Excessive intake of dietary trans-fat, saturated fat and cholesterol induces changes in lipid metabolism and leads to dyslipidaemia, which is one of the most prevalent chronic non-communicable diseases worldwide. Dyslipidaemia results from changes in lipid metabolism, being characterised by increased plasma levels of total cholesterol (TC), LDL and TAG and reduced levels of HDL. These may or may not occur simultaneously( Reference Xavier, Izar and Faria Neto 1 ). Furthermore, excessive dietary fat may lead to the accumulation of fat in the liver (liver steatosis)( Reference Kotronen and Yki-Jarvinen 2 ) that predisposes to the development of non-alcoholic fatty liver disease (NAFLD)( Reference Parafati, Lascala and Morittu 3 ) and atherosclerotic CVD (ACD)( Reference Gaggini, Morelli and Buzzigoli 4 , Reference Alheiros-Lira, Jurema-Santos and da-Silva 5 ).
Regular ingestion of fruit and dietary fibre (DF) has been recommended as primary prevention or treatment strategy for dyslipidaemia( Reference Anderson, Gregoire and Hegele 6 , Reference Grundy, Arai and Barter 7 ), NAFLD and ACD( Reference Raasmaja, Lecklin and Li 8 , Reference Yoo, Liu and Kim 9 ). Acerola (Malpighia emarginata D.C.), cashew (Anacardium occidentale L.) and guava (Psidium guajava L.) are tropical fruits consumed ‘in natura’ or processed to produce mostly juices and frozen pulp( Reference Martinez, Torres and Meneses 10 – Reference Santos, Santiago and Gadelha 12 ). Agro-industrial processing of these fruits generates a large amount of by-products (such as, peel, seeds and flesh) that if inappropriately discarded cause damage to the environment, requiring high costs to reduce their negative impacts( Reference Ayala-Zavala, Vega-Vega and Rosas-Domínguez 13 ).
Use of fruit industrial processing by-products in the human diet should be an interesting alternative destination because some of these materials contain high amounts of DF, as observed in by-products generated from acerola (37·32–80·42 g/100 g dry basis)( Reference Marques, Corrêa and Lino 14 ), cashew (76·20 g/100 g dry basis)( Reference Guedes-Oliveira, Salgado and Costa-Lima 15 ) and guava (88·7 g/100 g dry basis) processing( Reference Amaya-Cruz, Rodríguez-González and Pérez-Ramírez 16 ). In addition, high amounts of total phenolics have been observed in processing industrial by-products of acerola (7265·29 mg gallic acid equivalents (GAE)/100 g dry basis), cashew (6588·41 mg GAE/100 g dry basis) and guava (1987·19 mg GAE/100 g dry basis)( Reference Ribeiro da Silva, Teixeira de Figueiredo and Silva Ricardo 17 ). Consumption of DF may modulate the composition of intestinal microbiota and fermentation activity of beneficial bacteria (e.g. Bifidobacterium and Lactobacillus) found there( Reference da Silva, Cazarin and Bogusz Junior 18 , Reference Slavin 19 ), increasing the production of SCFA that contribute to the maintenance of intestinal health( Reference Barczynska, Slizewska and Litwin 20 ). Phenolic compounds have been also associated with positive effects on intestinal microbiota composition and SCFA production( Reference Parkar, Trower and Stevenson 21 – Reference Mosele, Macia and Romero 23 ). SCFA are saturated aliphatic organic acids exerting positive effects on intestinal and liver health and function, as well as on the regulation of lipid metabolism( Reference den Besten, van Eunen and Groen 24 – Reference Wang, Koonen and Hofker 26 ).
This study evaluated whether the consumption of by-products generated from industrial processing of acerola, cashew and guava can affect selected parameters that indicate the intestinal health and lipid metabolism of female Wistar rats with induced dyslipidaemia.
Methods
Acquisition and preparation of fruit processing by-products
By-products, mostly composed of mashed peels and seeds and small amount of flesh, generated from acerola, cashew and guava industrial processing were obtained from a fruit pulp processing industry (Polpa Ideal Indústria Ltda). The by-products were homogenised after freezing in liquid N2 and freeze-dried (−40°C, vacuum pressure <150 µmHg) for approximately 12 h in a bench lyophilizer model L-101 (LIOTOP). The freeze-dried material obtained was ground in a domestic blender, sieved through a 1·0-mm mesh and stored (−10°C) in glass containers under light protection.
Moisture, ash, protein and lipid content and the total energy value( 27 ) of these freeze-dried by-products were analysed in triplicate; total, soluble (SDF) and insoluble DF (IDF), respectively, were measured using an enzymatic-gravimetric method( Reference Prosky, Asp and Schweizer 28 ), and fructan content was measured by enzymatic hydrolysis( 27 ). Organic acids, sugars and phenolic contents were determined by HPLC under analytical conditions described elsewhere( Reference Duarte, Rodrigues and da Costa Lima 29 ). Nutritional composition and amounts of different phenolic compounds in freeze-dried acerola, cashew and guava processing by-products used in this study are shown in Table 1.
Table 1 Nutritional composition and phenolic compounds determined in freeze-dried acerola, cashew and guava industrial processing by-products (Mean values and standard deviations)

IDF, insoluble dietary fibre; SDF, soluble dietary fibre; TDF, total dietary fibre; GAE, gallic acid equivalent; ND, not detected.
a,b,c Mean values in the same row with unlike superscript letters were significantly different between the mean of each group (one-way ANOVA, P≤0·05, Tukey’s post hoc test).
Animals
In all, forty female adult Wistar rats, approximately 90 d old, were maintained individually in metabolic cages with water and food ad libitum at 21±1°C, relative humidity in the range of 50–55 % and alternating 12 h light–12 h dark cycles. The experimental protocol used in this study was approved by a Committee on Ethics in Animal Experimentation (Center of Biotechnology, Federal University of Paraíba, João Pessoa, Brazil) under the no. 0505/14. All experiments were performed in agreement with the guidelines of Brazilian College for Animal Experimentation (COBEA, Brazil).
Experimental design
After an acclimatisation period of 1 week, the female Wistar rats were randomly put into five groups: a healthy control group (HC, n 8), which received saline solution via gavage and was fed on an AIN 93M diet( Reference Reeves, Nielsen and Fahey 30 ) (Rhoster) throughout the experiment; a dyslipidaemic control group (DC, n 8), which received saline solution via gavage and was fed on a dyslipidaemic diet (Rhoster), containing 6 % lard, 5 % non-hydrolysed vegetable fat, 1 % cholesterol and 0·5 % cholic acid (online Supplementary Table S1), throughout the experiment; and three dyslipidaemic experimental groups that were fed a dyslipidaemic diet throughout the experiment and received supplementation with processing by-products of acerola (DEA, n 8), cashew (DEC, n 8) or guava (DEG, n 8).
Initially, the female rats forming the dyslipidaemic groups (DC, DEA, DEC and DEG) had undergone previous treatment by consuming a dyslipidaemic diet for 2 weeks( Reference Bouderbala, Lamri-Senhadji and Prost 31 ). After this period, the detection of dyslipidaemia was performed through biochemical examination of the lipid profile in all groups (online Supplementary Table S2). Afterwards, the female rats forming HC and DC groups received a saline solution via gavage for 28 consecutive days, and those forming the DEA, DEC and DEG groups received acerola, cashew or guava processing by-product at a dose of 400 mg/kg body weight (fruit by-products were diluted (1·6 %, w/v) in saline solution), respectively, via gavage( Reference Lakshmi, Sudhakar and Aparna 32 ) for the same time period. Gavage was performed twice a day at 4-h intervals in the morning and afternoon periods. Body weight and food consumption were assessed weekly.
On day 29 of treatment, the animals were fasted for 12 h and then anaesthetised by intraperitoneal injection of 1 ml of ketamine hydrochloride (75 mg) and 1 ml of xylazine hydrochloride (5 mg) per kg body weight. Following the euthanasia of animals via aortic transection, visceral fat samples were collected and weighed. Colon and liver were removed for histological analysis and faeces were collected from caecum for microbiological counts and quantification of organic acids and total fat. Serum was collected and maintained at room temperature (25±1°C) for determination of lipid profile.
Determination of lipid profile
Serum concentrations of TC and HDL were measured using the Trinder enzymatic method and the accelerator selective detergent method using Liquiform Cholesterol and HDL LE kits, respectively (Labtest). TAG levels were determined using the Trinder method with a TAG Liquiform kit (Labtest). All analyses followed the manufacturer’s recommendations, and absorbance was determined using a LabMax 240 Premium automatic analyser (Labtest) at 505 nm (TAG), 500 nm (TC) or 600 nm (HDL). LDL and VLDL values were calculated using previously described equations( Reference Friedewald, Levy and Fredrickson 33 ), as follows: LDL=TC–HDL–TAG/5; and VLDL=TAG/5.
Fat in liver and moisture, pH, fat and microbial counts in faeces
For three consecutive days, that is, on days 26, 27 and 28 of treatment, faecal samples were collected and stored at −20°C. For analysis, the samples were diluted in deionised water (1 mg/ml) and faecal pH was measured using a digital potentiometer (Q400AS; Quimis)( Reference Asvarujanon, Ishizuka and Hara 34 ). A separate portion of faecal sample was dried in an oven (320-SE; Fanem) at 105°C for 24 h to determine faecal moisture( 27 , Reference Huang, Tsai and Chow 35 ).
Another portion of faecal samples collected from caecum was diluted (1:9) in sterile peptone water and inoculated (20 µl), using the microdrop technique( Reference Miles, Misra and Irwin 36 ), on selective agar for counting Lactobacillus spp. (de Man, Rogosa and Sharpe (MRS); HiMedia), Bifidobacterium spp. (Bifidobacterium agar; HiMedia) and Enterobacteriaceae (MacConkey agar; HiMedia). Agar plates for counting Lactobacillus spp. and Bifidobacterium spp. were incubated under anaerobic conditions (Anaerobic System Anaerogen; Oxoid Ltd) and agar plates for counting Enterobacteriaceae were incubated under aerobic conditions, all at 37°C for 24–48 h. At the end of the incubation period, characteristic colonies on the selective media were counted, and the results were expressed as log of colony-forming units per g of faeces (log10 CFU/g)( Reference da Silva, Cazarin and Colomeu 37 ). Total lipid in faeces and liver was determined by cold extraction( Reference Folch, Lees and Sloane Stanley 38 ).
Quantification of organic acids in faeces
Contents of acetic, butyric, propionic, formic, citric, lactic and malic acids in faeces were quantified by HPLC using a 1260 Infinity LC system (Agilent Technologies) coupled to a PDA detector (G1315D; Agilent Technologies). For analysis, an Agilent Hi-Plax H (300×7·7 mm) column with 8·0 µm particle size was used; the column was protected with a PL Hi-Plax H (5×3 mm) guard column (Agilent Technologies). Column temperatures were maintained at 50°C. Each sample was diluted in ultrapure water filtered through a 0·45-µm-pore membrane, with an injection volume of 10 µl, at a flow rate of 0·5 ml/min and a runtime of 20 min. The phase was 4·0 mm H2SO4 in ultrapure water. Obtained data were processed using Open LAB CDS Cessation Edition (Agilent Technologies). HPLC sample peaks were identified by comparing their retention times with those of organic acid standards( Reference Ball and Lloyd 39 ). Duplicate injections were performed, and average peak areas were used for quantification. Standard of formic acid was obtained from Sigma-Aldrich, and standards of acetic, butyric, propionic, citric, lactic and malic acids were obtained from Vetec, all with a purity of ≥99 %. Ultrapure water was obtained from a Mille® system (EMD Millipore), and sulphuric acid was obtained from Merck.
Histopathological evaluation of the intestine and liver
Colon and liver fragments were excised, washed in saline solution (0·9 % NaCl), fixed in 10 % buffered formalin for 48 h and subjected to histological processing. Samples were dehydrated in an increasing ethanol series, clarified in xylene, embedded in paraffin and sectioned in a microtome to obtain 5 μm-thick slices. Sections were stained with haematoxylin–eosin (H&E) and periodic acid–Schiff (PAS) and analysed under a light microscope (Motif BA 200) at 4×, 10×, 20× and 40× magnifications. Morphological analysis of the intestine included evaluation of inflammatory processes presence, such as stasis, leucocyte migration, haemorrhage, vasodilation and necrosis, as well as evaluation of epithelial preservation, hypertrophy and hyperplasia of the outer muscular layer. The morphological analysis of liver included evaluation of the occurrence of degenerative processes by fatty degeneration and inflammatory parameters, including leucocyte migration, oedema, hyperaemia, haemorrhage, necrosis, preservation of liver parenchyma and presence of microthrombi.
Statistical analysis
Statistical power of 0·80 (80 %) was obtained by estimating forty adult female Wistar rats (eight females per group) when the minimally detectable effect size was 1·0 and the significance level was 0·05. Food intake, body weight and lipid intake data were compared by a two-way ANOVA for independent measures with a factor for group (five levels) and a factor for time (four or five levels). Other data with single factor for group were analysed via one-way ANOVA. When there was a difference between the obtained data, Tukey’s post hoc test was performed at a significance level of 5 % (P≤0·05). Results are expressed as the means and standard deviations. The software SigmaPlot 12.5 for Windows (Systat Software Inc.) was used to perform the statistical analysis and graphic design.
Results and discussion
Food intake and body weight
Food intake and body weight of the HC, DC, DEA, DEC and DEG groups were monitored during the 28-d experimental period (online Supplementary Fig. S1(a) and S1(b)). The HC group presented the highest food consumption during the monitored period (P≤0·05) when compared with the other groups. DEA, DEC, DEG and DC groups presented similar food consumption over time (P>0·05), with exception of the DEA group that presented lower food consumption than the HC group during the 1st week of the experimental period (P≤0·05). These results indicate that the dyslipidaemic diet consumption promoted greater satiety( Reference Duca, Zhong and Covasa 40 , Reference Perry and Wang 41 ) in the DEA, DEC, DEG and DC groups. The fruit by-products tested had little influence on the appetite of the animals, probably owing to the lower energy value provided by DF, which was detected in high amounts in these materials. These findings are similar to those found in a previous study that administered different amounts of prebiotic fibres in diet, and observed no difference in diet intake among the groups of animals receiving these fibres( Reference Parnell and Reimer 42 ).
Although consuming a high-lipid and high-energy diet (online Supplementary Table S1 and Supplementary Fig. S1(c)), the DC group showed body weight gain similar to HC group (P>0·05), which could be related to a lower dyslipidaemic diet intake by the DC group during the experimental period. Short-term dietary fat consumption is associated with satiety-inducing intestinal peptides expression, such as cholecystokinin, glucagon-like peptide-1, pancreatic polypeptide, peptide YY and oxymomodulin. Interestingly, these effects are attenuated with long-term dietary fat consumption( Reference Duca, Zhong and Covasa 40 , Reference Perry and Wang 41 ).
DEA, DEC and DEG groups showed body weight loss during the monitored experimental period (P≤0·05). Body weight loss among these groups followed the order DEG>DEA>DEC. Although the DEA, DEC and DEG groups consumed an amount of dyslipidaemic diet similar to the DC group, the DF of the fruit by-products contributed to the weight loss of the animals, as the consumption of the tested fruit by-products may have facilitated greater faecal excretion of dietary fat, implying i) lower energy absorbed and metabolised; ii) increased postprandial energy expenditure by favouring greater gastrointestinal motility; and iii) increased excretion of bile acids, inducing the mobilisation of body fat reserves for the bile acids hepatic synthesis( Reference Brownlee, Chater and Pearson 43 ). A previous study found that high-fat-diet consumption resulted in increased body weight in rats over time, whereas the same high-fat diet to which had been added 10 % dehydrated guava reduced rats’ body weight gain( Reference Esmael, Sonbul and Kumosani 44 ). Consumption of acerola juice has shown to be effective in preventing body weight gain in mice fed a cafeteria diet( Reference Dias, Leffa and Daumann 45 ).
Effects on lipid profile
The DC group exhibited higher TC, TAG and LDL levels compared with the HC, DEA, DEC and DEG groups (P≤0·05) (Fig. 1(a), (b) and (d)). The DEA and DEG groups presented lower VLDL levels compared with the DC group (P≤0·05) (Fig. 1(e)). The DEA and DEC groups presented increases in HDL levels (Fig. 1(c)) by 104·96 and 40·74 %, respectively, compared with the DC group (P≤0·05).
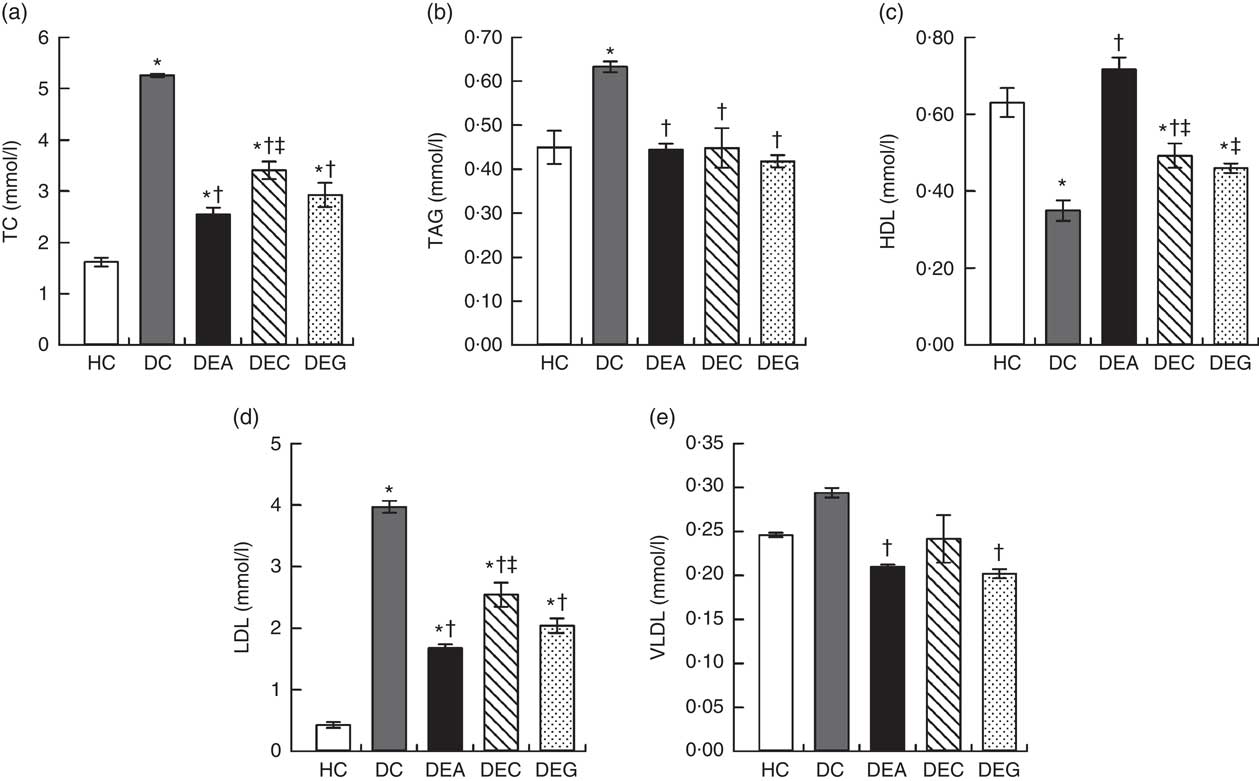
Fig. 1 Total cholesterol (a), TAG (b), HDL (c), LDL (d) and VLDL (e) of the healthy control animals (HC), non-treated dyslipidaemic control animals (DC) and dyslipidaemic animals that received acerola (DEA), cashew (DEC) or guava (DEG) industrial processing by-products. Values are means and standard deviations represented by vertical bars (one-way ANOVA, P≤0·05, Tukey’s post hoc test). * Significant difference compared with the HC group, † significant difference compared with the DC group, ‡ significant difference compared with the DEA group.
An increase in HDL levels is associated with increased protection against atherosclerosis, whereas an increase in LDL levels is associated with increased atherosclerosis risks. Similarly, high cholesterol and VLDL serum levels indicate a higher atherogenic risk( Reference Grundy, Arai and Barter 7 , Reference Kwok, Li and Cheng 46 ). Considering the results of this study, consumption of acerola, cashew and guava by-products, particularly the acerola by-product, was effective in preventing changes in lipid metabolism induced by the dyslipidaemic diet.
Improvement in lipid profile of dyslipidaemic animals receiving fruit processing by-products could be related to the fibre content and phenolic compounds (Table 1) found in these materials. SDF increases intraluminal viscosity, affects the enterohepatic circulation of bile acids and reduces fat absorption( Reference Kristensen, Jensen and Aarestrup 47 ). Phenolic compounds increase bile acids excretion and inhibit fat and cholesterol intestinal absorption by modulating expression and interacting with the intestinal cholesterol transporter Niemann-Pick C1-Like 1, which prevent the absorption of dietary cholesterol( Reference Caimari, Puiggros and Suarez 48 , Reference Dominguez-Avila, Wall-Medrano and Velderrain-Rodriguez 49 ). Excretion of bile acids in faeces induces the production of bile via cholesterol in the liver, lowering cholesterol plasma levels and cholesterol-transporting lipoproteins( Reference Zhang, Zhou and Fang 50 ).
Effects on pH, moisture, organic acids and microbial counts in faeces
The DC group showed a higher faecal pH than the HC group (P≤0·05) (Table 2). Faecal pH was lower in the DEA, DEC and DEG groups compared with the DC group; this suggests increased DF fermentation and organic acid production by colonic microbiota, causing faecal acidification in rats fed on the tested fruit by-products( Reference McOrist, Miller and Bird 51 , Reference Paturi, Butts and Monro 52 ). Consumption of a diet rich in resistant starch or fructo-oligossacharides has also shown an ability to decrease faecal pH in rats( Reference Hu, Le Leu and Christophersen 53 , Reference Rodriguez-Cabezas, Camuesco and Arribas 54 ).
Table 2 Faecal moisture (g/100 g), pH and organic acids (μmol/g) in healthy control animals, non-treated dyslipidaemic control animals and dyslipidaemic animals that received acerola, cashew or guava industrial processing fruit by-products (Mean values and standard deviations)
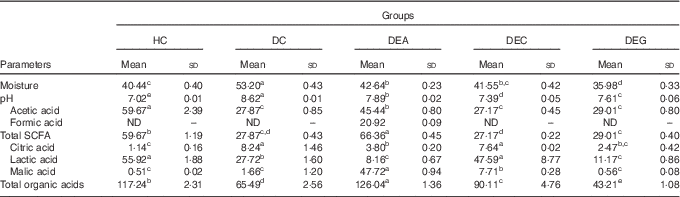
HC, healthy control; DC, non-treated dyslipidaemic control; DEA, dyslipidaemic group supplemented with acerola by-product; DEC, dyslipidaemic group supplemented with cashew by-product; DEG, dyslipidaemic group supplemented with guava by-product; ND, not detected.
a,b,c,d,e Mean values in the same row with unlike superscript letters were significantly different between the mean of the each group (one-way ANOVA, P≤0·05, Tukey’s post hoc test).
Faecal moisture was higher in the DC group than in the HC group (P≤0·05). The DEA group presented increased faecal moisture compared with the HC group (P≤0·05) (Table 2), which could be related to better water retention in the colon caused by a balanced DF intake. Increased faecal moisture was not observed in the DEG group. The guava by-products showed the highest IDF content and IDF/SDF ratio. In addition, the guava and cashew by-products presented high amounts of tannins( Reference Amaya-Cruz, Rodríguez-González and Pérez-Ramírez 16 , Reference Fonteles, Leite and Silva 55 ), which have been shown to have anti-secretory activity, inducing decreased intraluminal fluid, increased water reabsorption, reduced intestinal motility and, consequently, reduced moisture in faeces( Reference Liu, Zheng and Xu 56 ).
Diets presenting IDF/SDF ratio in the range of 1·0–2·3 typically present better capability of modulating physiological processes( Reference Martinez, Torres and Meneses 10 ). Supplementation with acerola by-products (IDF:SDF ratio: 2·42) should produce the best DF fractions balance, compared with cashew and guava by-products. Consumption of a diet containing an adequate DF balance might benefit intestinal functions by increasing faecal volume, as a result of increased water absorption( Reference Hu, Nie and Min 57 ), facilitating faecal excretion( Reference Rodriguez-Cabezas, Camuesco and Arribas 54 ).
Production of organic acids, particularly SCFA, is directly related to selective fermentation of potentially prebiotic components by colonic bacteria( Reference Yasmin, Butt and Afzaal 58 ). Although SCFA can be used as energy sources by the host, they can also act as energy consumption and metabolism regulators( Reference Salazar, Dewulf and Neyrinck 59 ). SCFA can improve blood lipid levels by suppressing hepatic lipogenesis and increasing oxidative metabolism( Reference Wang, Koonen and Hofker 26 ). The highest contents of acetic acid were found in faeces of the HC group, followed by the DEA group (P≤0·05). Formic acid was found only in the faeces of the DEA group. Highest total SCFA contents were found in the faeces of the DEA group (P≤0·05). Lactic acid was the organic acid found in highest amounts in the faeces of the HC, DC, DEC and DEG groups; malic acid was the organic acid found in the highest amount in the faeces of the DEA group (P≤0·05) (Table 2).
Considering that the acerola by-product exhibited high SDF content, the increased acetic acid content observed in the faeces of the DEA group could be related to increased colonic SDF fermentation( Reference da Silva, Cazarin and Bogusz Junior 18 ). Moderate amounts of acetic acid detected in the acerola by-product (Table 1) could be related to the detection of increased amounts of formic acid in faeces of the DEA group. Formic acid is used as substrate for acetic acid production by acetogenic bacteria via the Wood–Ljungdahl pathway( Reference Louis, Hold and Flint 60 ). In the present study, butyric and propionic acids were not detected in the animals’ faeces regardless of the consumption of acerola, cashew or guava by-products. One possible reason for this is that 95 % of SCFA produced in large intestine is quickly absorbed by colonocytes, whereas only the remaining 5 % is expelled in faeces( Reference den Besten, van Eunen and Groen 24 ).
The DC group exhibited higher faecal counts of Enterobacteriaceae (Fig. 2(b)) and lower counts of Bifidobacterium spp. and Lactobacillus spp. (Fig. 2(a) and (c)) than the HC, DEA, DEC and DEG groups (P≤0·05). The DEA group showed higher faecal counts of Bifidobacterium spp. and Lactobacillus spp. than the DC group (P≤0·05) and similar to HC group (P>0·05), suggesting that the DF and fructans present in acerola by-products (Table 1) may stimulate the increase in populations of these beneficial microorganisms in the intestine( Reference Huang, Tsai and Chow 35 , Reference Massot-Cladera, Costabile and Childs 61 ). Phenolic compounds present in acerola processing by-products (Table 1) might also contribute to these stimulatory effects, as up to 90 % of plant phenolics may reach the colon where they are used as substrates for production of small phenolic acids by intestinal microbiota( Reference Parkar, Trower and Stevenson 21 ). Small phenolic acids affect intestinal microbial composition and are used as substrates for organic acid production by colonic bacteria( Reference Parkar, Trower and Stevenson 21 , Reference da Silva, Cazarin and Colomeu 37 ). Bifidobacteria and lactobacilli can normalise fat metabolism through TAG synthesis and mineral absorption, which is also associated with a reduced incidence of colon cancer( Reference Delgado, Tamashiro and Pastore 62 ). Furthermore, DF can bind to phenolic compounds and, consequently, reduce their absorption in the small intestine. This permits a high proportion of ingested phenolic compounds to reach the large intestine to be fermented, contributing to the growth of beneficial bacterial species (e.g. Bifidobacterium and Lactobacillus) and inhibiting the growth of pathogenic bacteria (e.g. Clostridium perfringens and Enterobacteriaceae)( Reference Bondonno, Bondonno and Ward 63 ).
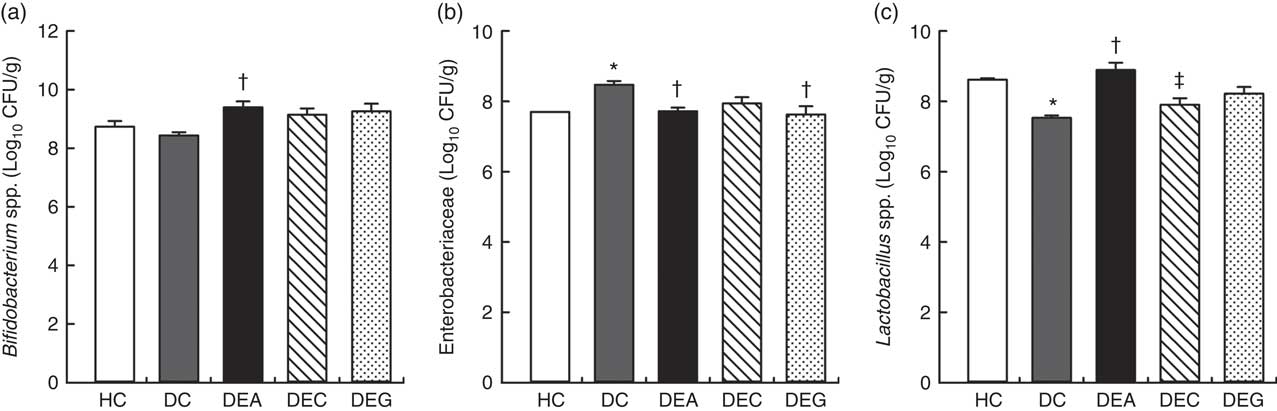
Fig. 2 Viable cell count of Bifidobacterium spp. (a), Enterobacteriaceae (b) and Lactobacillus spp. (c), in faeces of the healthy control animals (HC), non-treated dyslipidaemic control animals (DC) and dyslipidaemic animals that received acerola (DEA), cashew (DEC) or guava (DEG) industrial processing by-products. Values are means and standard deviations represented by vertical bars (one-way ANOVA, P≤0·05, Tukey’s post hoc test). CFU, colony-forming units. * Significant difference compared with the HC group, † significant difference compared with the DC group, ‡ significant difference compared with the DEA group.
The DEA and DEG groups presented lower faecal counts of Enterobacteriaceae compared with the DC group (P≤0·05). Increased counts of Bifidobacterium spp. and Lactobacillus spp. and reduced counts of C. perfringens was previously verified in the caecum contents of hamsters fed a diet supplemented with pineapple peel (rich in IDF). These results were related to increased production of SCFA and antimicrobial substances in hamsters’ intestine( Reference Huang, Tsai and Chow 35 ). Decreased luminal pH values, as observed in the DEA and DEG groups, were associated with changes in intestinal microbiota composition, hampering the growth of pH-sensitive pathogenic bacteria, such as Enterobacteriaceae( Reference den Besten, van Eunen and Groen 24 ).
Effects on visceral fat, total fat in faeces and liver
The visceral fat amount was higher in the DC group than in the DEA and HC groups (P≤0·05), indicating that supplementation with acerola by-products decreased the effects of excessive fat intake on visceral adipose tissue (Fig. 3(a)).
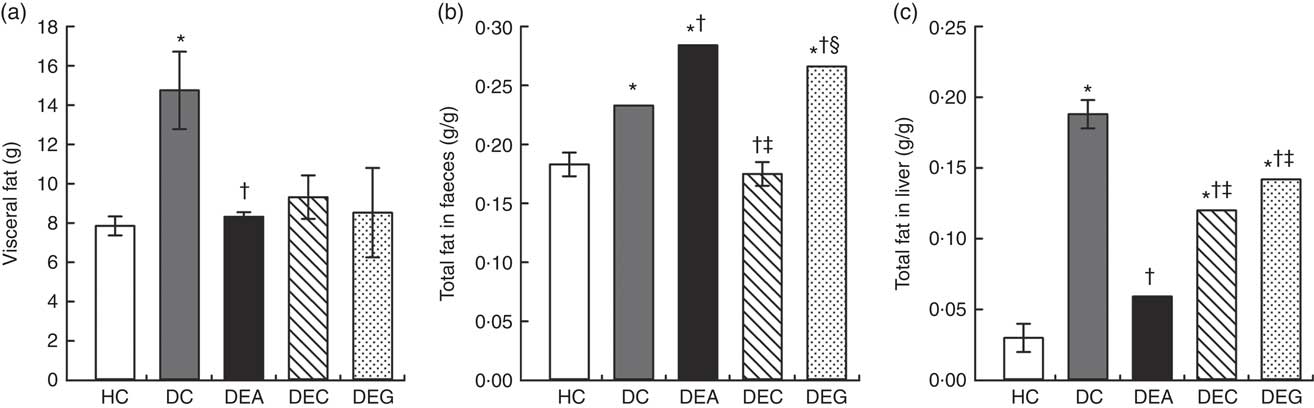
Fig. 3 Visceral fat (a), total fat in the faeces (b) and liver (c) of the healthy control animals (HC), non-treated dyslipidaemic control animals (DC) and dyslipidaemic experimental animals that received acerola (DEA), cashew (DEC) or guava (DEG) industrial processing by-products. Values are means and standard deviations represented by vertical bars (one-way ANOVA, P≤0·05, Tukey’s post hoc test). * Significant difference compared with the HC group, † significant difference compared with the DC group, ‡ significant difference compared with the DEA group, § significant difference compared with the DEC group.
Fat amount in faeces was higher in the DEA and DEG group compared with the HC, DC and DEC groups (P≤0·05) (Fig. 3(b)). High total fibre content in acerola and guava by-products may have favoured a decrease in fat absorption in the intestine( Reference Esmael, Sonbul and Kumosani 44 , Reference Perry and Ying 64 ) and, consequently, an increase in fat excretion in the faeces. DF and phenolic contents in bread containing an oven-dried or lyophilised extract of red grape processing by-products has been associated with increased fat excretion in faeces and less accumulation of visceral fat in hypercholesterolaemic rats( Reference Mildner-Szkudlarz and Bajerska 65 ). Fat excretion in faeces may have influenced the higher weight losses in the DEA and DEG groups because energy loss through faeces impacts directly on the amount of metabolisable energy in a diet( Reference Uebelhack, Busch and Alt 66 ).
The DC group exhibited higher amounts of liver fat than the HC, DEA, DEC and DEG groups (P≤0·05) (Fig. 3(c)). High-fat diet consumption can lead to the development of NAFLD, such as hepatic steatosis, which is characterised by fat accumulation in more than 5 % of liver cells( Reference Jump, Depner and Tripathy 67 ). In hepatic steatosis, there is increased formation of reactive oxygen species that damage hepatic tissue and cause mitochondrial dysfunction, reduction of β-oxidation and elevation of TAG production, which could justify the elevation of serum TAG levels in the DC group, as previously discussed( Reference Das, Mandala and Bhattacharjee 68 ).
The lowest amounts of liver fat were found in the DEA group (P≤0·05), indicating a hepatoprotective effect from supplementation with the acerola by-products. The hepatoprotective effects induced by the acerola by-products may also be related to the majority phenolics (catechin, myricetin, quercetin, rutin, 2,5-dihydroxybenzoic acid, syringic acid and salicylic acid) found in this material (Table 1). These compounds induce reduced liver fat deposition by decreasing fatty acid synthesis and lipogenic enzyme activity, as well as promoting cellular β-oxidation and lipophagy( Reference Parafati, Lascala and Morittu 3 , Reference Cho, Park and Jung 69 ). In addition, a lower amount of liver fat in the DEA group could indicate decreased activity of the lipogenic enzymes, such as lipoprotein lipase, involved in metabolism and transport of TAG-rich lipoproteins (chylomicrons and VLDL)( Reference Quinones, Miguel and Aleixandre 70 ).
Effects on histopathological characteristics of intestine and liver
Histopathological examination of H&E-stained colon samples revealed that consumption of dyslipidaemic diet caused epithelial destruction (Fig. 4(b), ▲) with the loss of organ integrity and epithelial conservation (![]() ). Colon samples stained with PAS showed loss of organ integrity, as evidenced by reduced goblet cell number (Fig. 5(b), ▲) in the DC group colonic mucosa. The HC group exhibited a normal colonic epithelial structure (Fig. 4(a) and 5(a)), and colon in the DEA, DEC and DEG groups (Fig. 4(c)–(e) and Fig. 5(c)–(e)) did not show morphological changes induced by the dyslipidaemic diet. The DEA, DEC and DEG groups showed maintenance of integrity of crypts, goblet cells and epithelial cells, indicating improved colon health. Goblet cells produce intestinal mucus and are among the most abundant cell types in colonic mucosa, exerting an important role in colon health(
Reference Kim and Ho
71
). DF and fructan amounts in acerola, cashew and guava by-products may have protected colon tissue from damage caused by a dyslipidaemic diet, as previously observed in rats fed a diet added with yacon root (rich in fructo-oligosaccharide) or fed a diet containing resistant starch(
Reference Hu, Le Leu and Christophersen
53
). The production of SCFA from the intestinal microbial fermentation of DF and phenolic compounds contained in acerola, cashew and guava by-products may have positively affected intestinal health, as SCFA are the main source of energy for the colonocytes(
Reference Wang, Koonen and Hofker
26
).
). Colon samples stained with PAS showed loss of organ integrity, as evidenced by reduced goblet cell number (Fig. 5(b), ▲) in the DC group colonic mucosa. The HC group exhibited a normal colonic epithelial structure (Fig. 4(a) and 5(a)), and colon in the DEA, DEC and DEG groups (Fig. 4(c)–(e) and Fig. 5(c)–(e)) did not show morphological changes induced by the dyslipidaemic diet. The DEA, DEC and DEG groups showed maintenance of integrity of crypts, goblet cells and epithelial cells, indicating improved colon health. Goblet cells produce intestinal mucus and are among the most abundant cell types in colonic mucosa, exerting an important role in colon health(
Reference Kim and Ho
71
). DF and fructan amounts in acerola, cashew and guava by-products may have protected colon tissue from damage caused by a dyslipidaemic diet, as previously observed in rats fed a diet added with yacon root (rich in fructo-oligosaccharide) or fed a diet containing resistant starch(
Reference Hu, Le Leu and Christophersen
53
). The production of SCFA from the intestinal microbial fermentation of DF and phenolic compounds contained in acerola, cashew and guava by-products may have positively affected intestinal health, as SCFA are the main source of energy for the colonocytes(
Reference Wang, Koonen and Hofker
26
).
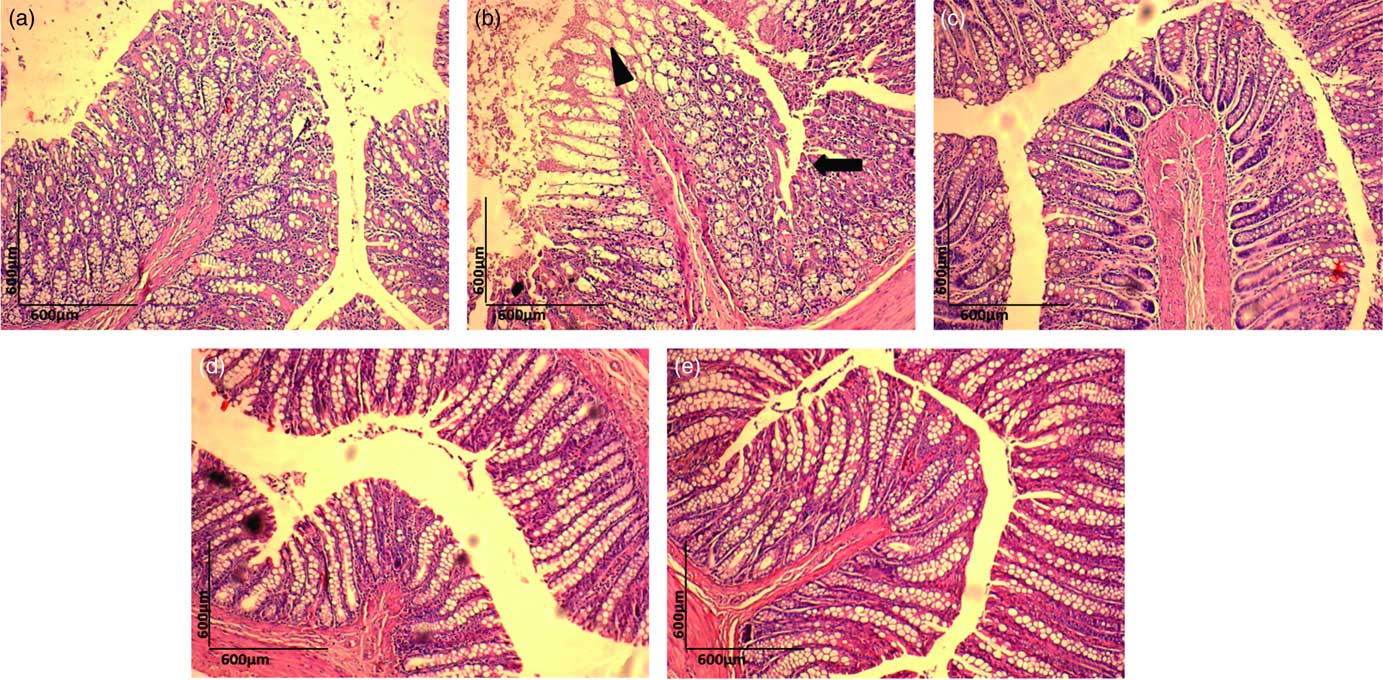
Fig. 4 Haematoxylin–eosin (H&E) staining (10×) for histopathological examination of the colon in the healthy control animals (a), non-treated dyslipidaemic control animals (b) and dyslipidaemic animals that received acerola (c), cashew (d) or guava (e) industrial processing fruit by-products.
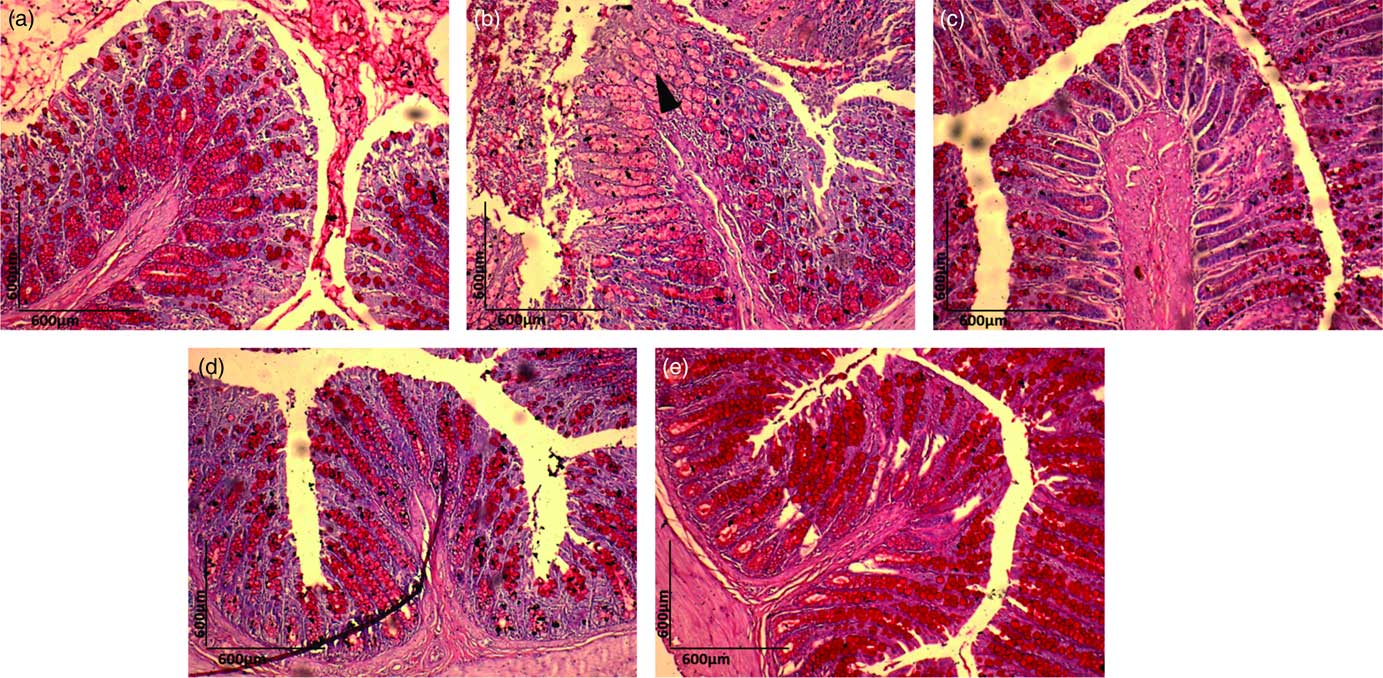
Fig. 5 Periodic acid–Schiff (PAS) staining (10×) for histopathological examination of the colon in the healthy control animals (a), non-treated dyslipideamic control animals (b) and dyslipidaemic animals that received acerola (c), cashew (d) or guava (e) industrial processing fruit by-products.
The liver architecture of the HC group was normal (Fig. 6(a)). However, the dyslipidaemic diet led to the fat accumulation in hepatocytes, and both hepatic steatosis (arrowhead) and blood stasis (black arrow) were observed in the DC group (Fig. 6(b)). The DEC (Fig. 6(d)) and DEG groups (Fig. 6(e)) exhibited reduction in steatosis compared with the DC group. The DEA group presented minor degenerative changes in the liver (Fig. 6(c)) compared with the DC, DEC and DEG groups. Previous studies on histopathological examinations of liver samples have found increased fat deposits in the livers of rodents fed a high-fat diet. These changes were reduced, however, after the animals received a high-fat diet with dehydrated Rosa laevigata Michx fruit, which contains a high amount of flavonoids( Reference Zhang, Zheng and Dong 72 ), as well as with dried oranges, strawberries and pomegranates( Reference Esmael, Sonbul and Kumosani 44 ). These hepatic protective effects were related to the existence of potential synergism among phenolic compounds present in tested materials. Fruit rich in phenolic compounds have also presented a protective effect against increased hepatic fat deposits in animals fed a high-fat diet( Reference Paim, Benjamin and Rondina 73 – Reference Pérez-Beltrán, Becerra-Verdín and Sáyago-Ayerdi 75 ). Phenolic compounds can minimise hepatic steatosis by regulating the activity of the antioxidant enzyme paraoxonase, which protects against the development of hypercholesterolaemia and atherogenesis( Reference Pereira, de Abreu and Guerra 74 ), as well as by decreasing hepatic lipogenesis through the activation of the hepatic cyclic AMP (30,50-monophosphate) (cAMP)/protein kinase A/cAMP-responsive element binding protein pathway( Reference Wang, Koonen and Hofker 26 ).
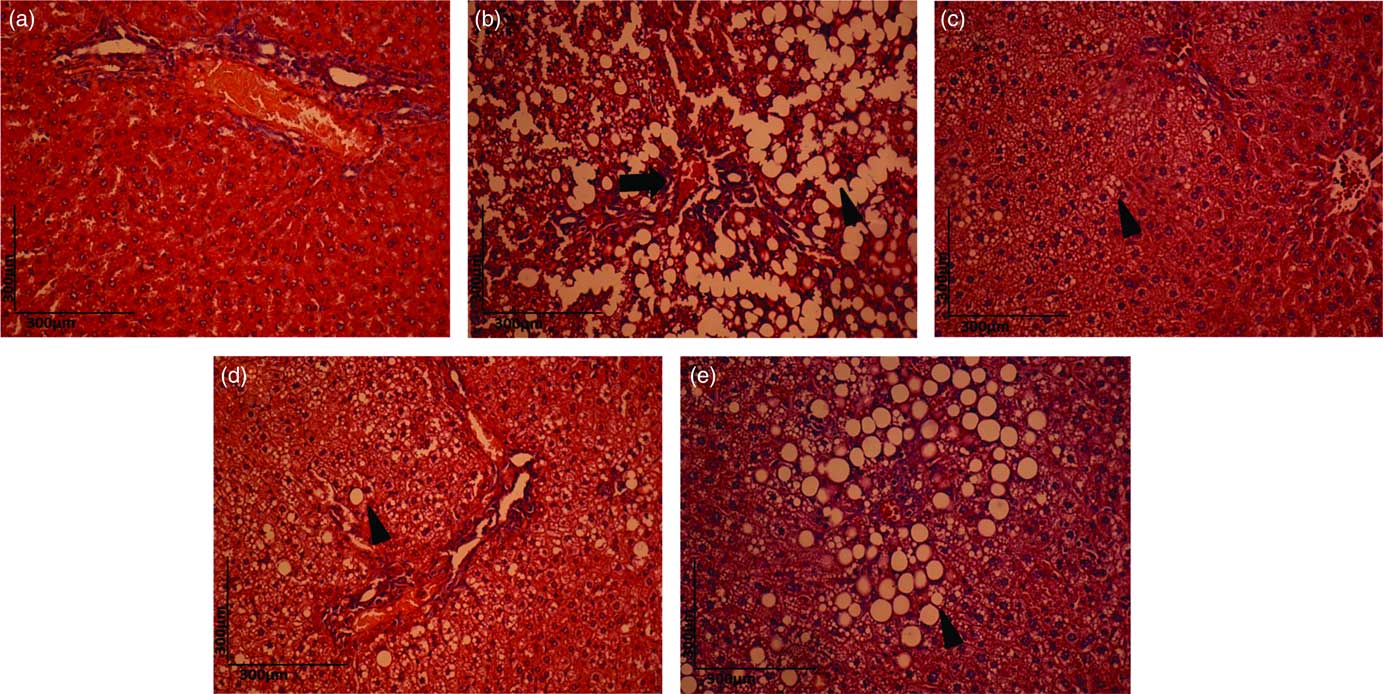
Fig. 6 Haematoxylin–eosin (H&E) staining (10×) for histopathological examination of the liver in the healthy control animals (a), non-treated dyslipidaemic control animals (b) and dyslipidaemic animals that received acerola (c), cashew (d) or guava (e) industrial processing fruit by-products.
Conclusions
In general, supplementation with acerola, cashew or guava industrial processing by-products reduced weight gain, faecal pH, liver fat accumulation, preserved colonic epithelial integrity and liver cell structure in dyslipidaemic female Wistar rats. Acerola and cashew processing by-products increased faecal moisture, and acerola and guava by-products increased fat excretion in faeces. The consumption of acerola processing by-products had the greatest effects on the monitored parameters. Its consumption of acerola by-products by dyslipidaemic female rats increased faecal counts of beneficial bacteria belonging to the Bifidobacterium and Lactobacillus genera, in addition to inducing higher organic acid production in the intestine, attenuating visceral fat accumulation and lipid profile changes. Protective effects of acerola by-products may be associated with its high amounts of phenolic compounds and other bioactive compounds and DF balance. These results encourage considering the safe use of acerola, cashew and guava by-products to protect the harmful effects caused by a dyslipidaemic diet on intestinal health and lipid metabolism. This use could be either as dietary supplements or as ingredients used in food products.
Acknowledgements
The authors thank the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; CAPES) for a scholarship to K. S. B. and the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico; CNPq) (grant no. 476302/2013-7) for financial support.
The authors thank Sidney Pratt for the revision of English.
K. S. B., A. S. S., E. L. d. S. and J. d. S. A. conceived the study, performed literature searches, wrote the manuscript and reviewed the final draft. M. d. S. L., B. R. L. d. A. M. and A. M. T. d. M. performed the characterisation analyses of fruit by-products and the statistical analysis of these results. K. S. B., A. F. A., L. A. d. S., P. P. L., J. A. d. S. G., L. T. T. and M. L. d. C. performed the biological assay and literature searches. K. S. B., M. L. d. C. and L. T. T. performed statistical analyses of the data and wrote the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517003282










