERIC terms, changing the default service site of PrEP care to one that is more convenient may improve both PrEP initiation and maintenance in care. CDC recommends for daily oral PrEP quarterly laboratory services to include HIV testing, 3-site gonorrhea/chlamydia testing (rectal, oral, urethral), and periodic creatinine monitoring. 22 All are conducted with traditional laboratory testing, and are achievable with self-collection of specimens at home and mailing those specimens to laboratories for testing. These tests are currently being run by a lab under CLIA-based validation protocols,Reference Siegler, Mayer and Liu 23 a procedure recommended by the CDC as a standard of care during the COVID-19 pandemic. 24
In our studies of home care for PrEP, participants have successfully self-collected specimens for each of these tests, meeting CLIA validation performance for laboratory developed tests and with the procedure receiving high acceptability ratings from persons in PrEP care. We anticipate that remote testing would not add costs beyond standard laboratory testing; in our experience, the costs of remote laboratory testing for PrEP, including mailing and materials, are lower than standard laboratory fees.
ERIC strategies provide a number of insights into how to make a national telehealth laboratory program successful. To optimize telehealth benefits, online clinician services should be covered for persons otherwise facing a PrEP gap. A centralized program website could facilitate PrEP care initiation with referral to online providers that meet program and state medical licensing requirements. Systems to facilitate Electronic Health Records (EHR) transfer, from centralized mail-in laboratories to recipient clinics, would assist scale-up of telemedicine at traditional clinics that are interested in participating in the program.
Audits should ensure that telehealth services are being provided to the same standard as in-person PrEP services, and should incorporate equity metrics to ensure program scale-up to communities most in-need of PrEP care to optimize the societal prevention impact of PrEP provision.
Discussion
There are many ways to cover PrEP laboratory service gaps faced by many people in need of prevention services. We suggest developing a two-track system — in-person and telehealth care — that would allow the most users to initiate and remain on PrEP. Moreover, we do not anticipate that covering both options would increase the per user cost of the system; instead, telehealth services may potentially be cost-saving given that both lab and facility costs may be lower for telemedicine. Implementation science frameworks and change strategies should be incorporated into a national system to cover PrEP laboratory services.
Future discussions and development of a national PrEP coverage system should consider these concepts and be informed by appropriate frameworks for implementation science to ensure a thorough exploration that seeks to minimize patient-level barriers to accessing PrEP.
Notes
The research reported in this publication was supported by the National Institute of Mental Health R01MH114692 and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH (U19HD089881), under ATN protocol 159. The research was facilitated by the Emory Center for AIDS Research (P30AI050409). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendices
Appendix 1 Cost of First Year of Laboratory Testing Services for HIV Pre-exposure Prophylaxis
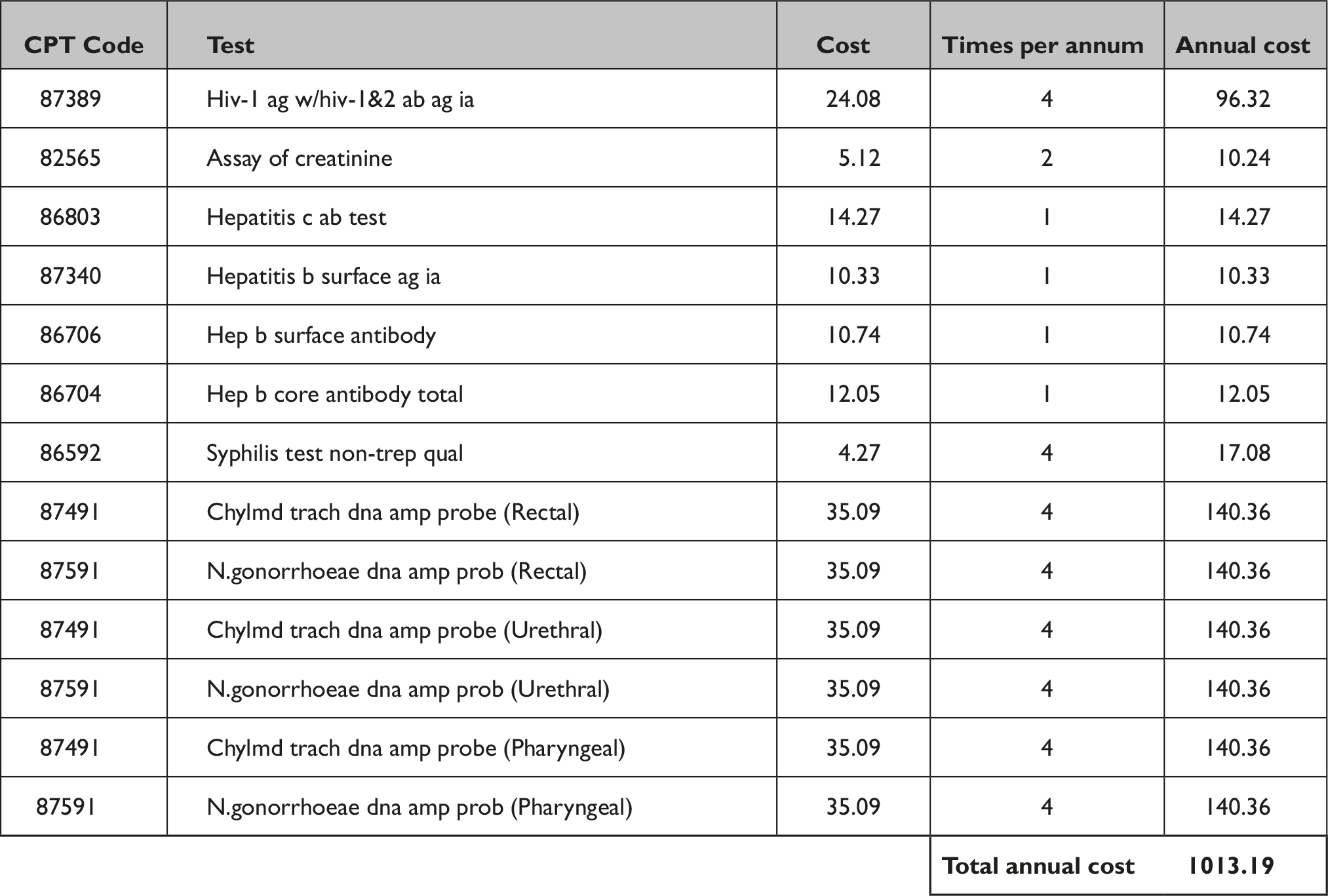
Source: CMS 2021 Clinical Diagnostic Laboratory Fee Schedule.
Appendix 2 Persons Not Covered by USPSTF Rules against Cost Sharing
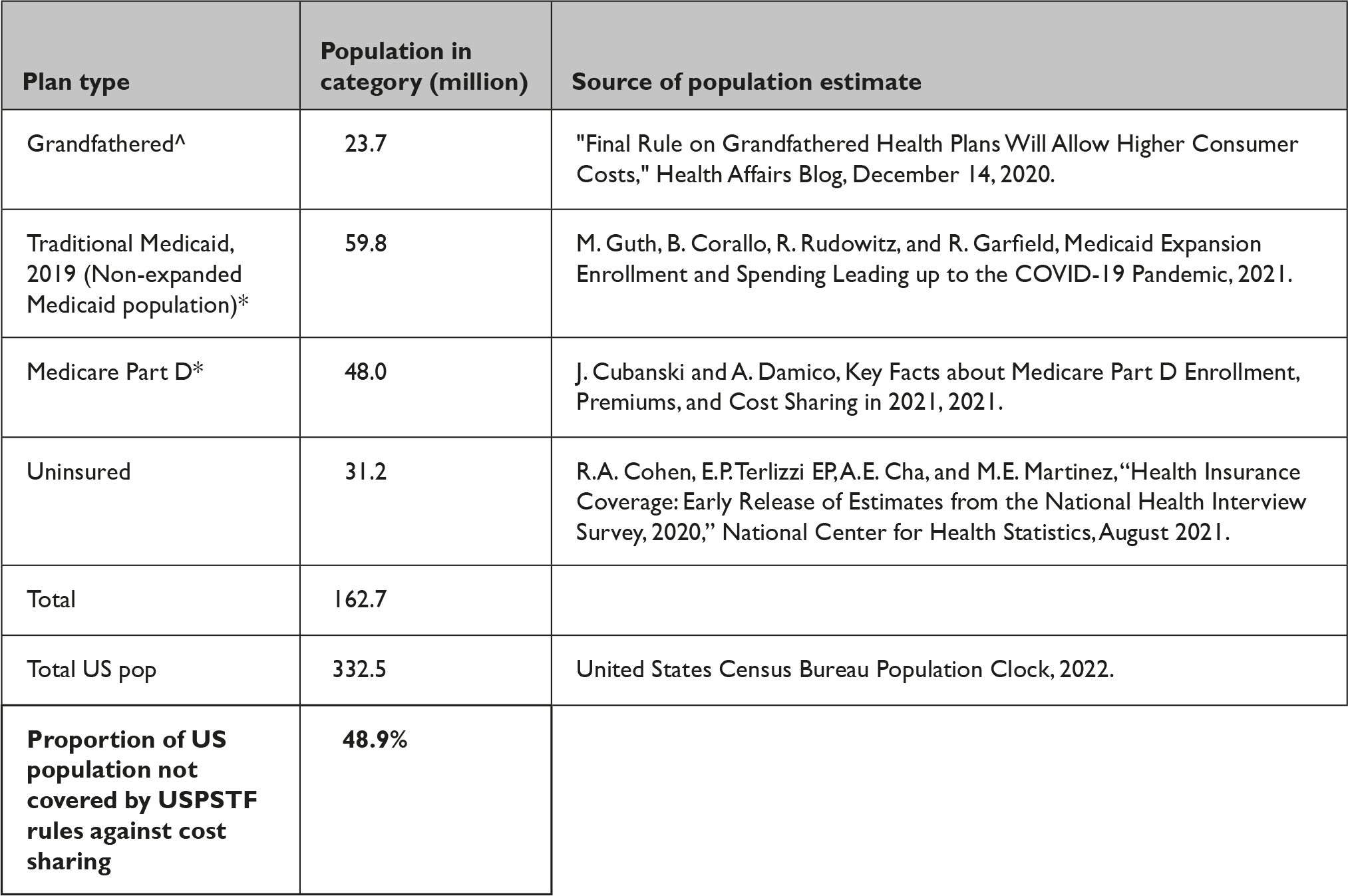
Notes:





