Vitamin K refers to fat-soluble vitamins with a 2-methyl-1,4-naphthoquinone nucleus, which includes the natural vitamers phylloquinone (vitamin K1) and menaquinone (vitamin K2) and the synthetic product menadione (vitamin K3). Vitamin K is an essential cofactor for the synthesis of functionally active liver-derived coagulation factors (II, VII, IX and X) in the body(Reference Tie and Stafford1,Reference Shearer, Fu and Booth2) . Thus, inadequate vitamin K may lead to vitamin K deficiency bleeding (VKDB)—a clinical entity characterised by haemorrhagic tendency(Reference Tie and Stafford1–Reference Araki and Shirahata3).
Though known for about a century, VKDB is still encountered globally(Reference Araki and Shirahata3–Reference Zurynski, Grover and Jalaludin9). The risk of VKDB is particularly high during early infancy(Reference Araki and Shirahata3,Reference Shearer4) . The onset may be ‘early’ (within first 24 h of birth), ‘classical’ (between 1 and 7 d of birth) or ‘late’ (between 2 and 12 weeks of life usually, but may manifest upto 6 months)(Reference Shearer, Fu and Booth2–Reference Shearer4). The incidence is higher in low- and middle-income countries, especially for late-onset VKDB(Reference Araki and Shirahata3,Reference Shearer4,Reference Sankar, Chandrasekaran and Kumar10) . The bleeding manifestations of VKDB are not always severe, but often produce complications (viz., intracranial haemorrhage, gastrointestinal haemorrhage, etc.) causing significant morbidity or even death(Reference Shearer, Fu and Booth2–Reference Shearer4,Reference Elalfy, Elagouza and Ibrahim6) . Thus, adequate vitamin K supplementation in newborns is vital. Towards that end, the WHO advocates intramuscular administration of vitamin K1 prophylactically to newborn babies soon after birth to prevent VKDB(11).
India is home to nearly one-fifths of the babies born in the world(12). Despite improvements in child health over the past decades(Reference Mathur and Reddy13,14) , the implementation of newborn vitamin K prophylaxis in India has been slow(Reference Rai, Luo and Tulchinsky15), with anecdotal evidence indicating that it is still not practiced routinely(Reference Kamate and Reddy16). Reliable estimates are unavailable, but VKDB appears to be quite prevalent in the country(Reference Williams, Jayashree and Bansal8,Reference Rai, Luo and Tulchinsky15,Reference Kamate and Reddy16) . In 2014, operational guidelines for prophylactic vitamin K supplementation in newborns were issued in India under a policy decision to prevent VKDB. As per these guidelines, a prophylactic birth dose of vitamin K1 (1 mg to neonates weighing ≥ 1000 g and 0·5 mg to neonates weighing <1000 g) was recommended intramuscularly for all newborns (not later than 24 h of delivery)(17). Providing vitamin K1 to neonates is also in alignment with Facility Based Integrated Management of Neonatal and Childhood Diseases (F-IMNCI) guidelines(18), and a component of the intervention package for immediate newborn care recommended under the India Newborn Action Plan(19). To inform these endeavours, this study was undertaken with the objective of determining the coverage of newborn vitamin K1 prophylaxis in India. Additionally, the variations in coverage across the country were explored.
Methods
Data sources and retrieval
The study used nationwide cross-sectional data from the Health Management Information System (HMIS). The HMIS is an initiative under the National Health Mission with technical assistance from the National Health Systems Resource Centre. It serves as a dedicated single-window web-based platform for gathering and monitoring India’s public health information(20,21) . The HMIS network mandates regular reporting of health-related information (in the form of count data) under specific headings (called ‘data elements’) from health facilities spread across the country, using standardised and uniform data reporting forms(20). The data elements pertain to a wide range of health-related events (e.g. infant deaths, complicated pregnancies, pregnant women with severe anaemia, institutional deliveries, etc.) and health services (e.g. Hb tests conducted, pregnant women receiving calcium supplements, vasectomies performed, condoms distributed, immunisation sessions held, etc.)(20,21) . This facility-level information is aggregated in a stepwise manner (i.e. successively at the block, district and state levels) for final compilation at the national level. Accordingly, all public health facilities (including subcenters, primary health centers and community health centers) are required to report their data regularly on a monthly basis (before the 5th of the following month) for compilation at the block level (Block Monthly Consolidated Report) and onward transmission to the District Programme Management Unit. The district-level public health facilities (viz., district hospitals, civil hospitals) report their monthly data directly to the district headquarters (District Programme Management Unit). The District Programme Management Unit aggregates this data along with all block-level monthly data from the district to generate the District Monthly Consolidated Report. This district report is submitted to the state headquarters, which then consolidate such reports from all the districts in the state to prepare the State Aggregated Report. Finally, all the state-level reports are consolidated to prepare the national report. The reporting and aggregation of data in HMIS are computerised. The HMIS also allows reporting of data from private health facilities, which may report either at the block level or directly at the district level(20).
The HMIS data for a particular financial year (i.e. April–March) is made available in the public domain (https://hmis.nhp.gov.in/#!/). In this study, relevant data elements (described below) pertaining to the thirty-six administrative units of India (twenty-nine states and seven union territories (UT)) were abstracted from the HMIS for the 2019–2020 reporting period (i.e. April 2019–March 2020). Since the HMIS collects and compiles deidentified data from health facilities in India and hosts it in the public domain, therefore ethical approval and informed consent were not required.
Definitions and data elements
Coverage of vitamin K1 prophylaxis in newborns was defined as the percentage of newborn children who were administered the birth dose of vitamin K1. It was determined using the data element ‘Child immunisation – Vitamin K1 (Birth Dose)’ as numerator and the sum of the data elements ‘Live Birth – Male’ and ‘Live Birth – Female’ as denominator (see online Supplemental Table 1).
The National Immunization Schedule under the Universal Immunization Programme in India recommends all newborn babies to be protected with the birth doses (also known as zero doses) of hepatitis B vaccine (HBV) and oral polio vaccine (OPV)(Reference Vashishtha and Kumar22,23) . Similar to vitamin K1 prophylaxis, the HBV birth dose is injected intramuscularly soon after delivery (and not later than 24 h). Due to the common schedule and route, vitamin K1 and HBV are administered to a newborn child at the anterolateral aspect of separate thighs(17,23) . The birth dose of OPV is provided orally as early as possible after birth (and within the first 15 d of life). While OPV was introduced into India’s routine immunisation since the 1980s, the inclusion of HBV and vitamin K1 prophylaxis into India’s routine newborn care services is relatively recent (since 2007 and 2014, respectively). Considering the operational similarities and dissimilarities, the coverage of vitamin K1 prophylaxis in newborns was compared and contrasted with that of HBV and OPV immunisation in newborns. As shown in Supplemental Table 1, the data elements ‘Child immunisation – OPV 0 (Birth Dose)’ and ‘Child immunisation – Hepatitis-B0 (Birth Dose)’ were used to assess the coverage of OPV and HBV birth doses, respectively.
Institutional birth rates (IBR), i.e. percentage of births occurring in health facilities, were determined from the number of births in health facilities (available from the data element ‘Number of Institutional Deliveries conducted Including C-Sections’) and the total number of births (given by sum of three data elements: ‘Live Birth – Male’, ‘Live Birth – Female’ and ‘Still Birth’) (see online Supplemental Table 1).
Data quality and validation
The HMIS has provisions for addressing data entry mistakes and systemic errors. These include quality-check mechanisms and guidelines, viz., black/zero examination, duplication checks, usage of consistent terminologies, standardised and uniform formats for reporting data, detection of outliers, visual checks, validation checks, etc. In addition, there are mechanisms in the HMIS to monitor the completeness and timeliness of the reported data (e.g. data status reports, data-filled summary reports, etc.)(21). The current study further interrogated the abstracted data using the following data validation rules: (a) number of newborns receiving vitamin K1 birth dose cannot exceed total live births; (b) number of newborns receiving OPV birth dose cannot exceed total live births; (c) number of newborns receiving HBV birth dose cannot exceed total live births and (d) number of institutional deliveries (including caesarean sections) cannot exceed total births (i.e. sum of live births and stillbirths). In five states/UT, at least one of these rules was violated, viz: (i) Goa (in one district); (ii) Gujarat (in twenty-six districts); (iii) Dadra & Nagar Haveli (in entirety); (iv) Daman & Diu (in one district) and (v) Lakshadweep (in entirety) (see online Supplemental Table 2). Data from these particular units (for both numerator and denominator) were deemed unsuitable and excluded from the study.
Subgroups
India is a vast and populous country with marked diversity in health and healthcare challenges(24,25) . Therefore, vitamin K1 coverage in this study was probed by various subnational groupings. The states/UT were classified for subgroup analysis into six geographical regions or zones (i.e. Eastern, Western, Northeastern, Central, Northern and Southern zones) as specified under States Reorganization Act 1956 and North Eastern Council Act 1971 (see online Supplemental Table 2).
The states/UT were also categorised by their socio-demographic index into three subgroups: low-, middle- and high-socio-demographic index (see online Supplemental Table 2). The socio-demographic index is a collective measure of developmental status that incorporates lag-distributed per capita income, total fertility rate in people < 25 years age and mean education of people aged ≥ 15 years age(24,25) .
Moreover, three ‘special developmental categories’ recognised by the government (viz., Empowered Action Group states, the Northeast (NE) states and the ‘Other’ states) were considered for subgroup analysis (see online Supplemental Table 2). In terms of development, the Empowered Action Group states (eight densely populated and poor-performing states with high disease burden) and the NE states (eight remote and hilly states in the northeastern corner of India, having substantial tribal population, limited industrialisation and poor health infrastructure) usually lag behind the ‘Other’ states (i.e. the remaining states and UT, other than the Empowered Action Group and NE states)(24,Reference Kumar and Singh26,Reference Arora, Swaminathan and Mohapatra27) . The special developmental schemes in India are often directed at the Empowered Action Group and NE states(24,Reference Arora, Swaminathan and Mohapatra27) .
Lastly, depending upon the IBR values (overall: 93·9 %; range: 59·1–99·4 %; median: 96·5 %), the states/UT were stratified into three groups by tertiles, viz., T1 or low IBR (< 93·8 %); T2 or medium IBR (93·8 to 97·8 %) and T3 or high IBR (> 97·8 %) (see online Supplemental Table 2).
Statistical analysis
The coverage (%) of vitamin K1 prophylaxis at birth among newborns was determined nationally (overall India) and subnationally (for the individual states/UT). The corresponding 95 % confidence interval (CI) was calculated by Wilson score method. As described above, the coverage of HBV and OPV birth prophylaxes were also estimated for comparisons with vitamin K1 prophylaxis. The variations in the coverage of vitamin K1, HBV and OPV among the states/UT were analysed with the help of coefficient of variation (CV) and Gini coefficient (GC). The CV (ratio of sD to mean, in percentage) indicated variability in coverage, with a higher CV % value denoting greater variability. Alternatively, the GC value reflected inequality in coverage(Reference Castillo-Salgado, Schneider and Loyola28–Reference Alonge and Peters30). Lorenz curves depicting inequalities in vitamin K1, HBV and OPV birth prophylaxis coverage were constructed for calculating the respective GC values (corresponds to twice the area between the Lorenz curve and the equality diagonal i.e. line of perfect equality). Farther the Lorenz curve is from the diagonal, greater the GC and greater the degree of inequality.
Variations in the coverage of vitamin K1 prophylaxis were also assessed across subgroups. The ‘between subgroup’ coverage variations under a grouping were quantified using coverage differences and coverage ratios, with the highest performing subgroup (in that grouping) serving as the reference. The accompanying 95 % CI estimates were derived by Taylor series expansion variance approximation method. On the other hand, the coverage variations ‘within a subgroup’ were captured using CV % and GC, computed from the coverage rates in the states/UT belonging to that particular subgroup. Data were analysed with the help of Microsoft Excel (Microsoft Office Professional Plus 2019, Microsoft Corp.) and OpenEpi v3.0.1 (http://www.OpenEpi.com) programs.
Results
A total of 20 208 804 live births documented with HMIS during the 2019–2020 reporting period were considered, out of which vitamin K1 prophylaxis was administered to 12 601 641 newborns (62·36 %; (95 % CI 62·34, 62·38)) (Table 1). The coverage ranged from 22·18 % (in Tripura) to 99·38 % (in Puducherry) among the different states and UT (Fig. 1). Eight states/UT—Arunachal Pradesh, Manipur, Nagaland, Tripura, Uttar Pradesh, Uttarakhand, Telangana and Andaman & Nicobar Islands—had coverage below 50 %. Of these, three states/UT (Tripura, Nagaland and Andaman & Nicobar Islands) had coverage below 25 %. Only a handful of states/UT (viz. Chandigarh, Gujarat, Goa, Puducherry and Tamil Nadu) achieved coverage values above 90 %. Coverage of newborn vitamin K1 prophylaxis differed by various subgroups (Table 1). A greater proportion of newborns received vitamin K1 in the Southern zone (76·66 %), high-socio-demographic index states (75·08 %), ‘Other’ states (74·03 %) and states/UT with high IBR (79·12 %) than the other subgroup(s) in their respective groupings.
Table 1 Coverage of vitamin K1 prophylaxis in newborns across India, overall and by subgroups, 2019–2020
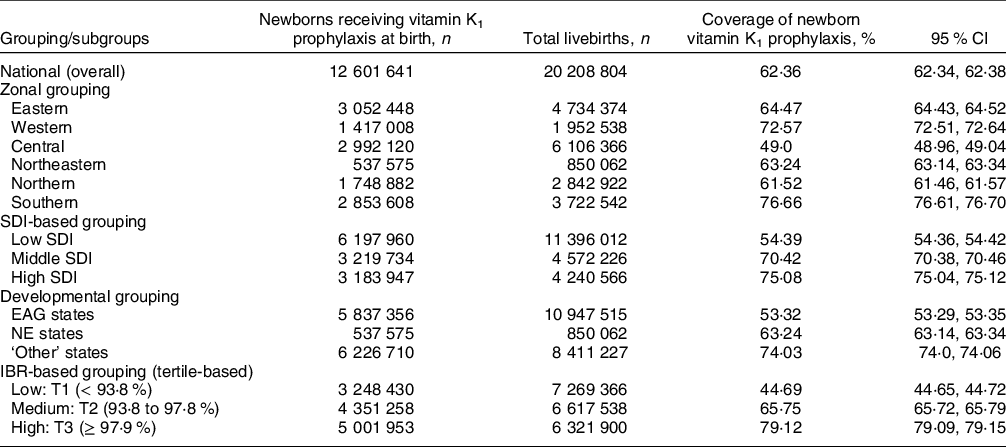
CI, confidence interval; EAG, empowered action group; IBR, institutional birth rate; NE states, northeast states; SDI, socio-demographic index.

Fig. 1 Coverage of vitamin K1 prophylaxis in newborn children at birth in the states and union territories of India, 2019–2020. (†Coverage in Gujarat, Goa and Daman & Diu were estimated in selected districts (details in methodology). Estimates for Dadra & Nagar Haveli and Lakshadweep were not calculated (n.c.). The state of Jammu & Kashmir was bifurcated in August 2019 into two union territories, namely ‘Jammu & Kashmir’ and ‘Ladakh’. The estimates reported against Jammu & Kashmir in the map are for both the union territories combined)
Notably, during the same reporting period, the coverage of the birth doses of OPV (86·70 %; (95 % CI 86·68, 86·71)) and HBV (71·41 %; (95 % CI 71·39, 71·43)) were superior to that of vitamin K1 (Table 2). Out of the three, vitamin K1 also displayed greater variations in coverage—in terms of both CV (33·74 %) and GC (0·17). Coverage inequality was the highest for vitamin K1, as seen from the Lorenz curves (Fig. 2). The superior coverage rates of OPV and HBV birth doses (as compared to vitamin K1 prophylaxis) were noticed in most states/UT of the country (see online Supplemental Table 3). However, in some states/UT (viz., Assam, Chandigarh, Goa, Gujarat, Himachal Pradesh, Jharkhand, Maharashtra, Meghalaya, Mizoram, Odisha), the coverage of vitamin K1 prophylaxis was better than that of HBV and/or OPV. In Tamil Nadu and Puducherry, the coverage rates for the three prophylaxes were fairly comparable.
Table 2 Coverage of the birth doses of vitamin K1, hepatitis B vaccine (HBV) and oral polio vaccine (OPV) among newborns in India, 2019–2020
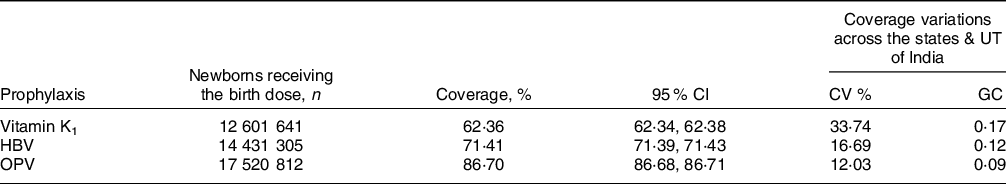
CI, confidence interval; CV, coefficient of variation; GC, Gini coefficient; HBV, hepatitis B vaccine; OPV, oral polio vaccine; UT, union territory.

Fig. 2 Lorenz curves representing the inequalities in the coverage of vitamin K1, hepatitis B vaccine (HBV) and oral polio vaccine (OPV) at birth across India
Grouping-wise comparisons revealed varying levels of ‘between subgroup’ and ‘within subgroup’ heterogeneities in vitamin K1 coverage (Table 3). The maximum ‘between subgroup’ differences were noticed across the IBR-based subgroups, wherein vitamin K1 was administered in the states/UT with low IBR to 34·43 % fewer newborns and 0·56 times less frequently than in the states/UT with high IBR (reference category). The states/UT with low IBR also had the highest ‘within subgroup’ co-efficient of variation (CV: 40·73 %), whereas the states in the Central zone exhibited the greatest coverage inequalities (GC: 0·18).
Table 3 Variations in the coverage of newborn vitamin K1 prophylaxis across India by subgroups, 2019–2020
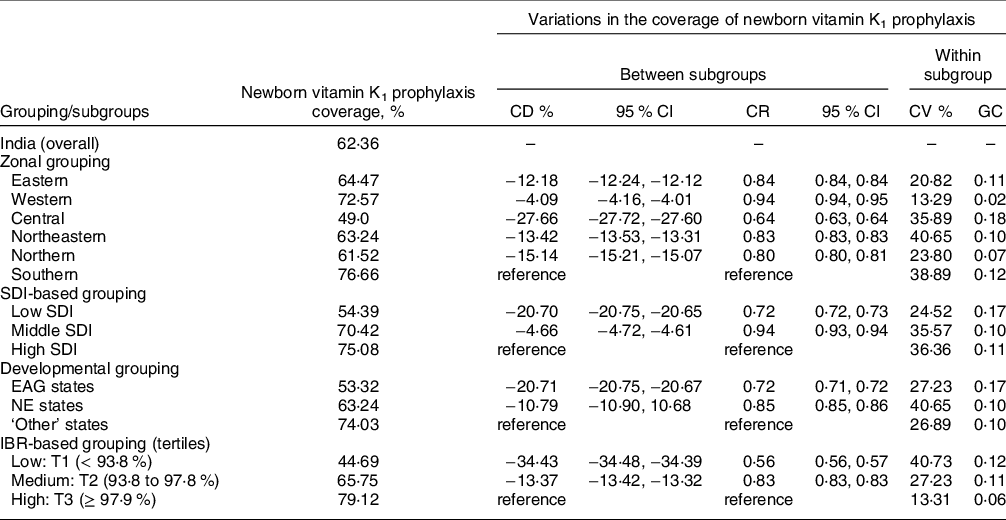
CD, coverage difference; CI, confidence interval; CR, coverage ratio; CV, coefficient of variation; EAG, empowered action group; GC, Gini coefficient; IBR, institutional birth rate; NE states, northeast states; SDI, socio-demographic index.
While analysing coverage variations between subgroups (in a particular grouping), the subgroup with highest coverage (%) was considered as reference.
Discussion
Considerable gaps and inequalities were observed in the coverage of vitamin K1 prophylaxis among newborns in India. Overall, less than two-third newborns in the country received vitamin K1 during the 2019–2020 period. The study findings have important policy implications. First, the fact that more than one-third babies were left out of vitamin K1 prophylaxis despite operational guidelines for universal coverage underscores the need for more effective implementation. Second, the reported differences and inequalities in vitamin K1 prophylaxis would guide regular monitoring and targeted allocation of resources in a need-based manner for redressing the unmet coverage gaps in various parts of the country. Third, given that a considerable proportion of newborns were not covered by vitamin K1 prophylaxis, it is likely that India has a sizeable burden of VKDB. As reliable estimates of VKDB-related burden are currently unavailable, systematic investigations should be initiated. Elsewhere in the world, VKDB surveillance programmes have helped in determining the VKDB-related burden and bolstering the VKDB prevention efforts(Reference Shearer4,Reference Zurynski, Grover and Jalaludin9,Reference Takahashi, Shirahata and Itoh31–Reference Shearer33) . The feasibility, applicability and benefits of establishing such programmes in the Indian context should be explored.
Although newborn vitamin K1 prophylaxis is regarded as a safe, reliable and cost-effective intervention against VKDB in many countries including India(Reference Shearer, Fu and Booth2–Reference Shearer4,Reference Sankar, Chandrasekaran and Kumar10,11,17,Reference Ng and Loewy34,Reference Mihatsch, Braegger and Bronsky35) , the challenges to implement it continue to exist(Reference Majid, Blackwell and Broadbent36). Reports from developed countries have increasingly documented instances of parents refusing vitamin K1 prophylaxis in their newborn children(Reference Schulte, Jordan and Morad7,Reference Zurynski, Grover and Jalaludin9,Reference Shearer33,Reference Marcewicz, Clayton and Maenner37–Reference Loyal and Shapiro39) . The common reasons include cultural and religious beliefs (e.g. clotting ability in newborns normalise by 8th d of life), poor awareness about VKDB and its prevention, apprehensions about harm to the baby (e.g. injury, pain, entry of germs, etc.), misinformation (e.g. vitamin K1 predisposes to cancer, etc.) and parental preference for ‘alternative’ and ‘natural’ lifestyle(Reference Shearer33,Reference Majid, Blackwell and Broadbent36–Reference Loyal and Shapiro39) . While these are globally relevant issues, a recent online survey brought to light some unique challenges that can hamper vitamin K1 prophylaxis in low- and middle-income countries, viz., high rates of home deliveries, perception among health workers that neonatal vitamin K prophylaxis is not a priority and supply chain shortcomings(Reference Coffey and Gerth-Guyette40).
The sizeable proportion of home deliveries in some states of India is indeed an important hindrance to achieving universal newborn vitamin K1 prophylaxis because it precludes the opportunity of contact with skilled manpower (viz., medical officer, staff nurse, auxiliary nurse midwife) for injecting vitamin K1, which is otherwise feasible during institutional deliveries. The differential coverage patterns that emerged across the IBR-based tertiles in this study substantiated that challenge. Geographical location, socioeconomic development and demographic variables were other important factors that affected the coverage. Training of community health workers to administer vitamin K1 injection at home may be a practicable solution to improve compliance(Reference Bang, Bang and Baitule41), at least in areas with high rates of non-institutional deliveries.
The more extensive and less variable coverage of OPV and HBV birth prophylaxes in contrast to vitamin K1 (during the same reporting period) suggests the presence of additional barriers to the successful implementation of the latter. It is true that OPV is relatively easier to administer (skilled manpower not required since the route is oral) and offers a greater window period (upto 15 d post-delivery). It is also likely that the greater awareness about OPV and HBV (due to regular immunisation drives and awareness campaigns against vaccine preventable diseases) had contributed to their enhanced coverage. However, there are some operational advantages in favour of vitamin K1 prophylaxis, as well. For instance, the stability of vitamin K1 at room temperature obviates the requirement of additional logistics/expenditure for storage and renders it suitable for routine practice even in resource-limited settings(17), unlike HBV and OPV that have stringent temperature requirements (stored at +2 to +8°C in ice-lined refrigerators) for maintaining cold chain(23). Moreover, the recommended vitamin K1 preparations are available as small dose vials (either 1 mg/1 ml or 1 mg/0·5 ml)(17) that facilitate single-usage and minimal wastage. On the contrary, OPV and HBV are available as multi-dose vials owing to which health workers may be reluctant to open a new vial for concerns of vaccine wastage (in spite of an ‘open vial policy’), more so when the number of deliveries in the health facility is low(23,Reference Khan, Shil and Mohanty42) . Therefore, considering the same route and similar timing of administration, the poor coverage of vitamin K1 (despite the operational advantages) vis-à-vis HBV is especially noteworthy and the reasons thereof merit closer examination. In particular, some populous states with low socio-demographic indicators (e.g. Bihar and Uttar Pradesh) and some states in remote and frontier locations (e.g. Andaman & Nicobar Islands, Arunachal Pradesh, Nagaland, Sikkim and Tripura) displayed stark differences in the coverage of vitamin K1 and HBV birth doses. In light of the above, the barriers are possibly specific to vitamin K1 prophylaxis and perhaps situated more proximally in the causal pathway. In the past, quality improvement interventions had led to improved neonatal health services (including vitamin K1 injection) in selected health institutions from India(Reference Sarin, Kole and Patel43). Nonetheless, from the implementation point of view, systematic steps are essential to identify and overcome the critical barriers that had affected the newborn vitamin K1 prophylaxis programme.
Interestingly, certain states (viz., Assam, Chandigarh, Goa, Gujarat, Himachal Pradesh, Jharkhand, Maharashtra, Meghalaya, Mizoram, Odisha) exhibited patterns that stood out as exceptions to the national trends. The coverage of vitamin K1 prophylaxis among newborns in these states was more than that of OPV and/or HBV. From the estimates in the individual states/UT and the various subgroups, it appears that a combination of factors (rather than a single factor) were responsible for the coverage disparities. Anecdotal experiences indicate that factors such as adequate and timely supplies, stockouts, availability of supporting infrastructure (e.g. when there is a need for cold-chain maintenance), knowledge and awareness, accountability, attitude and motivation and the perceived priorities of the health workers can play a critical role at the ground level in influencing newborn services(Reference Kamate and Reddy16,Reference Coffey and Gerth-Guyette40) . Besides, the first postnatal check is a good opportunity for contact with the newborn–mother pair during which health workers may offer services missed at the time of birth (if still within the recommended timeframe). Findings from the National Family Health Survey-4 report unveiled that the timing of the first postnatal check after birth (whether less than 4 h, 4–23 h, 1–2 d or 3–41 d) as well as the type of health provider providing that check (viz., doctor, nurse, midwife, lady health visitor, trained birth attendant, accredited social health activist, etc.) varies considerably across the different states/UT of India(44). It is plausible that the heterogeneity in the coverage of vitamin K1, HBV and OPV at birth were related to these factors, too. Health systems/service delivery in different states and UT of India have discernible differences with respect to key inputs and processes such as human resources, infrastructural facilities, quality accreditation, reporting mechanisms and utilisation of funds by the implementation agencies(45). Thus, such counter-intuitive patterns in a country like India (which has enormous variations and diversity in health and healthcare; often called ‘nations within a nation’(24,25) ) may be an outcome of the specific on-site scenarios in the concerned states/UT. It is also a reflection of the reality that a nationwide generalised ‘one-size fits all’ solution may not be applicable. For effective implementation of vitamin K1 prophylaxis throughout the country, customised interventions that take into account the challenges and context of the individual states/UT would be necessary.
Limitations
The HMIS is a major source of India’s health-related information that has expanded, evolved, improved and strengthened over the years. To the best of knowledge, HMIS is the only source of routinely collected and publicly accessible nationwide vitamin K1 prophylaxis data in India. However, there were certain limitations in the study. Although the HMIS currently collates data from nearly 200 000 health facilities all over the country, it still does not cover all health facilities (e.g. data from private health facilities is rather limited). That may have introduced some bias in the estimates. But that bias was potentially minimised by the extensive reach of the HMIS network (e.g. more than 20·2 million or nearly 80 % babies born in the country(12) were encompassed in this study). Besides, data quality for selected health indicators showed deviations between HMIS-reported data and data reported from surveys (e.g. National Family Health Survey-4) during a NITI Aayog evaluation(45). These deviations varied according to the evaluated indicators and also across the individual states and UT. Since the reporting of vitamin K1 prophylaxis was not evaluated in that exercise, its data quality is unknown and therefore may be interpreted with caution. The study had other limitations as well. It could not capture the ‘immediate reasons’ (viz., insufficient supply, poor awareness of health workers, parental refusal, etc.) responsible for the gaps in vitamin K1 coverage. It also could not ascertain the health outcomes in babies who received vitamin K1 prophylaxis v. babies who did not. These aspects were beyond the scope of this report, and require exploration through future studies.
Conclusion
In conclusion, this report describes the coverage of vitamin K1 prophylaxis among newborns in India. In spite of operational guidelines for universal prophylaxis, extensive gaps and inequalities in coverage were evident. Overall, more than one-third newborn children were not supplemented with prophylactic vitamin K1 during the 2019–2020 period. The situation was not uniform across the country, and varied according to geographical location, sociodemographic variables, developmental factors and rate of institutional deliveries. There were wide disparities in coverage amongst the different states and UT. Further sociobehavioural and health systems research are warranted to uncover the root causes and ‘immediate reasons’ that had hindered the practice of vitamin K1 supplementation in India. In addition, the health burden attributable to the gaps in coverage should also be investigated. Continuous vigilance, regular monitoring and multisectoral coordination will be crucial for a successful newborn vitamin K1 prophylaxis programme in the country.
Acknowledgements
Acknowledgements: I am grateful to the ICMR-Advanced Centre for Evidence Based Child Health (ACEBCH) at the Department of Pediatrics, PGIMER Chandigarh (India) for the wonderful capacity building activities and constant motivation. I also reminisce and thank Dr. Pallabi Konwar (Surveillance Medical Officer, NPHSP, WHO India) for the interesting discussions we had about the challenges that affect maternal and child health services in India. Further, I acknowledge the administrative support from Dr. K. Narain and Dr. S.K. Sharma. Financial support: No financial support was received for this work. Conflict of interest: There are no conflicts of interest. Authorship: K.B. conceived the study, analysed the data, interpreted the findings, drafted the manuscript and finalised it. Ethics of human subject participation: Not applicable
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021003670








