People with dementia are frequently admitted to an acute hospital. In the UK, at any given time, around 6% of people with dementia are in-patients in general hospitals, compared with approximately 0.6% of over-65s without dementia. Reference Russ, Shenkin, Reynish, Ryan, Anderson and Maclullich1 People with dementia are admitted to hospital two to three times more often than people of the same age without dementia. Reference Maslow, Silverstein and Maslow2 In the USA, admissions to hospital for people over the age of 85 years with dementia increased from 700 000 in 2000 to 1.2 million in 2008. Dementia is particularly common in patients in acute medical wards, with prevalences in this setting ranging between 40 and 43% in the UK, Italy and Switzerland. Reference Sampson, Blanchard, Jones, Tookman and King3-Reference Zekry, Herrmann, Grandjean, Meynet, Michel and Gold5 Studies from a range of countries have demonstrated how people with dementia in an acute hospital are at increased risk of mortality and adverse events, functional decline during their stay, higher risk of being discharged into care homes and longer length of stay. Reference Mukadam and Sampson6 In the UK, there have been increasing concerns about the care that frail older people receive when they are admitted to acute hospitals. Reference Francis7
The term ‘behavioural and psychological symptoms of dementia’ (BPSD) encompasses a range of symptoms including agitation, aggression, delusions, hallucinations, depression and apathy. These symptoms are common, multifactorial in origin and probably secondary to complex interactions between the severity of dementia, the environment and other illness. They are distressing for people with dementia and those who care for them. Family caregivers have given rich reports about how BPSD may worsen during hospital admission and how acute hospital staff struggle to adequately manage these, 8 however, we have little information on how common behavioural and psychiatric symptoms are in this setting. This is essential if we are to develop and evaluate management strategies for BPSD in the acute hospital, in particular effective non-pharmacological interventions, and to better justify the necessity of liaison psychiatry services within this setting. Our principal aim in this study was to examine the prevalence of BPSD in older people with unplanned medical admission to hospital. Our specific objectives were to (a) describe the prevalence and subtypes of BPSD in this population, and (b) examine the patient characteristics associated with BPSD. Our secondary aim was to explore associations between BPSD (including subtypes) and quality of care, length of stay, adverse events, discharge destination, mortality and costs of the hospital admission.
Method
Setting
For this longitudinal cohort study we recruited from two acute hospitals in London, UK. Both cover a large area encompassing socioeconomic and ethnic diversity, serving a population of two million people from six primary care trusts (healthcare commissioning bodies) and four mental health trusts. The hospitals have differing strengths and weaknesses in terms of their Care Quality Commission ratings and are at different stages of implementing the English National Dementia Strategy with varying provision of liaison psychiatry. This study was approved by the Central London research Ethics Committee 3, reference 10/H0716/79.
Participants
In both hospitals, all patients are admitted via accident and emergency services to the medical acute admissions unit before transfer to elderly care or medical wards (total of 20 wards). Two research assistants spent 5 months at each site, assessing within 72 h of admission all patients admitted to each unit under the care of the geriatricians (recruitment period 4 April 2011 to 6 March 2012). Clinical staff identified patients who met the following inclusion criteria: (a) aged 70 years or above with an unplanned acute medical admission; (b) able to give written informed consent or with an informal carer or ‘professional consultee’ available to give assent; (c) sufficient English language to complete the study ratings; (d) Abbreviated Mental Test Score (AMTS) Reference Hodkinson9 of ⩽7/10 (routinely measured on admission).
We excluded patients who indicated verbally or non-verbally that they did not wish to participate, those who were moribund, non-English speaking or where there were clinical concerns regarding them being approached.
Screening
All potential participants were screened for delirium using the Confusion Assessment Method (CAM). Reference Inouye, van Dyck, Alessi, Balkin, Siegal and Horwitz10 This has a sensitivity of over 94% and a specificity over 90% for detecting delirium and distinguishes accurately between delirium and dementia. Reference Inouye, van Dyck, Alessi, Balkin, Siegal and Horwitz10 Those who were not delirious and consented to the study were assessed using the Mini-Mental State Examination (MMSE). Reference Folstein, Folstein and McHugh11 If their score was ⩽l24 they were entered into the study. Patients with delirium were screened again 48 h later, if this had resolved they underwent testing with the MMSE. If they remained persistently delirious they were not eligible to participate as we could not establish whether or not they had an underlying dementia. However, patients with delirium who had a previous diagnosis of dementia from a specialist service (neurology, geriatrics, old age psychiatry) documented in their hospital notes were eligible.
Baseline study measures
Dementia diagnosis was confirmed using a structured clinical assessment based on operationalised DSM-IV criteria. 12 This comprised cognitive testing from the MMSE, structured review of the clinical notes and discussion with family and other carers. We only diagnosed new cases of dementia in the absence of delirium. Research staff did not give the diagnosis of dementia to the participant or their families. This was documented in their notes so the clinical team could manage this as per their usual procedures. Dementia severity was measured using the Functional Assessment Staging Scale (FAST). Reference Reisberg13 Reason for admission, comorbidities (Charlson Score) Reference Charlson, Pompei, Ales and MacKenzie14 and demographics were obtained from medical notes.
Assessment for BPSD
Participants were assessed for BPSD at baseline (during the first 72 h of admission) using the Behavioural Pathology in Alzheimer’s Disease (Behave-AD), Reference Reisberg, Borenstein, Salob, Ferris, Franssen and Georgotas15 a scale designed for prospective studies of behavioural symptoms in dementia. In addition we included information from discussions with family carers and ward staff and all available hospital notes. The scale covers seven domains of BPSD: paranoid and delusional ideation, hallucinations, activity disturbances, aggressiveness, diurnal rhythm disturbance, affective disturbance, anxieties and phobias. Scores can be generated for the presence or absence (0/1) or severity of symptoms (0, none; 1, mild; 2, moderate; 3, severe), giving a maximum score of 75. The scale also includes a global rating of how troubling the BPSD are to family carers or staff (0, not troubling; 1, mildly troubling or dangerous; 2, moderately troubling or dangerous; 3, severely troubling or intolerable).
Subsequent assessments
Patients were reviewed every 4 (± 1) days with the Behave-AD, until discharge or they were deemed medically fit and ‘awaiting placement’ in a care home. Hospital notes were examined and discussions held with clinical staff to identify any BPSD that had occurred in the 4 (± 1) days since the previous assessment. Interrater reliability for the Behave-AD was checked for 35 random participants. Agreement ranged from 84.9 to 97.1% (kappa (κ) = 0.60-0.75). It was possible that BPSD may have occurred secondary to incident delirium. To perform a sensitivity analysis for this we undertook regular delirium assessments using the CAM every 4 (± 1) days at the same time as the BPSD assessment at the second hospital site (n = 113).
Other clinical measures
Data on length of admission, mortality and change of residence on discharge from hospital (from own home to a care home) were collected from hospital notes.
Adverse events
These were recorded using validated pre-set criteria, defined as ‘an unintended injury caused by medical management rather than by the disease process and which is sufficiently serious to lead to prolongation of hospitalisation or to temporary or permanent impairment or disability to the patient at time of discharge’; these included falls. Reference Vincent, Neale and Woloshynowych16
Quality of care
We used the ACOVE (Assessing Care of Vulnerable Elders) indicators: standardised quality indicators in general hospital care and ‘geriatric-prevalent’ conditions (for example dementia and delirium). Reference Arora, Johnson, Olson, Podrazik, Levine and Dubeau17 They are a set of IF/THEN statements for 17 conditions, for example, ‘IF patient has an indwelling catheter placed either on admission or during hospitalization THEN there should be documented indication of need for catheter’. We used a standard method to calculate percentage adherence for each study participant. Reference Arora, Johnson, Olson, Podrazik, Levine and Dubeau17
Sample size
We assumed a point prevalence of BPSD of 31% from a community-based sample of people with dementia. Reference Lyketsos, Steinberg, Tschanz, Norton, Steffens and Breitner18 We aimed to recruit 250 patients (125 from each hospital) to ensure a 95% confidence interval for prevalence estimates of BPSD with an acceptable 6% precision.
Data analysis
We used simple descriptive statistics for the demographic features of the cohort. We calculated the prevalence (and 95% confidence intervals) of BPSD of any severity at the baseline assessment and then cumulatively at any time during the admission. We calculated the prevalence of individual types of BPSD (and 95% confidence intervals) as described in the seven domains of the Behave-AD scale. The continuous variable length of admission and ACOVE scores were dichotomised by cutting at the median score. We examined associations between participant characteristics, other clinical measures and the presence of BPSD using Fisher’s exact test and with the severity of BPSD using analysis of variance (ANOVA).
We also explored the association between BPSD severity (mean Behave-AD score over admission) and quality of care (ACOVE score), adverse events, length of stay, mortality during admission and discharge to institutional care (for those living in their own home before admission). The association was modelled using linear or logistic regression, with Behave-AD mean score (for each BPSD type and total) as independent variable. Thus, for continuous measures this gives the mean difference for a one-point increase in the Behave-AD. For binary measures (adverse events, mortality and change of residence to a care home) we calculated the odds ratio of the outcome for a one-point increase in the Behave-AD. Length of stay had a skewed distribution and was log-transformed for this analysis.
Sensitivity analysis for the impact of delirium
To examine whether BPSD prevalence estimates were altered by participants developing delirium, we conducted a sensitivity analysis, recalculating the prevalence of BPSD for participants at hospital site 2, excluding those who had delirium at any assessment during their admission.
Economic data
Data of sufficient quality were available from one hospital site on 98 consecutive patients enrolled into the study prior to 31 December 2011. These included charges accruing to the hospital for each participant’s admission: staff contact time, prescription medication use, hospital overheads and other running costs. We compared the cost of admission for patients who had any BPSD at baseline against costs for patients with no BPSD at baseline using a non-parametric Mann-Whitney test. Costs are reported as 2011-2012 values. As is common with healthcare data, costs were highly skewed (skewness, 2.88) because a small number of patients consumed a disproportionately large level of resources. As a result, the assumptions required for standard statistical approaches based on normally distributed data do not hold. Therefore a Bayesian parametric approach to analysis of costs was followed. The relationship between cost and mean BPSD score was estimated, taking into account the skewness, by using linear regression and non-parametric bootstrapping with 5000 resamples and reporting bias-corrected and accelerated 95% confidence intervals. Reference Barber and Thompson19
Ethical issues
Our participants were acutely ill, had dementia, delirium or both and were not able to give informed consent. Our consent procedure complied with capacity legislation governing England and Wales while balancing the need to recruit a representative cohort of people with dementia (Mental Capacity Act 2005). If a patient agreed to participate, we conducted a structured assessment of their capacity to consent. If they had capacity, written informed consent was obtained. If they did not have capacity, we attempted to identify their next of kin, carer or another close person to give proxy assent. This could be given verbally over the telephone and we sent this personal consultee the assent form in the post to sign and return. If these forms were not returned, the participant’s data were withdrawn and destroyed at the end of the study. If we could not contact a next of kin within 48 h of initial screening we approached a professional consultee for assent (a senior member of the clinical care team who was not directly involved in the research or the patient’s care). Reference Scott, Jones, Blanchard and Sampson20 The researchers had regular clinical supervision and a protocol to follow if they witnessed suboptimal care or distressing incidents.
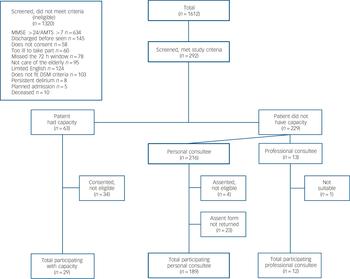
Fig. 1 Study flow chart.
MMSE, Mini-Mental Sate Examination, AMTS, Abbreviated Mental Test Score.
Results
Recruitment
A total of 1612 people were screened (Fig. 1). Of these, 292 met inclusion criteria. The most common reasons for exclusion were that the MMSE score was >24 or AMTS score >7 (n = 634) or patients were discharged before they could be assessed (n = 145). Of the 292 recruited to the study, a further 62 were excluded because they did not fulfil study inclusion criteria at baseline assessment or because family carers who gave telephone assent did not post back signed assent forms. There were 230 participants in the cohort (117 from hospital 1 and 113 from hospital 2). Median length of admission was 12 days (range, 2-72; interquartile range (IQR), 7-23 and median number of study assessments per participant was 3 (range, 1-20; IQR, 2-5. No participants dropped out during the study.
Study participants
There were no significant differences between the characteristics of participants from hospital sites 1 and 2 with respect to gender, proportion of those with a prior diagnosis of dementia, usual place of residence or comorbidity on the Charlson score. Participants from hospital 1 were significantly older (t = –2.26, P = 0.025) and more were of white British origin, 82% compared with 69% at hospital 2 (χ2 = 25.9, P = 0.004). Few participants had missing data: three (1.3%) had data missing on the CAM at baseline, and four (1.7%) sets of notes were not available for review post-discharge.
Participants were predominantly female (66%), with a mean age 87.2 years (s.d. = 5.9) and of white British ethnicity (76%) (Table 1). A known diagnosis of dementia prior to the hospital admission was present in 70% (Table 2). Most were admitted from their own home and 63% had moderate to severe functional impairment as a result of their dementia. During their stay, 13% died. Of those who survived and were admitted from their own home, 23.4% were subsequently discharged into a care home. The most common coded causes of admission were pneumonia/chest infection (26.7%), urinary tract infection (15.7%), fall or fracture (11.3%) and cardiac events (9.6%). At the initial study assessment (within 72 h of admission), 11.4% had delirium.
Behavioural and psychiatric symptoms of dementia
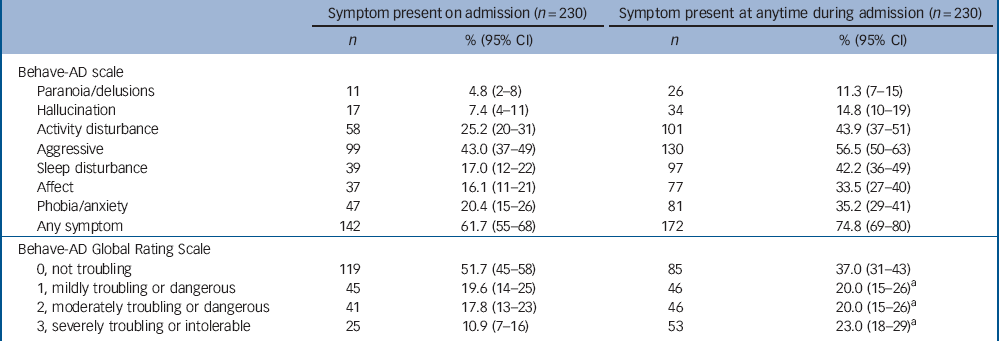
At the first study assessment, 62% (95% CI 55-68) had BPSD. The most common symptoms at baseline were aggression 43% (95% CI 37-49) and activity disturbance 25% (95% CI 20-31). Considering the whole admission 75% (95% CI 69-80) of participants had BPSD at some point, with 57% having aggression (95% CI 50-63) and 44% activity disturbance (95% CI 37-51) (Table 3). The least common BPSD were paranoia and hallucinations. In total, 47% of the cohort experienced three or more BPSD symptoms during their admission. At the first assessment, 29% of participants had experienced BPSD that were moderately or severely troubling to staff or other carers, this increased to 43% for the whole admission.
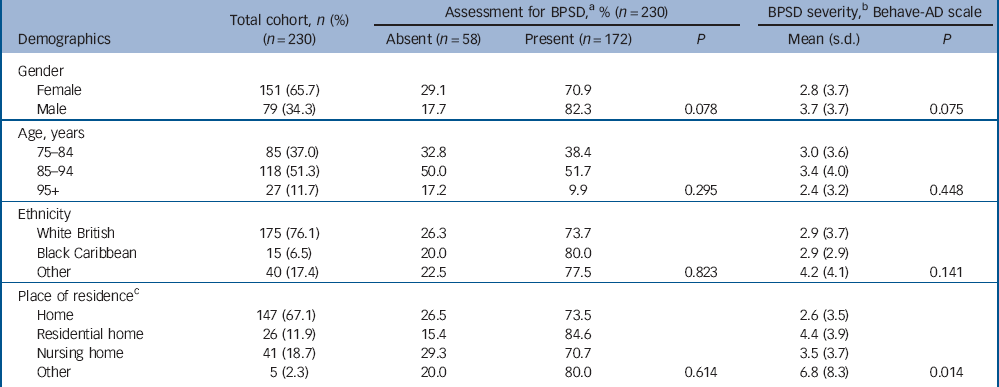
Table 1 Cohort demographics and associations with behavioural and psychiatric symptoms of dementia (BPSD) for 230 older people in the acute hospital
| Demographics | Total
cohort, n (%) (n = 230) |
Assessment for BPSD,Footnote a % (n = 230) | BPSD severity,Footnote b Behave-AD scale | |||
|---|---|---|---|---|---|---|
| Absent (n = 58) | Present (n = 172) | P | Mean (s.d.) | P | ||
| Gender | ||||||
| Female | 151 (65.7) | 29.1 | 70.9 | 2.8 (3.7) | ||
| Male | 79 (34.3) | 17.7 | 82.3 | 0.078 | 3.7 (3.7) | 0.075 |
| Age, years | ||||||
| 75-84 | 85 (37.0) | 32.8 | 38.4 | 3.0 (3.6) | ||
| 85-94 | 118 (51.3) | 50.0 | 51.7 | 3.4 (4.0) | ||
| 95+ | 27 (11.7) | 17.2 | 9.9 | 0.295 | 2.4 (3.2) | 0.448 |
| Ethnicity | ||||||
| White British | 175 (76.1) | 26.3 | 73.7 | 2.9 (3.7) | ||
| Black Caribbean | 15 (6.5) | 20.0 | 80.0 | 2.9 (2.9) | ||
| Other | 40 (17.4) | 22.5 | 77.5 | 0.823 | 4.2 (4.1) | 0.141 |
| Place of residenceFootnote c | ||||||
| Home | 147 (67.1) | 26.5 | 73.5 | 2.6 (3.5) | ||
| Residential home | 26 (11.9) | 15.4 | 84.6 | 4.4 (3.9) | ||
| Nursing home | 41 (18.7) | 29.3 | 70.7 | 3.5 (3.7) | ||
| Other | 5 (2.3) | 20.0 | 80.0 | 0.614 | 6.8 (8.3) | 0.014 |
a. At any assessment during hospital admission.
b. Mean score over admission.
c. Data available for n = 219 only.
Sensitivity analyses for the impact of delirium
Recalculating the prevalence of BPSD in participants from hospital site 2, excluding those with delirium at any study assessment point had a small effect, decreasing the prevalence estimates for hallucinations (from 14.8 to 10.1%), activity disturbance (from 43.9 to 36.4%), sleep disturbance (from 42.2 to 35.4%), affective disturbance (from 33.0 to 28.3%) and overall prevalence of BPSD of any type (from 75.0 to 68.7% (see online Table DS1)).
Associations with behavioural and psychiatric symptoms of dementia
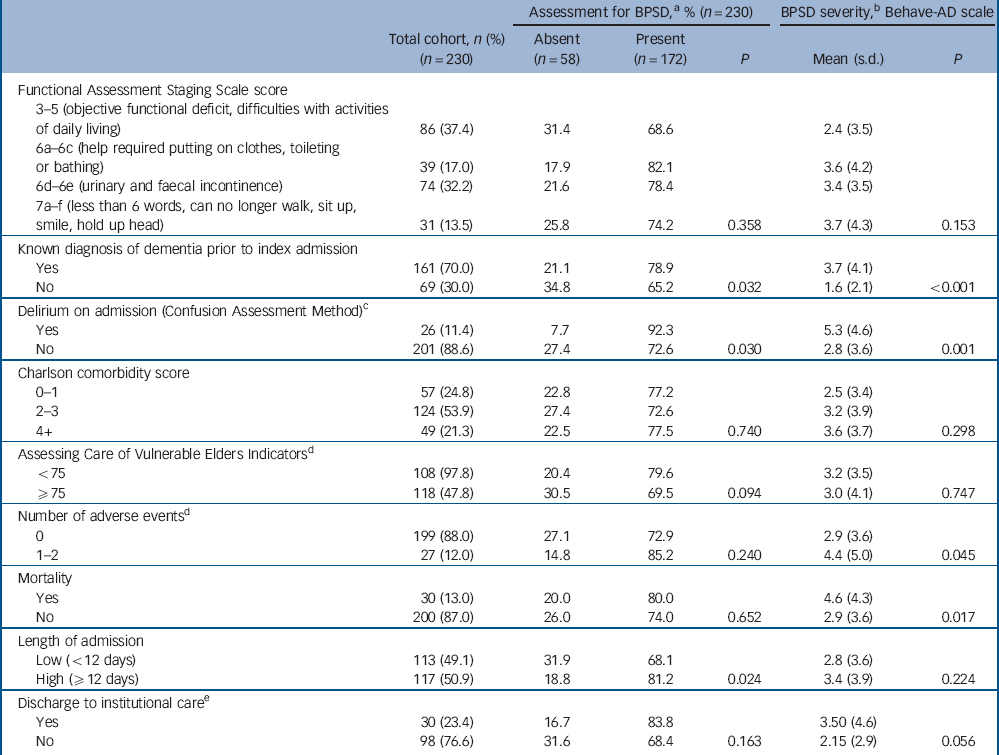
Associations between patient characteristics and BPSD are reported in Tables 1 and 2. We found that BPSD were more common in men, those admitted from residential or nursing homes, those with a prior diagnosis of dementia and the presence of delirium on admission. Adverse events and mortality were also associated with the total severity of BPSD. Exploring this further in regression analyses (Table 4) suggested a possible association between paranoia and activity disturbance and adverse events. Our analysis did not indicate an association between the length of admission and mean severity of BPSD during the hospital stay.
Costs
For 98 patients from hospital 2 with complete cost data, the mean cost of admission per patient was £14 464 (s.d. = 15 795) and median cost per patient £9923 (IQR £5195-15 960). The mean total cost of admission for participants without BPSD at baseline was £12 150 (observed sample skewness, 0.69) and the median cost £10 094 (IQR £4491-£16 821). For those with BPSD the mean cost was £15 639 (observed sample skewness, 2.69) and the median cost £9755 (IQR £5946-£14 937). The Mann-Whitney test of differences in costs between those with and without BPSD at baseline was not significant (χ2(1d.f.) = 0.40, P = 0.842). The association between total cost of the admission to hospital and mean BPSD score was not significant (average increase in cost for each one-point increase of mean BPSD was £215.45, bootstrap 95% CI -348.09 to 1020.37, P = 0.542).
Table 2 Cohort clinical characteristics and associations with behavioural and psychiatric symptoms of dementia (BPSD) for 230 older people in the acute hospital
| Assessment for BPSD,Footnote a % (n = 230) | BPSD severity,Footnote b Behave-AD scale | |||||
|---|---|---|---|---|---|---|
| Total cohort,
n (%) (n = 230) |
Absent (n = 58) |
Present (n = 172) |
P | Mean (s.d.) | P | |
| Functional Assessment Staging Scale score | ||||||
| 3-5 (objective functional deficit, difficulties with activities of daily living) | 86 (37.4) | 31.4 | 68.6 | 2.4 (3.5) | ||
| 6a-6c (help required putting on clothes, toileting or bathing) | 39 (17.0) | 17.9 | 82.1 | 3.6 (4.2) | ||
| 6d-6e (urinary and faecal incontinence) | 74 (32.2) | 21.6 | 78.4 | 3.4 (3.5) | ||
| 7a-f (less than 6 words, can no longer walk, sit up, smile, hold up head) | 31 (13.5) | 25.8 | 74.2 | 0.358 | 3.7 (4.3) | 0.153 |
| Known diagnosis of dementia prior to index admission | ||||||
| Yes | 161 (70.0) | 21.1 | 78.9 | 3.7 (4.1) | ||
| No | 69 (30.0) | 34.8 | 65.2 | 0.032 | 1.6 (2.1) | <0.001 |
| Delirium on admission (Confusion Assessment Method)Footnote c | ||||||
| Yes | 26 (11.4) | 7.7 | 92.3 | 5.3 (4.6) | ||
| No | 201 (88.6) | 27.4 | 72.6 | 0.030 | 2.8 (3.6) | 0.001 |
| Charlson comorbidity score | ||||||
| 0-1 | 57 (24.8) | 22.8 | 77.2 | 2.5 (3.4) | ||
| 2-3 | 124 (53.9) | 27.4 | 72.6 | 3.2 (3.9) | ||
| 4+ | 49 (21.3) | 22.5 | 77.5 | 0.740 | 3.6 (3.7) | 0.298 |
| Assessing Care of Vulnerable Elders IndicatorsFootnote d | ||||||
| <75 | 108 (97.8) | 20.4 | 79.6 | 3.2 (3.5) | ||
| ⩾75 | 118 (47.8) | 30.5 | 69.5 | 0.094 | 3.0 (4.1) | 0.747 |
| Number of adverse eventsFootnote d | ||||||
| 0 | 199 (88.0) | 27.1 | 72.9 | 2.9 (3.6) | ||
| 1-2 | 27 (12.0) | 14.8 | 85.2 | 0.240 | 4.4 (5.0) | 0.045 |
| Mortality | ||||||
| Yes | 30 (13.0) | 20.0 | 80.0 | 4.6 (4.3) | ||
| No | 200 (87.0) | 26.0 | 74.0 | 0.652 | 2.9 (3.6) | 0.017 |
| Length of admission | ||||||
| Low (<12 days) | 113 (49.1) | 31.9 | 68.1 | 2.8 (3.6) | ||
| High (⩾12 days) | 117 (50.9) | 18.8 | 81.2 | 0.024 | 3.4 (3.9) | 0.224 |
| Discharge to institutional careFootnote e | ||||||
| Yes | 30 (23.4) | 16.7 | 83.8 | 3.50 (4.6) | ||
| No | 98 (76.6) | 31.6 | 68.4 | 0.163 | 2.15 (2.9) | 0.056 |
a. At any assessment during hospital admission.
b. Mean score over admission.
c. Data available for n = 227 only.
d. Data available for n = 226 only.
e. Data available for n = 128 patients who were admitted from home and underwent a change of residence on discharge.
Table 3 Prevalence of behavioural and psychiatric symptoms of dementia on admission, and at any point during admission for 230 people in the acute hospital
| Symptom present on admission (n = 230) | Symptom present at anytime during admission (n = 230) | |||
|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | |
| Behave-AD scale | ||||
| Paranoia/delusions | 11 | 4.8 (2-8) | 26 | 11.3 (7-15) |
| Hallucination | 17 | 7.4 (4-11) | 34 | 14.8 (10-19) |
| Activity disturbance | 58 | 25.2 (20-31) | 101 | 43.9 (37-51) |
| Aggressive | 99 | 43.0 (37-49) | 130 | 56.5 (50-63) |
| Sleep disturbance | 39 | 17.0 (12-22) | 97 | 42.2 (36-49) |
| Affect | 37 | 16.1 (11-21) | 77 | 33.5 (27-40) |
| Phobia/anxiety | 47 | 20.4 (15-26) | 81 | 35.2 (29-41) |
| Any symptom | 142 | 61.7 (55-68) | 172 | 74.8 (69-80) |
| Behave-AD Global Rating Scale | ||||
| 0, not troubling | 119 | 51.7 (45-58) | 85 | 37.0 (31-43) |
| 1, mildly troubling or dangerous | 45 | 19.6 (14-25) | 46 | 20.0 (15-26)Footnote a |
| 2, moderately troubling or dangerous | 41 | 17.8 (13-23) | 46 | 20.0 (15-26)Footnote a |
| 3, severely troubling or intolerable | 25 | 10.9 (7-16) | 53 | 23.0 (18-29)Footnote a |
Behave-AD, Behavioural Pathology in Alzheimer’s Disease.
a. Maximum level reached by participant during admission.
Table 4 Clinical associations with severity of behavioural and psychiatric symptoms of dementia for 230 older people in the acute hospital
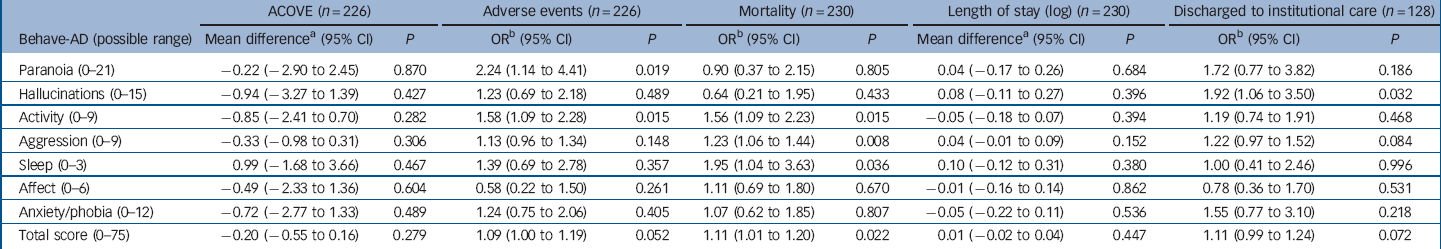
| ACOVE (n = 226) | Adverse events (n = 226) | Mortality (n = 230) | Length of stay (log) (n = 230) | Discharged to institutional care (n = 128) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Behave-AD (possible range) | Mean differenceFootnote a (95% CI) | P | ORFootnote b (95% CI) | P | ORFootnote b (95% CI) | P | Mean differenceFootnote a (95% CI) | P | ORFootnote b (95% CI) | P |
| Paranoia (0-21) | –0.22 (–2.90 to 2.45) | 0.870 | 2.24 (1.14 to 4.41) | 0.019 | 0.90 (0.37 to 2.15) | 0.805 | 0.04 (–0.17 to 0.26) | 0.684 | 1.72 (0.77 to 3.82) | 0.186 |
| Hallucinations (0-15) | –0.94 (–3.27 to 1.39) | 0.427 | 1.23 (0.69 to 2.18) | 0.489 | 0.64 (0.21 to 1.95) | 0.433 | 0.08 (–0.11 to 0.27) | 0.396 | 1.92 (1.06 to 3.50) | 0.032 |
| Activity (0-9) | –0.85 (–2.41 to 0.70) | 0.282 | 1.58 (1.09 to 2.28) | 0.015 | 1.56 (1.09 to 2.23) | 0.015 | –0.05 (–0.18 to 0.07) | 0.394 | 1.19 (0.74 to 1.91) | 0.468 |
| Aggression (0-9) | –0.33 (–0.98 to 0.31) | 0.306 | 1.13 (0.96 to 1.34) | 0.148 | 1.23 (1.06 to 1.44) | 0.008 | 0.04 (–0.01 to 0.09) | 0.152 | 1.22 (0.97 to 1.52) | 0.084 |
| Sleep (0-3) | 0.99 (–1.68 to 3.66) | 0.467 | 1.39 (0.69 to 2.78) | 0.357 | 1.95 (1.04 to 3.63) | 0.036 | 0.10 (–0.12 to 0.31) | 0.380 | 1.00 (0.41 to 2.46) | 0.996 |
| Affect (0-6) | –0.49 (–2.33 to 1.36) | 0.604 | 0.58 (0.22 to 1.50) | 0.261 | 1.11 (0.69 to 1.80) | 0.670 | –0.01 (–0.16 to 0.14) | 0.862 | 0.78 (0.36 to 1.70) | 0.531 |
| Anxiety/phobia (0-12) | –0.72 (–2.77 to 1.33) | 0.489 | 1.24 (0.75 to 2.06) | 0.405 | 1.07 (0.62 to 1.85) | 0.807 | –0.05 (–0.22 to 0.11) | 0.536 | 1.55 (0.77 to 3.10) | 0.218 |
| Total score (0-75) | –0.20 (–0.55 to 0.16) | 0.279 | 1.09 (1.00 to 1.19) | 0.052 | 1.11 (1.01 to 1.20) | 0.022 | 0.01 (–0.02 to 0.04) | 0.447 | 1.11 (0.99 to 1.24) | 0.072 |
Behave-AD, Behavioural Pathology in Alzheimer’s Disease; ACOVE, Assessing Care of Vulnerable Elders.
a. Mean difference in dependent variable for each one-point increase on the BEHAVE-AD, from linear regression.
b. Odds ratio of outcome for each one-point increase on the BEHAVE-AD, from logistic regression.
Discussion
Behavioural and psychiatric symptoms were common in people with dementia in the acute hospital, affecting 75% of participants at some point during their stay. Moderately or severely troubling BPSD occurred in 43% of participants and aggression (57%) and activity disturbance (44%) were the most common symptoms. Over a third of participants had symptoms of sleep disturbance, depression, phobia or anxiety at some time during their stay.
Systematic reviews of the prevalence of BPSD in community-dwelling older people with dementia give widely ranging results, depending on which tools are used and the length of the observation period. Reference van der Linde, Stephan, Savva, Dening and Brayne21 However, in our sample aggression and activity disturbance were more common than in people with dementia living in the community, Reference Steinberg, Shao, Zandi, Lyketsos, Welsh-Bohmer and Norton22 or those living in residential or care homes Reference Wetzels, Zuidema, de Jonghe, Verhey and Koopmans23 and higher than in large UK population samples of people with dementia. Reference Savva, Zaccai, Matthews, Davidson, McKeith and Brayne24
The acute hospital is a challenging environment for people with dementia and BPSD are multifactorial in origin. The combination of an unfamiliar, disorientating and often noisy environment with physical illness and the need for staff to undertake physical care tasks may have increased the likelihood that BPSD would occur. Reference Schnelle, Ouslander, Simmons, Alessi and Gravel25 Studies undertaken in care homes have demonstrated that agitation is often preceded by verbal and physical interactions with staff Reference Burgio, Butler, Roth, Hardin, Hsu and Ung26 and physical aggression is more likely to occur when providing personal care. Reference Bridges-Parlet, Knopman and Thompson27
We found a possible association between BPSD (activity disturbance and paranoia) and the risk of adverse events. These symptoms may be more common in individuals who are ‘ambulatory’ cognitively impaired and who are at higher risk of adverse events, such as falls. Reference Watkin, Blanchard, Tookman and Sampson28 More severe BPSD (activity disturbance, aggression and sleep disturbance) were associated with mortality. This may be mediated by delirium and its associated behavioural disturbances. Alternatively, ‘terminal restlessness’ is a common phenomenon in people who are dying and our behavioural rating scale may have detected these symptoms. In clinical practice, it may be difficult to distinguish between delirium and terminal restlessness. Reference Sampson, Leurent, Blanchard, Jones and King29,Reference Breitbart and Alici30
Dementia is known to increase the length of hospital stay in a range of countries and types of acute care services. Reference Mukadam and Sampson6 However, our analysis did not indicate an association between BPSD at admission and length of stay. It may be that length of stay for people with dementia is more strongly influenced by external factors, such as the speed at which discharge care packages can be arranged and the availability of social care at home. Reference Bourne31
The mean cost of admission was higher for people with BPSD at baseline (£15 639 compared with £12 150 in those without BPSD). However, data were highly skewed and median costs were not different between the two groups. These figures reflect the actual costs to the hospital of providing in-patient care and are charges accrued to the hospital for staff contact time, prescription medication use, hospital overheads and other running costs. Because of variation in clinical practice and different local cost structures, these data are less suitable for drawing wider conclusions about the cost of providing care at a national level. Length of stay has the strongest influence on costs per stay and this may be determined by factors outside the control of the hospital. These costs may be useful in developing future economic evaluations of service improvements for people with dementia in the acute hospital.
Strengths and limitations
It is possible that the participants may not be representative of the wider population of people with dementia in acute hospitals. However, we believe it is very likely that our results could be generalised; we recruited from two large acute hospital trusts that cover a population of over two million people. Previous research conducted in this location found a dementia prevalence of 42%, which is similar to that found in other acute hospital populations. Reference Laurila, Pitkala, Strandberg and Tilvis4,Reference Zekry, Herrmann, Grandjean, Meynet, Michel and Gold5 In addition our prevalence estimates for behavioural problems at admission are similar to those of Goldberg et al, Reference Goldberg, Whittamore, Harwood, Bradshaw, Gladman and Jones32 who used the Neuropsychiatric Inventory in older people admitted to hospital. Finally the coded causes of admission in our cohort reflect other UK statistics. Reference Natalwala, Potluri, Uppal and Heun33 We attempted to reduce selection bias by screening all people who met our inclusion criteria and carefully documenting reasons for exclusion. Using ‘professional consultees’ enabled us to recruit participants who may otherwise have been excluded because they could not consent for themselves and did not have a carer or family member to give assent for their participation.
Diagnosing dementia in the acute setting is challenging because delirium is common in this population. However, it was important to attempt this, as many people with dementia in the acute hospital have not received a prior diagnosis. To reduce the risk of misclassification of delirium as dementia we only diagnosed new cases of dementia in the absence of delirium, screening for delirium with the CAM version, which gives maximum sensitivity. A recent systematic review showed the CAM to have high specificity (96-100%) and moderate sensitivity (77%) in distinguishing between these conditions. Reference Morandi, McCurley, Vasilevskis, Fick, Bellelli and Lee34 We conducted a sensitivity analysis, excluding participants with delirium as this may cause behavioural disturbance. This did not markedly alter our prevalence estimates for BPSD. It is, however, possible that incident delirium occurred between study assessments.
Research in the acute hospital can be difficult. We reviewed patients every 4 days, using clinical notes, and interviewed families and ward staff in detail to ascertain whether BPSD had occurred, but recall bias may have led to overreporting of ‘troublesome’ behaviours, for example aggression, and underreporting of tearfulness, depression or other forms of distress. Although less challenging for staff, these are important to people with dementia and their carers.
We conducted multiple analyses and some of our findings may be as a result of chance. Our study may have been underpowered to explore associations between BPSD and other factors, particularly for less frequent events such as mortality. It is possible that there are residual confounding factors that we did not consider. Factors such as admission diagnosis may have an impact on clinical outcomes; however, reasons for admission in older people are often complex and multifactorial. For example pneumonia may be caused by a fall, precipitated by a urinary tract infection; thus, defining a single cause of admission may not be clinically relevant. In addition, agitation and behavioural problems may themselves precipitate acute hospital admission. Reference Toot, Devine, Akporobaro and Orrell35 The direction of these associations is complex; adverse events may be more common in longer hospital stays but this may be because there is more time at risk.
Clinical implications
The results of this study demonstrate a high prevalence of behavioural and psychiatric symptoms in people with dementia in the acute hospital, particularly aggression and activity disturbance. Excluding participants with delirium in a sensitivity analysis did not markedly reduce our estimates. Despite this, many UK acute hospitals and their staff do not have access to specialist psychiatric advice and support or liaison services for older people. In addition the skill-mix of staff on acute general hospital wards may not be optimal to manage the range or severity of BPSD that occur. Our participants were very functionally impaired; 32% were at FAST stage 6d-e (doubly incontinent) and 14% at FAST stage 7a-f (unable to walk, smile or hold their head up). This finding supports recent concerns in the UK that there may be inadequate staff numbers to undertake basic care tasks in such dependent and vulnerable patients. 36 Symptoms such as aggression and activity disturbance are highly distressing for the person with dementia, their families, carers, other in-patients and acute hospital staff.
The majority of acute hospital nurses want more training and support in managing BPSD, particularly aggression (which occurred in over half of our participants). 8 However, there have been few evaluation studies or clinical trials on how we can best educate staff and implement changes to improve care. Reference Harwood, Goldberg, Whittamore, Russell, Gladman and Jones37 The severity of the behavioural and psychiatric problems found in this study, particularly that of aggression and activity disturbance, suggest more complex structured interventions for BPSD, similar to those that have been shown to be successful in care homes Reference Kovach, Noonan, Schlidt, Reynolds and Wells38,Reference Testad, Ballard, Bronnick and Aarsland39 may be required. Our results provide strong evidence for the necessity of specialist interventions for BPSD in the acute hospital setting, and for psychiatric liaison teams and specialists in dementia care, to support hospital staff in managing these.
Acknowledgements
We would like to thank Victoria Vickerstaff for her assistance with analyses, Hedwig Hendriks for help with data collection, our Alzheimer’s Society Quality Research Monitors Barbara Di Vita, Sylvia Wallach and Lynn Whittaker, the staff from Health Services for Older People at the Royal Free NHS Trust and the North Middlesex University Trust (Dr Sophie Edwards and Dr Dan Lee, Consultants in Care of the Elderly) and particularly our professional consultees Jo James, Jenny Kenward, Pippa Street, Bridget Cooney, Jane Dunne, and Dr Ada Chime.








eLetters
No eLetters have been published for this article.