SAKK – Contact Details
Country
Switzerland
Chairs
O. Pagani, IOSI, Oncology Institute of Southern Switzerland, Ospedale Beata Vergine CH-6850 MENDRISIO, SWITZERLAND. Tel: +41 79 208 77 85 Fax: +41 91 811 30 27 Email: [email protected]
Dr S. Aebi, Universitätsspital Bern, Inselspital PT2, 3010 BERN, SWITZERLAND, Tel: +41 31 6324114 Fax: +41 31 3821237 Email: [email protected]
Website
SAKK – Study Details
Title
Randomized phase III trial of Herceptin followed by chemotherapy plus Herceptin versus the combination of Herceptin and chemotherapy as palliative treatment in patients with HER2-overexpressing advanced/metastatic breast cancer.
Protocol SAKK 22/99
Coordinator(s)
A. Goldhirsch, IOSI, Oncology Institute of Southern Switzerland, c/o Ospedale Italiano, Via Capelli, CH-6962 VIGANELLO-LUGANO, SWITZERLAND, Tel: +41 91 811 7923 Fax: +41 91 811 7925 Email: [email protected]
O. Pagani, IOSI, Oncology Institute of Southern Switzerland, Ospedale Beata Vergine, CH-6850 MENDRISIO, SWITZERLAND. Tel: +41 79 208 7785 Fax: +41 91 811 3027 Email: [email protected]
Summary
Primary Objective
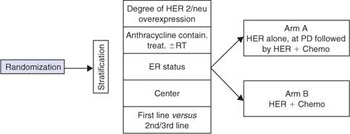
- To compare efficacy, toxicity and quality of life of the sequential administration of Her alone followed, at PD, by the combination with Chemotherapy (Arm A) versus the upfront combination of Herceptin and Chemotherapy (Arm B) in patients with advanced/metastatic breast cancer.
Secondary Objective
- To investigate the predictive value of serum HER2/neu ECD levels on clinical outcome, the effects of Her on estrogen receptor, and the association of immunoprofiles of erbB-1, erbB-2, erbB-3 and erbB-4 with clinical outcome.
Scheme
Update
- Activation date: 30 August 1999.
- Accrual at the end of September 2006: 100 patients.
Related Publications
None available
Topics
- HER2 positive patients
- Innovative schedules
- Metastatic breast cancer
- Predictive markers
- Taxanes
- Trastuzumab
- Vinorelbine
- Capecitabine
Keywords
Metastatic breast cancer, chemotherapy, targeted therapy, trastuzumab
***************************************************
Title
Trastuzumab monotherapy followed by the combination of trastuzumab and letrozole in post-menopausal women with ER-positive, HER-2 positive advanced breast cancer resistant to a nonsteroidal aromatase inhibitor.
A multicenter two-step phase II trial
Protocol SAKK 23/03
Coordinator(s)
Dr D. Köberle, Kantonsspital St Gallen ST GALLEN, SWITZERLAND. Tel: +41 71 494 11 11 Fax: +41 71 494 63 25 Email: [email protected]
Summary
Objectives
- The primary objective is to determine the efficacy of combined letrozole (L) and trastuzumab (T) in postmenopausal women with ER-positive, HER-2 positive advanced breast cancer resistant to sequential monotherapy of a nonsteroidal aromatase inhibitor followed by trastuzumab.
- The secondary objectives are to evaluate the safety profile of this combination by recording the adverse drug reactions and abnormal laboratory values during treatment and to investigate predictive markers for response to treatment with letrozole and trastuzumab in HER-2 positive, ER-positive positive tumors.
Scheme

Update
- Activation date: 12 May 2005.
- Accrual at the end of September 2006: 4 patients.
Related Publications
None available
Topics
- HER2 positive patients
- Hormonal therapy
- Hormone receptor positive breast cancer
- Innovative schedules
- Metastatic breast cancer
- Postmenopausal patients
- Predictive markers
- Trastuzumab
- Aromatase inhibitors
Keywords
Metastatic breast cancer, targeted therapy, trastuzumab, aromatase inhibitors, postmenopausal patients
***************************************************
Title
Phase I–II trial of capecitabine and vinorelbine in elderly patients (>65 years) with metastatic breast cancer with and without bone involvement.
Protocol SAKK 25/99
Coordinator(s)
D. Hess, Kantonsspital St Gallen, Medizinische Klinik C, 9007 ST GALLEN, SWITZERLAND. Tel: +41 71 494 1111 Fax: +41 71 494 6121 Email: [email protected]
Summary
- In order to evaluate the influence on hematologic and mucosal toxicity, the protocol was divided into two trials, one including patients with bone involvement and one without.
Phase I
- Identification of the maximum tolerated dose (MTD) of capecitabine and vinorelbine in elderly patients (>65 years) with metastatic breast cancer.
Phase II
- Evaluation of the efficacy and tolerability of capecitabine and vinorelbine in elderly patients (>65 years) with metastatic breast cancer.
- As a secondary objective, to assess the time to treatment failure (TTF) of capecitabine and vinorelbine as a first line chemotherapy. Ultimately the goal of this trial was to assess the efficacy and toxicity of the tested dose levels and schedule in this elderly population as a “pilot” for a later adjuvant trial in high-risk elderly patients after surgical treatment.
Phase II End Points
- Response rate, toxicity and time to treatment failure, quality of life.
Scheme
- Capecitabine po d 1–14
- Vinorelbine iv d 1 and d 8
- Cycles to be repeated every 21 days for a maximum of 6 cycles
Update
- Activation date: 10 March 1999.
- Accrual of patients with bone involvement closed on 8 December 2004: total accrual 46 patients.
- Accrual of patients without bone involvement closed on 7 September 2005: total accrual 24 patients.
Related Publications
Capecitabine and vinorelbine in elderly patients (≥65 years) with metastatic breast cancer: a phase I trial (SAKK 25/99).
Hess D, Thurlimann B, Pagani O et al. Ann Oncol 2004; 15: 1760–1765. Capecitabine and vinorelbine in elderly patients (> or = 65 years) with metastatic breast cancer: a phase I trial (SAKK 25/99)
Topics
- Elderly patients
- Metastatic breast cancer
- Treatment tailoring
- Vinorelbine
- Capecitabine
Keywords
Metastatic breast cancer, elderly patients, chemotherapy, dose finding
***************************************************
Title
Open multicenter phase II trial evaluating the antitumour efficacy of Faslodex (Fulvestrant) in postmenopausal women with advanced breast cancer failing non-steroidal or steroidal aromatase inhibitors.
Protocol SAKK 21/00
Coordinator(s)
Dr L. Perey, Centre Pluridisciplinaire d'Oncologie, 1011 LAUSANNE, SWITZERLAND. Tel: +41 21 314 11 Email: [email protected]
Summary
Primary Objective
- Objective response rate (=CR + PR) of Faslodex treatment.
Secondary Objectives
- Duration of clinical benefit (=CR + PR + SD ≥ 24 weeks), time to progression, duration of response, time to treatment failure and safety and tolerability of Faslodex treatment.
- Objective response rate according to Her-2/neu status.
The trial involved three levels of stratification:
Stratum A
Anastrozole or letrozole or aminoglutethimide responsive patients defined as patients who progressed while on anastrozole or letrozole or aminoglutethimide treatment given for advanced disease after initial objective response or disease stabilisation of at least 24 weeks.
Stratum B
Anastrozole or letrozole or aminoglutethimide resistant patients defined as patients who did not respond to anastrozole or letrozole or aminoglutethimide given for advanced disease or showed disease stabilisation lasting less than 24 weeks.
Stratum C
Eligible patients for whom strata A and B are not applicable, that is received anastrozole or letrozole or aminoglutethimide as adjuvant therapy.
Scheme
Faslodex (Fulvestrant) 250 mg intramuscular every month
Update
- Activation date: 13 March 2000.
- Accrual closed on 23 June 2005: a total of 90 patients have been entered (25 in Switzerland).
Related Publications
Perey L, Paridaens R, Hawle H et al. Clinical benefit of fulvestrant in postmenopausal women with advanced breast cancer and primary or acquired resistance to aromatase inhibitors: final results of phase II Swiss Group for Clinical Cancer Research Trial (SAKK 21/00). Annals of Oncology 2006; doi: 10.1093/annonc/mdl341
Topics
- Hormone receptor positive breast cancer
- Metastatic breast cancer
- Postmenopausal patients
- Hormonal therapy
Keywords
Metastatic breast cancer, postmenopausal patients, endocrine treatment
***************************************************
Title
Phase I/II trial of capecitabine with weekly paclitaxel for advanced breast cancer
Protocol SAKK 26/00
Coordinator(s)
Dr S. Aebi, Institute of Medical Oncology, University of Bern, Inselspital, 3010 BERN, SWITZERLAND. Tel: +41 31 632 41 14 Fax: +41 31 382 12 37 Email: [email protected]
Summary
Objectives
- To identify the maximum tolerated dose (MTD) of capecitabine in combination with paclitaxel in patients with metastatic breast cancer.
– Phase I: – dose finding.
– Phase II: – evaluation of efficacy and toxicity.
- To test the dose just below the MTD identified in the phase I study for clinical efficacy and toxicity in the same population. The final goal is to find a well-tolerated drug combination for patients with metastatic breast cancer.
Scheme
- Capecitabine po d 1–14
- Paclitaxel iv d 1, 8 and 15
- Cycles to be repeated every 21 days for a maximum of 6 cycles
Update
- Activation date: 22 May 2000.
- Accrual closed on 21 September 2004: a total of 35 patients were enrolled.
Related Publications
None available
Topics
- Metastatic breast cancer
- Treatment tailoring
- Capecitabine
- Taxanes
Keywords
Metastatic breast cancer, chemotherapy, dose finding


