Introduction
Coronavirus disease (COVID-19) has remained a Public Health Emergency of International Concern (PHEIC) almost one and half years after the pandemic was declared a PHEIC in January 2020 by the World Health Organization [Reference Parker1]. To date, nations are still under increased pressure to overcome the spiralling global spread of the deadly novel COVID-19, which was responsible for more than 266 million infected individuals and over 5.2 million deaths worldwide as of 7 December 2021 [2, Reference Cucinotta and Vanelli3]. The wide and unprecedented spread of COVID-19 caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has been attributed to its ability to spread via respiratory droplets, aerosol, and secretions facilitated by high levels of globalisation and international travel [4].
On 21 March 2020, the Ugandan Ministry of Health reported the first case of confirmed COVID-19 in Uganda from a returning passenger through Entebbe International Airport. Uganda continued to register a few cases of COVID-19 composed mainly of cross-border truck drivers from neighbouring countries until June 2020 when community transmissions increased marking the first wave of COVID-19 cases [Reference Kitara and Ikoona5, Reference Keni, Alexander, Nayak, Mudgal and Nandakumar6]. To curtail the spread of the disease, the Government of Uganda instituted public health interventions including border closure, institutional lockdown, quarantine, and testing of returnees, contact tracing, and abolishing of public gatherings [Reference Ferguson, Laydon, Nedjati Gilani, Imai, Ainslie, Baguelin, Bhatia, Boonyasiri, Cucunuba Perez, Cuomo-Dannenburg, Dighe, Dorigatti, Fu, Gaythorpe, Green, Hamlet, Hinsley, Okell, Van Elsland, Thompson, Verity, Volz, Wang, Wang, Walker, Walters, Winskill, Whittaker, Donnelly, Riley and Ghani7–Reference Ssali9]. Following the end of the first COVID-19 wave, which subsided in January–February 2021, most of the instituted control measures were eased, especially lockdown measures such as public transport operations, while others such as worship places and school re-openings were relaxed, allowing the public to resume normal routines that supported their social and economic activities [Reference Odour10, Reference Bongomin, Fleischer, Olum, Natukunda, Kiguli, Byakika-Kibwika, Baluku and Nakwagala11]. However, few SARS-CoV-2 infections were still being reported in the communities, and later, unknown factors triggered an exponential rise of COVID-19 cases in different parts of the country, marking the start of the second wave. Most of the cases were reported in the capital city, Kampala, regional cities, and border districts, with over 900 cases daily and reaching a positivity rate of 17% by June 2021 [12]. The period between March and June 2021 is believed to have marked a clear emergence of the second wave of COVID-19 in Uganda with the highest recorded number of cumulative cases of up to 90,000 and with over 2,000 deaths as of 20 July 2021 [Reference Kitara and Ikoona5].
Uganda like other countries globally was affected by different variants throughout the period of the COVID-19 outbreak [Reference Parker1].
The variants of concern included B.1.1.7, B.1.351, B.1.617.2, and B.1.525, which had been reported in Nigeria and the UK [2]. These were first observed in Uganda on 5 March 2021 in Kampala and were consistently observed until December 2021 [Reference Cucinotta and Vanelli3]. The A.23.1 was a lineage that emerged in Uganda in the summer of 2020 and later spread in Uganda and globally (Bugembe et al. [Reference Bugembe, Phan, Ssewanyana, Semanda, Nansumba, Dhaala, Nabadda, O’Toole, Rambaut, Kaleebu and Cotten13]). The first wave in Uganda was mostly spread by the A.23.1 variant, which later decreased in frequency around February 2021 [Reference Cucinotta and Vanelli3]; later, there was the Omicron variant, which emerged from Wuhan in China [4, Reference Vitiello, Ferrara, Auti, Di Domenico and Boccellino14]. The second wave was mostly aided by B.1.1.7, B.1.351, and B.1.617.2, which had their origins in South Africa and the UK [Reference Kitara and Ikoona5]. The second was mostly spread by the Delta variant, which contributed to high hospitalisation since March 2021, and this formed the focus of our investigation. The number of COVID-19 cases in the second wave was strikingly high and more fatal, and there was a high incidence in several districts, but there was no/limited data to explain the factors associated with the observed high incidence of and impact of COVID-19 among the population. To address this gap, we conducted a retrospective cross-sectional study on recovered, confirmed COVID-19 positive PCR-RT/RDT cases from March to June 2021 from 10 districts that had registered the highest number of COVID-19 cases in the second wave to explain the factors associated with the observed spread of COVID-19 among the population [Reference Kitara and Ikoona5, Reference Mugisha, Ssebuliba, Nakakawa, Kikawa and Ssematimba15].
Materials and methods
Study Location
The study was conducted in 10 selected districts in Uganda. Uganda is a landlocked country that lies between 10 29’ South and 40 12’ North latitude, 290 34′ East and 350 0′ East longitude [Reference El-Sadr and Justman16]. Uganda has a population of 41.6 million people, based on the Uganda National Household Survey (UNHS) conducted in 2019/20 by the Uganda Bureau of Statistics (UBOS). More than half (54%) of the population is below 18 years of age. Uganda, just like other Sub-Saharan African countries, has a weak healthcare system, characterised by low clinician-to-patient ratio, limited laboratory capacity, poor administration, and limited resources [Reference Peter, Keita and Nkengasong17, 18].
Study setting
In this study, we selected 10 districts (Figure 1) representing the main geographic regions that had the highest number of COVID-19 cases as reported by the MOH [Reference Kitara and Ikoona5]. The selected districts were the border districts (Busia and Tororo) with Points of Entry (PoE); major road highways for transit of cargo across districts (Mbale, Gulu, Luwero, Soroti, and Moroto districts); and highly populated regional city districts (Wakiso, Gulu, Mbarara, and Kampala) [Reference Olukya19, Reference Bellhouse and Kulperger20].

Figure 1. Location of study districts in Uganda.
Study population
The study population included patients or caregivers (especially for children below 18 years) of people who had suffered and recovered from COVID-19, either after HBC or after discharge from health facilities. During the investigations, we noted that some of these had died, while for those who were unwell and on treatment, whether at home or in hospitals and on treatment, we interviewed the caretakers/caregivers. The retrospective cross-sectional study was done as part of the outbreak investigation from March 2021 to June 2021 for PCR/RDT confirmed cases.
Sample size and sampling procedure
Sampling
We selected 10 districts based on their high population densities, high incidences of COVID-19 cases from March 2021 to June 2021 exceeding 300 cumulative cases in the study period, and having PoEs within the districts. We used a computer-based simple random sampling technique [Reference Holler, Eriksson, Jensen, van Wijhe, Fischer, Søgaard, Israelsen, Mohey, Fabricius, Jøhnk and Wiese21] to identify 120 COVID-19 cases from each district. This was sampled from the Ministry of Health database of all confirmed and reported COVID-19 cases. On obtaining the sample size, we followed each of the sampled cases, and we used their laboratory investigation forms that were available at respective health facilities in the study districts. We only considered cases that had COVID-19-positive RDT/PCR results (sample form Appendix A). The 1,120 positive COVID-19 PCR tests were done through the routine Ministry of Health testing in a bid to detect COVID-19 among populations. Such people either presented with signs and symptoms related to COVID-19 (suspects) or were contacts of the COVID-19 cases. The Government of Uganda, through the Ministry of Health, made the testing available and mandatory for those who presented as above [Reference Keni, Alexander, Nayak, Mudgal and Nandakumar6]. This was aimed at identifying cases early and linking them to care in a bid to minimise mortalities. Such people could voluntarily identify themselves to any testing centres for COVID-19 or the community health workers (CHWs) would identify them and refer them for this service. This testing was highly mobilised by the Government of Uganda and the Ministry of Health [Reference Ferguson, Laydon, Nedjati Gilani, Imai, Ainslie, Baguelin, Bhatia, Boonyasiri, Cucunuba Perez, Cuomo-Dannenburg, Dighe, Dorigatti, Fu, Gaythorpe, Green, Hamlet, Hinsley, Okell, Van Elsland, Thompson, Verity, Volz, Wang, Wang, Walker, Walters, Winskill, Whittaker, Donnelly, Riley and Ghani7]. The COVID-19 champion was the President of the Republic of Uganda, His Excellency Yoweri Kaguta Museveni, who made several presidential addresses and provided strong political leadership in the bid to fight the COVID-19 pandemic in Uganda [Reference Keni, Alexander, Nayak, Mudgal and Nandakumar6].
As this was voluntarily done, women sought more care than males according to our results.
The information extracted was then used to systematically sample (Table 1) and locate the recovered COVID-19 cases who were interviewed in the community.
Table 1. Recruitment protocol

We further conducted 38 key informant interviews (KIIs) and 19 in-depth interviews of purposively selected participants in all districts (Appendix C). The key informants comprised COVID-19 District Task Force (DTF) members based on their knowledge and active participation in the COVID-19 outbreak response interventions (Appendix B). In-depth Interviewees were participants who had either had COVID-19 19 and recovered or had lost a COVID-19 case in the family.
Data collection, management and analysis
Data collection and management
a) Quantitative data
Quantitative data was collected by trained and experienced epidemiologists using open-ended semi- structured questionnaires (Appendix A uploaded on the mWater portal (@mWater,2021), using an open-source cloud-based web application that was deployed on Android tablets. First, we obtained the details of the case listings in Microsoft Excel from the national database of COVID-19 cases at MOH that guided the selection of target districts with cases ranging from 100–150 cases per district. We then proceeded to the targeted districts to further access records for COVID-19 cases for verification and selection of participants.
The field teams further accessed laboratory investigation forms of the COVID-19 PCR and RDT-positive cases from the laboratories of the selected health facilities in each of the selected districts to extract data on variables such as personal details of the patients such as name, phone number, village, next of kin, and clinical symptoms. The collected information was then used to locate the recovered COVID-19 cases in respective communities guided by the Village Health Teams (VHTs). The selected cases were called via telephone to arrange appointments before the visits. On the day of the visit, the investigation team members took the potential respondents through the consenting process using Appendix D. Data from each participant was collected from a community COVID-19 case interviewer-administered questionnaire that was adopted from the MOH standard tool which assessed the socio-demographics and clinical characteristics of the COVID-19-positive cases. The live COVID-19 cases who consented to the study provided the information, but for those who had died, the next of kin provided this information. The next of kin were also taken through the same consenting process. All the data collected on tablets was uploaded daily onto an mWater portal server secured with passcodes that was only accessed by the principal investigators.
b) Qualitative data
An in-depth and key informant guide (Appendix C) was used to conduct interviews with members of the communities in the selected districts who had contracted COVID-19 and the DTF members, respectively. The main theme explored was drivers of the COVID-19 transmissions and spread during the second wave. Verbal consent was obtained from all participants before the commencement of any interview. From each district, four respondents (two male and two female) who had contracted COVID-19 were interviewed during in-depth interviews. Both the KIIs and in-depth interviews were audio-recorded using smartphones and tablets and the audios transcribed verbatim into Microsoft Word, that were only accessed by the study team.
Data analysis
Quantitative data was exported and cleaned using MS Excel 2016 (Microsoft Corporation, Redmond, WA) and all the data records that had missing data were eliminated at the cleaning stage. Data was analysed using STATA 15.0 statistical software (StataCorp, Texas). Descriptive analyses were performed for demographic characteristics, and clinical characteristics of the COVID-19 cases were presented as frequencies, proportions, and means where appropriate. The outcome variable was binary: being symptomatic (coded 0) or asymptomatic/not symptomatic (coded 1). To assess the association between the outcome variable and the explanatory variables, we considered a generalised linear model of the Poisson family with a logarithm as the conical link function with a robust error variance. This resulted in Crude Prevalence Ratios (CPR) at 95% confidence intervals. Furthermore, variables with a threshold P-value less than 0.05 (P-value <0.05) at bivariate analyses were subjected to the multivariable regression analyses to adjust for confounding, thus establishing Adjusted Prevalence Ratios (APR). At multivariable analysis, only variables with a P-value less than 0.05 were considered significant. Both the CPR and APR have been reported. Qualitative data was analysed using manual thematic analysis, diverging, converging, and emerging themes with representative quotes that were obtained during the analysis. The outputs of these findings are presented in Table 8 in the appendices.
Results
Response
A total of 1,120/1,200 RT-PC and RDT COVID-19 confirmed cases from 10 districts in Uganda completed the survey with a 93% response rate.
Characteristics of COVID 19 cases
Social demographics
Of the COVID-19 cases interviewed, more than half 51.5% (577/1120) were females. Although we found increased numbers of cases across all age groups, more occurrences were among the young and middle age groups (30–39 years) at 26.8% (300/1120). Overall, we found increased cases of up to 62% among the age group 39 years and below (Figure 2). When we adjusted for age, the majority of the cases were between 40 years and above. We further found that most of the respondents were in the business class, 25.9% (290/1,120), followed by students, 17.2% (193/1,120), farmers/peasants, 17.1% (192/1,120), and health workers, 12.4% (139/1,120). The detailed socio-demographic characteristics of the interviewed cases are summarised in frequencies and percentages (Table 2).

Figure 2. Adjusted age distribution of study participants.
Table 2. Socio-demographic characteristics and history of COVID-19 cases

Note: The significance of asterisks are 0.005.
Most respondents had visited various places or attended social gatherings: markets (20.3%), clinics/hospitals (17.6%), places of worship (10.7%), high-risk towns or districts (10.7%), and mass gatherings such as funerals (13.7%) before developing/testing for COVID-19. 21.5% (241/1120) had contacts with COVID-19-like symptomatic persons, while 18.2% (204/1,120) did not have any contact, and 59.9% (671/1,120) did not know of any contact with anyone with COVID- 19-like symptoms 2 weeks before the onset of symptoms (Table 1).
Symptoms
Most cases (79.9%, 895/1120) acknowledged having developed COVID-19 symptoms at a certain point during the course of illness, while a small proportion (17.8%,199/1120) were asymptomatic (Figure 3).

Figure 3. Symptoms experienced during illness.
a) Admission status
Only 9.1% of the COVID-19-positive cases were admitted to health facilities (Table 3). According to age group, most cases 31.3% (130) with underlying conditions were aged 40 years and above. However, an increased number of young people (13–39 years), ranging from 13% (12) to 21% (63), reported having underlying conditions (Table 3). Among the cases aged 40 years and above, 31.3% (130) had underlying conditions, and many of them who were admitted either required oxygen, ventilation, or admission to the ICU as summarised in Table 3. The most commonly encountered underlying conditions were high blood pressure, diabetes, and asthma.
Table 3. Number of cases, underlying conditions, and need for admission by age group

Note: Underlying conditions included high blood pressure, diabetes, and asthma.
b) Vaccination status
The majority of the cases investigated (78.4% or 878 cases) had not received any COVID-19 vaccine, with only 14.8% (166) having received one dose of AstraZeneca vaccine and only 4.1% (46) with two doses received among the vaccinated group (Table 4). Furthermore, slightly above average (58.7%) participants (542/924) were willing to take the COVID-19 vaccine. The elderly survivors aged 40–49 years (PR = 1.43, 95% CI 1.10–1.84) and ≥ 50 years (PR = 1.52, 95% CI 1.18–1.96) were more willing to receive COVID-19 vaccination.
Table 4. Vaccination status of COVID-19 patients

c) Survival status
From the data, 3.7% (41/1079) of the cases died of COVID-19 during the second wave. The elderly, 50 years and above, were eight times more likely to die after adjusting the prevalence ratio 8.0 (1.04–61.52). We further noted cases of death in the age group starting from 20–49 years of age but with slightly more numbers among persons who were 30–39 years, represented by the prevalence ratio of 3.8 (CI: 0.47–31.1). Additionally, participants who were vaccinated with at least one dose of the vaccine were six times more likely to survive compared to those not vaccinated as per adjusted prevalence ratio 6.1 (3.24–11.57) as shown in Table 5.
Table 5. Factors associated with survival among the COVID-19 cases

a The significance was 0.05 at 95% CI.
b The significance of asterisks are 0.005.
d) Being asymptomatic
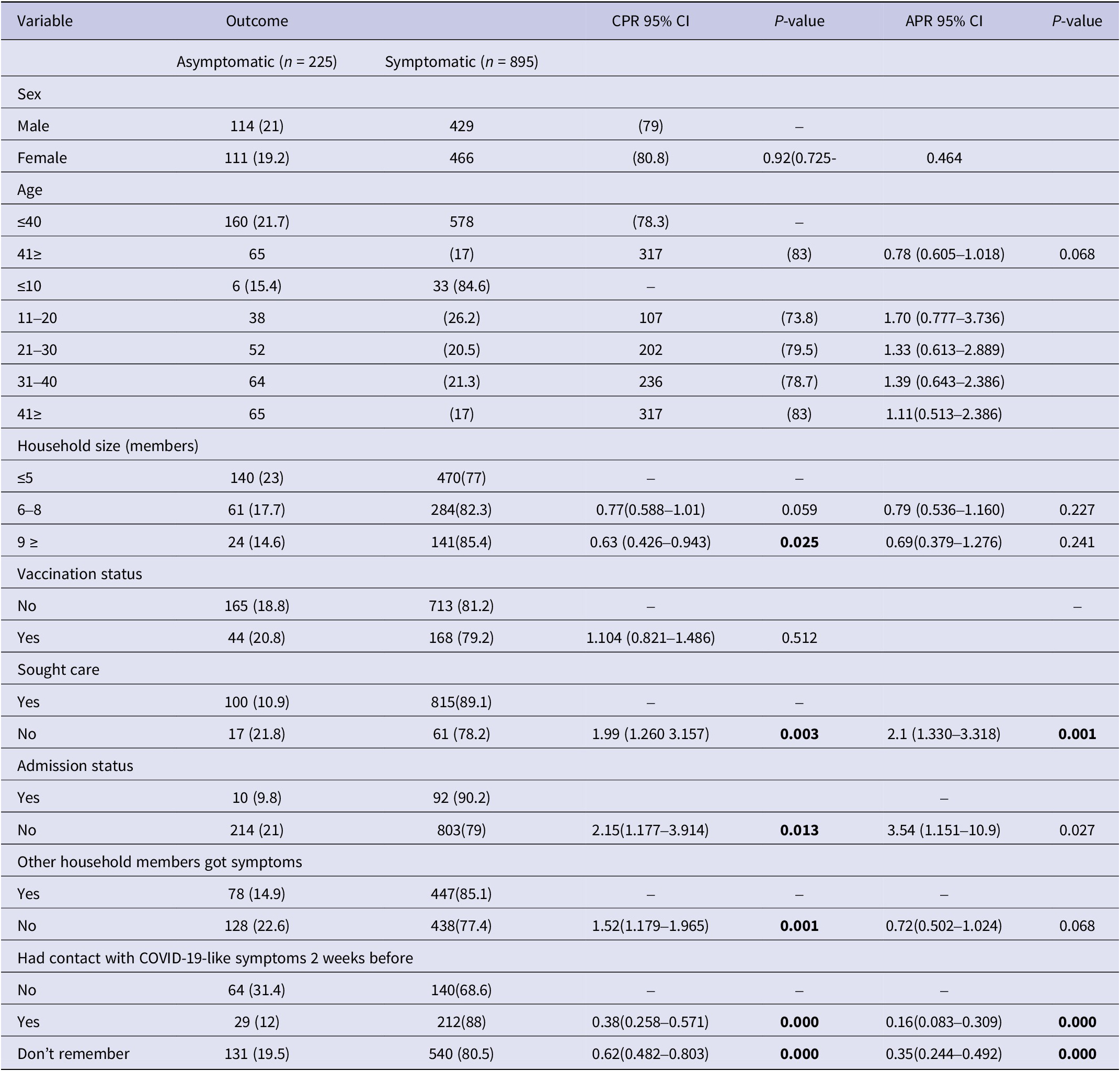
A small proportion (17.8%, 199/1120) were asymptomatic (Table 1). At bivariate analysis, results showed that not seeking care (CPR 1.99, P-value 0.003), not being admitted (CPR 2.15, P 0.013), and other household members not having symptoms (CPR 1.52, P 0.001) were positively associated with being asymptomatic among the COVID-19 cases. While a household size of greater than nine members (CPR 0.63, P 0.025), and having contact with others 2 weeks before testing (CPR 0.38, P 0.000) were likely to be symptomatic among the COVID-19 cases. The details of the bivariate analysis are presented in Table 6.
Table 6. Showing characteristics of asymptomatic patients
e) Health-seeking behaviours
Most respondents (81.7% or 915 cases) sought care after noticing symptoms of COVID-19. A total of 79.4% (823) tested after feeling COVID-19-like signs and symptoms followed by those who had been in contact with a confirmed case (19% or 197 cases). Participants from the central region (prevalence ratio 0.94; 0.94–0.99 95% CI) were less likely to seek care for COVID-19 symptoms, while health workers (PR 1.06; 1.01–1.12) and persons with underlying health problems (PR 1.04; 1.01–1.09) had more proactive health-seeking behaviours (Table 7). Being asymptomatic was found to be associated with not seeking healthcare (APR 2, P < 0.001) (Table 6).
Table 7. Intention to receive COVID-19 vaccines among health-seeking participants

Key emerging issues from key informant interviews
Key issues emerged from KIIs that could have contributed to the wide spread of SARS-CoV-2, including
infections under HBCM, social gatherings, myths, misconceptions and misinformation, politics,
schools, weak health systems, and stigma as summarised in Table 8.
Table 8. Emerging issues and respondent quotes from KIIs and in-depth COVID-19 interviews

Discussion
In this study, we assessed the factors associated with the observed wide spread and impact of COVID-19 among the Ugandan population during the second wave of SARS-CoV-2 infections between March and June 2021 from 10 districts in Uganda. In the second wave of COVID-19, we had a slightly higher proportion of female cases compared to males. Our results represent a shift from the first wave, where males were the most affected [Reference Isralowitz, Nicholson, Ferlic-Stark, Piedra, Blunck, Fragoso, Bond, Santarcangelo, Ye, McBride and Aideyan22, Reference Avadhanula23] as has been reported elsewhere [Reference Kirenga24, Reference Guan, Eastin and Eastin25].
We found that the majority of the cases reported having several and varying symptoms during the course of the disease where most of them reported cough, headache, runny nose, fever, and general body weakness as previously reported [26, 27]. We further observed poor healthcare-seeking behaviours among the COVID-19 cases in our study, where 18.3% of cases never sought care at all and 81.7% sought care after experiencing COVID-19 symptoms. Even those who sought healthcare went after experiencing advanced stages of the disease with severe symptoms like difficult breathing as verified by the information from in-depth interviews. Whereas the studied COVID-19 cases presented themselves for testing having experienced COVID-19-like symptoms, the biggest proportion (91.3%) were sent back home for home-based care management (HBCM) as designated COVID-19 treatment units were overwhelmed with severe cases. The Ministry of Health had established and approved HBCM guidelines [Reference Etheridge and Spantig28, 29] and rolled them out to decongest designated COVID-19 treatment units. Unfortunately, the HBC guidelines were rolled out without a proper strategy for implementation and supervision, and hence families with COVID-19 cases were not sure of what to do, lacked supervisory support, and were not able to adhere to SOPs within the guidelines. Our findings are in agreement with other studies conducted in the United Kingdom that showed that women were twice more likely to get COVID-19 [Reference Felice, Di Tanna, Zanus and Grossi30], although it differs from another study in China where it was found that most of the affected persons were aged 50–55 years old [Reference Gao, Sanna, Tsai and Wen31]. Such discrepancies in studies could be explained by the fact that there is previously documented high care-seeking behaviour exhibited by women than men [Reference Tran, Vu, Le, Pham, Phan, Latkin and Ho32, Reference Saah, Amu, Seidu and Bain33]. Potentially we could see otherwise a different impression if all gender sought care the same way, and therefore these results could be skewed and biased, and not representative of the real-life experience and distribution of COVID-19 in populations [Reference Mahmud and Riley8, Reference Ssali9]. There is a need for gender-specific massive sensitisation of the public about new policies on COVID-19 diagnosis, treatment, and vaccination by the relevant authorities to increase compliance and uptake of COVID-19 control measures, including the current vaccination programme and booster doses. In our study, the change in gender infection status with more females being infected and together with their social roles in families and communities facilitates close interactions with households and communities with more likelihood of increasing transmissions. We further noted increased cases among all age groups with more cases recorded in the young people aged 19 to 39 years that constituted the highest percentage (62%) of infections in the second wave. Again, our results reflect a change in the risk groups in the second wave, where young people including school-going age children were infected and probably escalated the spread of infections in their communities. Previously in several studies, the virus was more reported in adults aged 40 years and above including disease severity presentation [Reference Huang, Wang, Li, Ren, Zhao, Hu, Zhang, Fan, Xu, Gu, Cheng, Yu, Xia, Wei, Wu, Xie, Yin, Li, Liu, Xiao, Gao, Guo, Xie, Wang, Jiang, Gao, Jin, Wang and Cao34]. In our current study, we found that the virus was affecting all age groups, especially the young ones. We also report mortalities ranging from 1–3.7% among the infected young ones aged 13 to 39 years, which was not the case in the first wave. We strikingly noted high cases of underlying conditions (high blood pressure and diabetes) among the young COVID-19-positive cases aged 20–39 years. This observation is surprising and may explain the increased numbers of severe cases and hospitalisations observed and reported in the second wave. Whereas it has been severally reported that COVID-19 remains limited in young ones in terms of numbers, disease presentation, and clinical outcomes, our study suggests otherwise. We think that there has been limited attention and focus on this age group as most cases would probably remain asymptomatic and rarely tested. During KIIs, it was reported that the COVID-19 positivity rate was high, up to 70%, among students returning from boarding schools upon closure of schools in the second lockdown in June 2021. Hence, our results call for a shift in outbreak response strategies to address the current disparities and prioritise women and young generations for interventions like vaccinations and specific awareness messages targeting this category to prevent further spread of infections.
Our study further noted that there is a need to have infection prevention and control (IPC) measures to mitigate health facility–acquired nosocomial infections which may arise due to less observance of IPC guidelines. In our qualitative results, the respondents expressed fear that unprotected health workers may pose a risk for COVID-19 transmission to patients during health-seeking care. The exposed health workers before testing positive continued to interact with other patients, members of their families, and communities, an exposure factor for virus transmission. Our results are in agreement with other studies, including one of the national surveys in Italy where it was found that over 74% of the people were health workers and many of them were women [Reference Wada, Okabe and Shobugawa35]. In China, health workers were found to be positive for COVID-19 and many of them had signs and symptoms [Reference Varma36]. One more critical area of concern identified during our study were social gatherings that continued to take place unabated despite government directives on social gatherings like burials, weddings, churches, bars and restaurants, salons, markets, public transport, and schools. SOPs like wearing facemasks, social distancing of at least 2 meters, minimum numbers recommended of some social functions, and hand washing with soap/sanitisers were not being observed, ignored, or even completely forgotten. Respondents of KIIs and in-depth interviews castigated that the non-adherence to SOPs for social gatherings accelerated the number of cases in most communities observed in the second wave. Even the schools that were opened in a staggered manner with prior preparations and clear instructions to curtail transmissions became a seedbed for COVID-19 transmissions. The schools flaunted instructions and some concealed information about COVID-19 cases for fear of being closed. By the time the schools were closed again in June 2021, the cases both identified and unidentified were very high and further contributed to community transmissions upon returning home. As much as our school situation and operational settings may be different with so many boarding schools compared to other regions of the world, schools (students and teachers) had been reported as one of the super-spreaders of SARS-CoV-2 [Reference Schmidt37, 38]. The social gatherings were further fuelled by stigma, social media misinformation, and falsifications that circulated widely about COVID-19 that affected many of the instituted prevention measures as also reported elsewhere [Reference Nakkazi39]. At the time of the study and during the study period, COVID-19 vaccine access was extremely very low and only 4% of the studied COVID-19 cases had received two doses of AstraZeneca vaccine. At the national level, only less than 2% of the targeted population had received two doses of the vaccine [40]. Hence, the biggest percentage of the population remained naïve and vulnerable to SARS-CoV-2 infections and associated severe disease outcomes, especially among the elderly and those with comorbidities. In addition to having a vulnerable population, Uganda also registered and reported the existence of COVID-19 variants (Delta, Eta, Alpha, Beta, and local strain) in June 2021 [Reference Diesel41, Reference Malik42]. Low vaccination coverage together with the emergence of COVID-19 variants could have contributed to the high numbers of COVID-19 cases and associated mortalities registered in June 2021 alongside other factors already described in this study. Our study differed from other studies including the one conducted in the United States which showed that COVID-19 vaccination was up to 57%, with the majority of them at least receiving a single dose of vaccination during the same period of this study [Reference Vasireddy43, Reference O’Toole44].
Our study had a number of strengths. First, we visited different districts in Uganda which are geographically spaced and this gave a better picture of what was happening across the entire country. Secondly, we used both quantitative and qualitative methods, and this helped us to probe further on some salient issues that could have emerged from the quantitative findings. We also used a standard MOH case investigation tool, and this helps our results to be generalised across the country. We further visited quite a reasonable sample size that is representative of the COVID-19 cases at that time. We also conducted both bivariate and multivariate logistic regression for our project. We also had a good response rate of 93%, and this was a deliberate effort by the research team. Lastly, we interviewed frontline health workers and supervisors that helped us to get real-life facts on the spread of the disease during this period. We also acknowledge several limitations given its retrospective nature. First, we reviewed secondary information at the testing centres and laboratories, and this exposed us to incomplete and inaccurate documentation in such places. We also did not statistically determine the sample size for our study given the emergency we were in. Second, we had few confirmed cases by post-mortem as this was done for a few financially stable persons. We also failed to document the age and gender of the 7% non-respondents during our data collection, and yet this would inform the presentation of results. Lastly, we interviewed people during the key informants and in-depth interviews after they had gone through COVID-19 signs and symptoms, and this is likely to have contributed to recall bias among the participants.
Conclusions
Our research found that various factors, including demographic, patient, health facility and service, social, and economic-related elements, contributed to the emergence and persistence of the second wave of COVID-19 from March to June 2021.
Specifically, young, asymptomatic individuals not under home-based care, those working or studying in schools, and those who were not vaccinated were major drivers of the second wave. To effectively manage future waves of COVID-19, proactive efforts should be made to enhance home-based care services, strictly observe SOPs in schools, and increase vaccination rates. To continue protecting communities from emerging variants of SARS-CoV-2, all stakeholders, including policy makers, healthcare workers, non-governmental organisations, the public, and researchers, must work together to implement vigilant surveillance services at the community and home levels and increase vaccination uptake. This will help to minimise the health, social, and economic impacts of COVID-19.
Data availability statement
The data collected using different forms and associated data for results presented in this manuscript can be downloaded at the mWater Portal on request.
Acknowledgments
We would like to thank all the respondents for providing the necessary information that made this study a success. We sincerely appreciate Tigist Menkir for her valuable feedback that improved our manuscript. Further appreciation goes to the district leadership who actively participated in this study. We want to thank the STI Secretariat of the Republic of Uganda for entrusting us with the mandate of Epidemic preparedness at the STI Secretariat and Presidential Scientific Initiative on Epidemics (PRESIDE) and allocating us funds in the financial year 2020/21 that enabled us to conduct this study. We also appreciate a team of field epidemiologists who supported us in data collection. Our sincere appreciation goes to the districts’ leadership who accorded us an audience and permitted us to visit the cases.
Author contribution
A.W.W., B.N., M.N., G.N., T.T., A.B.E., M.M., L.M.: Conceptualised the study, designed study protocol, processed ethical approvals, conducted data collection, and developed the first draft of the manuscript; Do.B., B.A., C.N., M.D.N., R.W.M.: Participated in data collection, data analysis, reviewed the first manuscript draft; S.T.W., Do.B., C.N., R.W.M., Da.B.: Technically guided in data analysis, reviewed the manuscript revisions; Da.B. and L.M. verified all underlying data; M.M. mobilised resources for study and gave technical supervision for the field teams. All authors reviewed and approved the final version of the manuscript.
Financial support
No external funding for this study. Limited funds as part of outbreak investigation were obtained from the Government of Uganda through Statehouse, under the STI – Secretariat, Presidential Scientific Initiative on Epidemics (Epidemics Unit). The Epidemics Unit is mandated to collect and analyse all epidemiological data to inform national policies in the control and management.
Competing interest
The authors declare no competing interests. All the authors confirm that they have had full access to all the data in the study and accept the responsibility of submitting it for publication.
Institutional review board statement
The study was undertaken as part of the COVID-19 rapid response surveillance under the Government of Uganda through Statehouse, under the STI – Secretariat, Presidential Scientific Initiative on Epidemics (Epidemics Unit). The protocol was waived off for the Institutional Review Board by the Technical Inter-sectoral Committee and Enforcement on COVID-19 Response as it was considered an outbreak investigation to explain why COVID-19 cases were increasing steadily. Permission was also obtained from districts’ leadership at the sub-national level who provided letters of administrative clearance.
Informed consent statement
All study participants consented to participate in the study, and the data obtained was secured and kept under lock and key. The field team individually signed the confidentiality agreement before the commencement of the study.
Appendix A: Outbreak Investigation of Epi Characteristics, drivers, and exposures of COVID-19 in Uganda’s selected districts,_2021

Appendix B: Composition of Key Informant and Focus Group Discussion Team members

Appendix C: Key informant, in-depth interview guide, and consent form key informant guide (sub-study 2)
For this objective, key informant interviews will be conducted among the District Task Force members: RDC, L.C V, DHO, DSFP, DLFP; Head of case management (private and public): VHT, L.C.1.
What in your opinion is the response to COVID? (probe about the tenets of the response)
In your opinion, how would you generally describe and rate the response to COVID-19 in your district?
Have you played any role in the response? (probe on their experience working in the response, what has your role been, challenges, mitigation strategies, support network)
Do you have any comment on the COVID-19-related deaths? (probe on community and facility deaths)
Describe the situation of COVID-19 vaccination in your community (vaccine hesitancy, uptake, availability, myths, side effects…).
In your opinion, what are the drivers of the COVID-19 infections (risk factors/ factors associated with infections)
What are the treatment places/centres for COVID-19 in your district/ community? (probe: describe their location, capacity for case management).
What has worked well in the response? (probe on availability of equipment, vaccines, treatment beds, ICU capacity, logistics, health workers, laboratory capacity)
What has not worked well? What can be done to improve the current and future responses?
Appendix D: In-depth Interview Guide
We shall conduct four in-depth interviews with participants that have ever contracted COVID-19; two men and two women. For either gender, we shall have two participants that will have been treated in a health facility and those that underwent home-based care.
What in your opinion is the response to COVID? (probe about the tenets of the response)
In your opinion, how would you generally describe the response to COVID-19 in your district?
Describe your experience when you contracted COVID-19 (when did you contract COVID-19, where do you think you contracted it from, did you test, for how long were sick, where were you treated from)
Do you have any comment on the COVID-19-related deaths (probe on community deaths)
Describe the situation of COVID-19 vaccination in your community (vaccine hesitancy, uptake, availability, myths, side effects…).
In your opinion, what are the drivers of the COVID-19 infections in your community?
What are the treatment places/centres for COVID-19 in your district/community? (probe: describe theirs)
What has worked well in the response? (probe on availability of equipment, vaccines, treatment beds, ICU capacity, logistics)
What has not worked well?
What can be done to improve the current and future responses?
Appendix E: Consent form Outbreak Investigation and assessment of the challenges faced by the Public Health system in containing SARS-CoV-2 in Uganda, June 2021
Introduction
My name is………………………………………, a member of a team conducting a COVID-19 outbreak investigation and an assessment of the challenges faced by the Public Health system in containing COVID-19 in Uganda, June 2021 under the Presidential Scientific Initiative on Epidemics (PRESIDE) under the Science, Technology and Innovation Secretariat (www.sti.go.ug). You are selected to participate in this investigation to share your experience with COVID-19 infection. This study will seek your views about the drivers, strengths, gaps/challenges faced by the Public Health system in containing COVID-19 in Uganda.
Your views will enable the COVID-19 National Response team understand why the country is experiencing a rapid increase in the transmission of this disease so as appropriate actions/interventions can be put in place to curb the infections.
Confidentiality
The information you will share with investigation team shall be kept very confidential. All your responses will be kept anonymous and your personal identifiers like names, telephone, gender, position/role/designation will not appear anywhere in the final report. For the key informant and in-depth interviews, we request to record your audio submission(s) to enable later transcribing which will enable report generation. Regarding potential benefits, there will be no monetary benefits for you to participate in this study, however, your shared experience will contribute to the National Task Forces’ efforts in developing and implementing interventions to respond to the COVID19 Pandemic.
Potential Risks and Discomfort
You have a right to answer or decline responding to some questions which you may not be comfortable with. You may choose to end the interview or withdraw from this investigation at any during its course.
Do you have any questions?
Do you agree to participate in this study?
If yes,
Signature_______________________________.
Contact Person for Questions
If you have further questions or inquiries about this investigation, feel free to contact you may contact the following lead researchers on this study: Abel Wilson Walekhwa, +256752206865.














