Recommendations about the amounts and kinds of dietary fat to achieve good health without altering the risk of cardiovascular complications have been derived from many studies over many years. Epidemiologic studies typically reflect the general trends relating to lifestyle in which the diet is a major factor but over long periods of time. In contrast, short-term, well-designed interventional studies with appropriate controls can define changes relating to specific interventions on blood plasma lipoproteins, which have proven influences on the risk of CVD. Recent dietary fashions for body mass reduction and improved health have appealed to the public. A high-fat, high-protein diet has gained popularity for short-term mass loss( Reference Astrup, Larsen and Harper 1 ) even though the public is aware that the increased cholesterol and saturated fat intake are generally accepted to have adverse effects. This document will briefly review the insights over the past three decades that relate the impact of dietary lipids on lipoprotein metabolism as it applies to the person with mild dyslipidaemia and those persons who have inherited metabolic disorders in lipoprotein metabolism.
Dyslipidaemia
Dyslipidaemia may be defined as any change of lipids or lipoproteins, quantitatively or qualitatively, in the blood that signifies an increased risk of disease. The lipid profile, whether in a state of health or disease, is the result of interplay between genes and the environment with varying contributions in each individual. Nutrition is a powerful connection between these two processes. Important nutritional considerations include energy consumption, macro-nutrients and micro-nutrients as well as interaction between these nutrients and genes, over variable periods of time and with different requirements at physiologic or pathologic changes.
Vascular disease is generally multi-factorial and relatively low in the population at large until an elderly state is reached although lifestyle-related factors may increase risk. The risk is very high and practically unifactorial in individuals with severe metabolic errors such as familial hypercholesterolaemia in which the average age of death from myocardial infarction was at the age of 43 years for men( Reference Slack 2 ). The monogenic disorders bringing about atherosclerosis are usually easy to identify in a family: severe hypercholesterolaemia is a clear indicator of an adverse outcome, but the polygenic disorders are less predictable and may be further compounded by modulating genes and lifestyles.
The chief concern about dyslipidaemia is atherosclerosis. The pathophysiology has been studied intensively since the response to injury hypothesis( Reference Ross 3 ). It is an inflammatory process that is modifiable. Epidemiologic studies reveal that lifestyle factors including diet, obesity and certain common disorders such as hypertension, diabetes and chronic kidney disease play significant roles and (partially) successful intervention with pharmaceutical agents culminated in clinical guidelines for persons at risk, such as recently published in South Africa( Reference Klug, Raal and Marais 4 ).
The pathophysiology of atherosclerosis is one of gradual progression. The process commences with endothelial damage and the penetration of lipoproteins and leucocytes finally lead to plaque formation where rupture and thrombosis can suddenly cause complications( Reference Falk 5 ). To combat the complications of dyslipidaemia it is desirable that the risk be identified early, and that intervention addresses all risk factors and not only the predominant one. The evidence-base for medication to reduce the risk of complications( 6 ) is better than that for nutritional interventions although it appears that a low-fat diet has cardiovascular benefits exceeding the degree of blood cholesterol reduction( Reference Stamler and Shekelle 7 ). It should be borne in mind that there is a postprandial exposure to chylomicrons and remnants that may affect vascular function, including effects attributable to thermally stressed lipid( Reference Williams, Sutherland and Mc Cormick 8 ). However, in the fasted state, it is mainly the lipoproteins from the liver that are observed and of particular interest is LDL on which guidelines, including the South African guidelines, focus. Depending on the contextual risk of the patient, the treatment target for LDL cholesterol is <3 mm/l, <2·5 mm/l and <1·8 mm/l in the usual, high risk and very high risk settings, respectively( Reference Klug, Raal and Marais 4 ). It is noted that a change of LDL cholesterol by 1 mm/l lowers the cardiovascular events by 22 %.( 6 ) It has become clear that hyperlipidaemias where the TAG is higher than the ideal (1·7 mm/l) and low HDL cholesterol also confer risk and may be targets for intervention as well.
The prediction of CVD in people who do not have identifiable monogenic disorders is difficult. Epidemiologic evidence from the seven countries’ study( Reference Keys, Menotti and Aravanis 9 ) indicated a relationship between the cardiovascular mortality and the plasma cholesterol concentration. Within a population there appears to be an exponential rise in risk in the range seen in a developed country( Reference Stamler, Wentworth and Neaton 10 ). Risk prediction can further be refined by sub-dividing cholesterol to HDL cholesterol and LDL cholesterol( Reference Drexel, Amann and Rentsch 11 ) but postprandial phenomena and the nature of fatty acids are less amenable to analysis and study.
Impact of dietary fats on the plasma lipid profile
In people with mild dyslipidaemia there can be significant differences in TAG excursion after a meal. Longer postprandial TAG exposure in people with the atherogenic lipoprotein phenotype( Reference Austin, Kim and Vranizan 12 ) comprising modest hypertriglyceridaemia, mildly depressed HDL cholesterol concentration and small dense LDL may not be recognised by conventional tests. Several biological determinants have been found for the atherogenic lipoprotein phenotype: it is more common in males and with increasing age, and is strongly influenced by BMI or waist:hip ratio. During pregnancy this change happens physiologically as a result of increasing TAG concentration. The atherogenic lipoprotein phenotype may also display a familial trait. Even though high carbohydrate diets may increase VLDL concentration somewhat and fail to raise HDL, the lesser postprandial exposure to remnants of TAG-rich postprandial lipoproteins may confer benefit in the atherogenic lipoprotein phenotype as well.
The response to ingested cholesterol is also known to be variable and to be saturable. Considering the amount of cholesterol in food, the plasma volume and the rapid clearance from the circulation, the effect of a cholesterol-rich feed on plasma cholesterol is negligible when viewed over hours to a day. However, with sustained cholesterol feeding the modulation of the LDL receptor reduces hepatic uptake of plasma LDL and plasma LDLC rises with a mean of about 1·5 mm/l( Reference Hopkins 13 ) to achieve a new steady state between production and clearance of LDL. There are normal subjects who have different steady states with normal variants in apoE( Reference Dallongeville, Lusier-Cacan and Davignon 14 ) and apoAiv( Reference McCombs, Marcadis and Ellis 15 ).
The nature of the dietary fatty acids also influences the response. Saturated fatty acids promote the LDL elevation and PUFA reduce the LDL increase on cholesterol feeding( Reference Grundy and Denka 16 ). Cholesterol feeding increases LDL and usually also HDL cholesterol, saturated fats in the diet also tend to increase LDL and HDL. MUFA may decrease LDL somewhat, leaving HDL cholesterol normal. PUFA, some of which are essential, tend to decrease HDL and LDL cholesterol. The n-3 PUFA may, especially with mild hypertriglyceridaemia, increase the LDL cholesterol( Reference Tinker, Parks and Behr 17 ). Trans-unsaturated fatty acids are known to decrease HDL cholesterol, whereas SFA increase LDL cholesterol( Reference Mozzafarian, Katan and Ascherio 18 ). Table 1 summarises information on lipoprotein responses to cholesterol and different classes of fatty acids, bearing in mind that dietary manipulation may not only increase one entity but could also decrease another.
Table 1. Collation of plasma lipoprotein responses to various dietary lipid changes
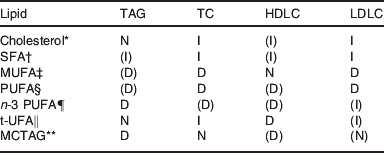
TC, total cholesterol; HDLC. HDL cholesterol; LDLC, LDL cholesterol; t-UFA, trans unsaturated fatty acids; MCTAG, medium-chain TAG. D, decrease; I, increase; N, neutral; () variable responses.
* Response to cholesterol feeding is increased by SFA and decreased by PUFA.
† SFA promote coagulation and inflammation.
‡ MUFA have low oxidation potential.
§ PUFA from plants provide essential fatty acids, mostly of n-6 type, but some α-linolenic acid is a C18 n-3 PUFA.
¶ n-3 PUFA delay coagulation and enhance repair after inflammation.
‖ t-UFA occurs at low concentration in products from ruminants and at higher concentrations in partially hydrogenated PUFA.
** MCTAG release fatty acids that proceed to the liver through the portal vein and are more readily taken into mitochondria for oxidation, useful in management of hypertriglyceridaemia.
Although the effects of dietary lipids on lipoproteins may be relatively small in most people, they can certainly have significant impact in a lifetime and may also influence other processes that govern outcome. The role of n-3 PUFA in inflammation as well as the healing response has recently been recognised( Reference Serhan and Chiang 19 ). There are also epidemiologic studies that indicate reductions in heart disease with increases in consumption of n-3 fatty acids as fish( Reference Kromhout, Bosschieter and de Lezenne Coulander 20 ) or supplementations( Reference Saravanan, Davidson and Schmidt 21 ). An n-3 index has been suggested( Reference Harris 22 ) by relating the sum of EPA and DHA to the sum of all the fatty acids in red cell membrane. If <4 %, then supplementation of 1–3 g n-3 PUFA/d is recommended. If >8 %, it is viewed as desirable and no action needs to be taken. Between 4 and 8 % requires 0·5–1 g/d n-3 PUFA supplementation. The benefit f such an approach has not been verified in an interventional study but additional intake of n-3 fatty acids over a diet containing several portions per week may be worthwhile in very high risk cases.
Another dietary component that may modulate the plasma cholesterol concentration is the consumption of plant sterols and stanols. Plant sterols have about 3 % uptake and plant stanols (saturated B-ring) have an uptake of about 0·3 %. These molecules displace cholesterol from the micelle in the gut and impair uptake of cholesterol into the enterocyte at the level of Niemann–Pick C1-like protein 1. Once taken in the plant sterols are preferentially secreted by adenosine binding cassette G5 and G8 into the intestinal or biliary tract( Reference Calpe-Berdiel, Escola-Gil and Blanco-Vaca 23 ). Lessening the absorption of the cholesterol in the intestinal lumen reduces the delivery of cholesterol to the liver and consequently there is an up-regulation of LDL receptors that within 4 weeks will lower the LDL cholesterol by approximately 9 %. The finding( Reference Assmann, Culen and Erbey 24 ) that higher plant sterol concentrations predict vascular disease may refer more to higher sterol absorption than an adverse effect as a recent review did not find the consumption or plasma concentration of plant sterols to be predictive of vascular disease( Reference Genser, Silbernagel and Debacker 25 ). While recognising that no outcome studies are available on the use of plant sterols their use is nevertheless recommended( Reference Katan, Grundy and Jones 26 ). Importantly, plant sterols are contra-indicated in patients with phytosterolaemia, a rare disease that is recessively inherited and masquerades clinically similar to familial hypercholesterolaemia.
Hypertriglyceridaemia and other dyslipidaemias
The management of dyslipidaemia focuses mainly on atherosclerosis but hypertriglyceridaemia is important to treat as there is a high risk of pancreatitis as well, with significant morbidity and mortality. Most of the fatty acids in the diet are in TAG but phospholipids and, to a very limited extent, cholesterol ester also contributes to fatty acid intake. Fatty acids are re-assembled into TAG in the enterocyte and are transported in chylomicrons. It is not generally appreciated that the plant oils are pure TAG which lend taste, texture and satiety to food but may be harmful if consumed in large amounts in cases of hypertriglyceridaemia. The dietary variation in TAG intake is large: 20–200 g/d. If a bolus of fat of 85 g (approximately 100 mm) is consumed, with complete absorption and distribution into 3 litres plasma, the change in plasma concentration will be 33 mm/l if no clearance occurs. This is the case in familial chylomicronaemia of which lipoprotein lipase deficiency is the commonest cause. Inherited as a recessive condition this may be unexpected in a family and the diagnosis may be delayed. The lipaemic blood will be evident in the neonatal period and eruptive xanthomata will usually precede the onset of pancreatitis. Recurrent pancreatitis will cause failure to thrive and can bring about exocrine and endocrine failure of the pancreas. The response to the low-fat diet is dramatic. For an adult, restricting the intake of TAG to 25 g daily will usually avoid recurrent pancreatitis. Infants and children need special dietary assistance to ensure optimal energy supply as well as complete nutrition.
Rare but severe disorders such as phytosterolaemia and adrenoleukodystrophy require very special dietary lipid modifications. Phytosterolaemia may cause severe hypercholesterolaemia that could be mistaken for familial hypercholesterolaemia except that it is recessively inherited. Adrenoleukodystrophy is an X-linked recessive disorder in which very long-chain fatty acids accumulate but do not alter the conventional measurements of plasma lipoproteins. The diagnosis and management of these disorders are best undertaken at specialised lipid clinics.
Conclusion
Atherosclerosis is generally multifactorial and is significantly influenced by lifestyle in which diet is important from the point of view of energy as well as other macro-nutrient intakes. The nature of fatty acids, even while appearing to have a low impact on lipoprotein concentrations, may play a role in the pathophysiology of atherosclerosis through other mechanisms. These mechanisms include postprandial vascular exposure and reactivity, coagulation and inflammation. The human individual has not evolved for avoidance of atherosclerosis but with current expectations of longevity it is important to ensure good nutrition that will avoid, or at least minimise, atherosclerosis and its complications.
How does the information gathered about diet and dyslipidaemia translated into practice for the person with mild dyslipidaemia? The ideal diet has the appropriate energy intake to sustain a lean body. Attempts to lose weight by low carbohydrate diets with increased protein intake, and usually also increased saturated fat intake may increase the risk of vascular disease( Reference Lagiou, Sandin and Lof 27 ). The intake of TAG is important for essential fatty acids and ensuring adequate nutrition for other nutrients will, except in the vegan, result in some cholesterol intake. In specific metabolic conditions very restricted diets may be required to ameliorate the disorder, mostly together with medication. The healthy diet in general comprises cereals, vegetables, fruit, legumes and nuts with marine products as a source of protein, while poultry and occasional red meat will not be problematic. Dairy products should preferably be low in fat unless consumed in small amounts. Nutriceuticals that may be of value are n-3 fatty acids and phytosterol spreads. The usual westernised profile of LDL hypercholesterolaemia can be viewed to be in a continuum with more severe LDL hypercholesterolaemias. Thus, the success of an intensively counselled ‘portfolio’ diet on plasma biochemical parameters( Reference Jenkins, Jones and Lamarche 28 ) in the setting of hyperlipidaemia could be translated to less severe dyslipidaemias in persons at risk for vascular disease, by advocating the use of plant sterols, soya protein, viscous fibres and nuts.
While this approach will have a dramatic impact on population statistics, patients with monogenic disorders need more accurate diagnosis and more aggressive treatments. Such problems are best evaluated at special centres such as lipid clinics. Uncommon and rare disorders such as hypertiglyceridaemia and phytosterolaemia, respectively, should be accompanied by much specialised dietary management. In the future, it is hoped that research in nutrition and disease will be able to identify more subtle patterns relating to the lipid profile, including fatty acid profiles and possibly provocative tests and interactions with genes, so that dietary advice can be tailored for all individuals.
Acknowledgements
The author has participated in interventional studies with pharmaceutical lipid modifying drugs as well as a sitosterol-containing spread. The author thanks F. Hassan for assistance in typing of this manuscript. This paper was not financially supported by any company.



